CALIFORNIA GIANT SALAMANDER
Dicamptodon ensatus (Eschscholtz 1833)

California giant salamander, Santa Cruz County, California. Courtesy of Nicholas Hess.

Status Summary
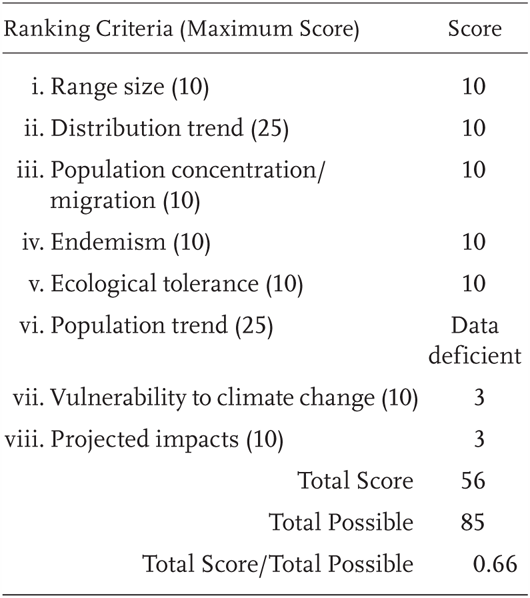
Dicamptodon ensatus is a Priority 3 Species of Special Concern, receiving a Total Score/Total Possible of 66% (56/85). This species was not previously considered a Species of Special Concern (Jennings and Hayes 1994a).
California Giant Salamander: Risk Factors
Identification
Dicamptodon ensatus is a large (6.3–17.3 cm SVL) robust salamander with a very large head and stout limbs. The dorsal coloration is a coppery tan to dark brown irregular marbled pattern on a tan to light reddish brown background. The venter is paler and usually unmarked, although marbling often extends onto the chin, throat, and under the legs. The marbling coloration is often brighter in young metamorphs compared to adults. The tail is laterally compressed, the skin is smooth, and post-metamorphic juveniles and adults lack tubercles on their feet (Stebbins 2003).
Larvae are of the stream type, with short bushy gills and a tail fin that begins at the insertion of the hind limbs and extends posteriorly to the tail tip. Larval dorsal coloration is light brown, and ventral coloration is white to yellowish white (Nussbaum 1976). There is also a pale eye stripe behind each eye, and the snout is depressed (Petranka 1998). The toe tips of larvae are black and cornified (Petranka 1998).
In California, D. ensatus is largely indistinguishable from the more widely distributed coastal giant salamander (D. tenebrosus) based on morphology alone. However, both geographic range and genetic markers distinguish these two species.
Taxonomic Relationships
Good (1989) split California Dicamptodon into two species, D. tenebrosus in the north and D. ensatus in the south, on the basis of allozyme data. A 4.7 km hybrid zone exists between the two species approximately 10 km north of Gualala in Mendocino County (Good 1989). Otherwise, the two species are allopatric.
Life History
Adult Dicamptodon ensatus are terrestrial and return to streams to breed during the fall rainy season (Kessel and Kessel 1943a) and in the spring (Stebbins 2003). One D. ensatus nest of approximately 70 eggs was found under a submerged wooden plank in a rapidly flowing stream in the Santa Cruz Mountains, San Mateo County, during June (Henry and Twitty 1940). Female D. tenebrosus guard nests through hatching (Nussbaum et al. 1983), and an adult female D. ensatus was found near the Santa Cruz Mountains nest (Henry and Twitty 1940), suggesting that both species may guard their eggs. Eggs in early developmental stages are pure white and approximately 5.5 mm in diameter (Petranka 1998). The larval stage lasts approximately 18 months, with larvae growing 8–12 mm in TL per month during the warmer months in their first year. Larvae reach 10 cm TL within a year of hatching and metamorphose in late summer at 13–14 cm TL (Kessel and Kessel 1943a, Kessel and Kessel 1943b, Kessel and Kessel 1944). The prevalence of paedomorphosis in this species is unknown, although it can be quite common in D. tenebrosus. A paedomorphic population of D. ensatus has been reported from caves on the UC Santa Cruz campus (B. Sinervo, unpublished data).
Bury (1972) reported gut contents of 12 adults from Del Norte, Humboldt, and Marin Counties (i.e., a mix of D. ensatus and D. tenebrosus). Eight out of 12 specimens contained one or more vertebrates, including California slender salamanders (Batrachoseps attenuatus), lizards, mice, shrews, and voles. Other prey included large invertebrates such as land snails and smaller invertebrates such as beetles and crickets (Bury 1972). Cannibalism has been documented in adults (Anderson 1960). No diet data from larvae are available for this species, though they are presumed to have similar diets to larval D. tenebrosus (Petranka 1998), which primarily consume aquatic insects and other invertebrates (Parker 1994).
Habitat Requirements
Dicamptodon ensatus occurs in mesic coastal forests (oak woodland and coniferous forest; Petranka 1998), and coastal chaparral habitat is used in southern Marin County and San Mateo County (N. Waters, pers. comm.). Very little is known about terrestrial habitat use by adults and metamorphs, although adults are occasionally found surface active or under cover objects in wet conditions (Petranka 1998). One unusual record exists of an adult D. ensatus in a tree vole (Arborimus pomo) nest 2.4 m off the ground, the only account of arboreality in this species (D. Hamilton and W. Roberts, unpublished data in Forsman and Swingle 2007).
Breeding and larval development occurs in cold permanent and semipermanent streams (Petranka 1998). Larval habitat use is poorly studied. In one stream, small larvae were found in slow-moving water near the banks during heavy flows, and as flows decreased they moved into the main stream channel where larger larvae occurred (Kessel and Kessel 1943a, Kessel and Kessel 1943b).
Distribution (Past and Present)
Dicamptodon ensatus is endemic to California, occupying a small range from sea level to 900 m in elevation along the coast in two isolated areas near San Francisco Bay (Stebbins 2003). North of the Bay, they occur in the outer Coast Ranges from near the southern border of Mendocino County south through Marin County, and the inner Coast Ranges in Napa, Sonoma, Lake, and Solano Counties (Good 1989). South of the Bay, they occur in the Santa Cruz Mountains in San Mateo, Santa Clara, and Santa Cruz Counties (Good 1989; N. Waters, pers. comm.). Dicamptodon ensatus has not been recorded in the East Bay (Stebbins 2003). Nussbaum (1976) mentioned an unconfirmed sight record from the Santa Lucia Mountains in Monterey County. Multiple surveys by several researchers over the decades have attempted to verify this account with no individuals detected (N. Waters, pers. comm.). While extirpations have not been documented, urbanization, agriculture, and timber harvest have likely resulted in some population losses, particularly due to development in the southern part of the range (Bury 2005; S. Barry, pers. comm.)
Trends in Abundance
Given the paucity of information, this species is currently considered data deficient for the population trend metric. However, it is likely that abundance has been reduced in habitats disturbed by urbanization, roadbuilding, logging, or water diversions (Bury 2005).
Nature and Degree of Threat
The Santa Cruz Mountains isolate is currently largely contained within a network of public parkland, though the extent of possible losses in this region due to past development is poorly understood (N. Waters, pers. comm., S. Barry, pers. comm.). Coast Range populations in the north are likely subject to negative effects from timber harvest and development, though this area is less urbanized than the southern part of the range. Disturbances such as clear-cutting and road construction can lead to lower abundances in Dicamptodon tenebrosus (Corn and Bury 1989, Welsh and Ollivier 1998). Other threats include fragmentation of riparian habitat, water diversions for municipal and agricultural use, and road mortality (N. Waters, pers. comm.).
Climate change may negatively impact D. ensatus, although uncertainty in climate projections coupled with limited ecological information makes assessing risk difficult. Mean annual temperature is expected to increase while projected changes in precipitation are likely modest, leading to warmer and possibly drier conditions in northwestern and central California (reviewed in PRBO 2011). At the same time, upwelling is expected to intensify (Snyder et al. 2003, Lebassi et al. 2009). This may increase fog development and contribute to cooler, moister conditions along the coast, potentially ameliorating effects of warming or drying within the range of D. ensatus. The frequency and extent of wildfire is expected to increase in the region encompassing the southern part of the range, with predicted increases in area burned of up to 50% (Fried et al. 2004, Lenihan et al. 2008, Westerling and Bryant 2008). How fire regime will change in the northern part of the range is less well understood (reviewed in PRBO 2011). Effects of wildfire on D. ensatus are unknown, though mortality and habitat degradation due to fire has been documented in other stream-breeding amphibians (e.g., Gamradt and Katz 1997, Pilliod et al. 2003). In northwestern California, vegetation communities are expected to shift from moist conifer to drier mixed evergreen forest, with reductions in Douglas fir and redwood forest in particular (Lenihan et al. 2008, PRBO 2011), which may impact the availability of D. ensatus habitat.
Status Determination
Dicamptodon ensatus is an endemic, ecologically specialized salamander with a small geographic range that is restricted to an area with a high human population density. These factors combine to place it at high risk of habitat loss and disturbance. However, data are not available to determine whether ongoing declines and population losses have occurred, resulting in a Priority 3 designation for this species.
Management Recommendations
We know little about the basic biology of this species, which makes it difficult to formulate management recommendations beyond minimizing disturbances to existing habitat. Habitat protection may be particularly important for small headwater streams where siltation and other stream disturbances are known to severely impact other Dicamptodon species. Construction and use of roads should be eliminated or minimized within D. ensatus habitat, particularly during the breeding season. Riparian buffer vegetation should be retained in areas that are developed or harvested, though efficacy of buffers and optimal buffer widths for this taxon are unknown.
Monitoring, Research, and Survey Needs
Distribution, abundance, habitat requirements, and life history of Dicamptodon ensatus all need further study. Most research to date has focused on the more widespread D. tenebrosus to the north and was conducted before the two species were recognized as distinct. This substantial knowledge gap needs to be addressed with basic ecological studies. Nothing is known about dispersal in this species, especially the importance of movement through terrestrial habitats. Both mark–recapture and landscape genetic studies are needed for D. ensatus. Studies are also needed that examine the efficacy of streamside buffers in ameliorating the effects of disturbance on stream habitats. Such studies should be replicated both north and south of San Francisco Bay, given that these are completely isolated population segments living in different habitats. Distributional surveys are particularly needed in the Inner Coast Range portion of the northern range (N. Waters, pers. comm.).
While larvae are easy to find by searching aquatic habitats, transformed D. ensatus are infrequently encountered using typical amphibian survey techniques. For example, only 12 individuals were captured in 18,032 trap nights over 3 years of pitfall trapping along 840 m of drift fence in suitable habitat at Point Reyes National Seashore (G. Fellers and D. Pratt, unpublished data, in Fellers et al. 2010). In the same study, no Dicamptodon were detected under 84 coverboards during nearly 2000 coverboard checks. However, culvert removal using heavy equipment uncovered aggregations of >20 adults at the same study sites, suggesting that terrestrial sampling may severely underestimate abundance (Fellers et al. 2010). Another account from Santa Cruz County reported several adults and eggs getting washed out of a drill hole made 6 m into a hillside to access a subterranean spring (Dethlefsen 1948). These reports suggest that metamorphosed individuals may be largely subterranean in their habits, a possibility that needs further investigation.