SOUTHERN TORRENT SALAMANDER
Rhyacotriton variegatus Stebbins and Lowe 1951

Southern torrent salamander, Mendocino County, California. Courtesy of Robert Thomson.

Status Summary
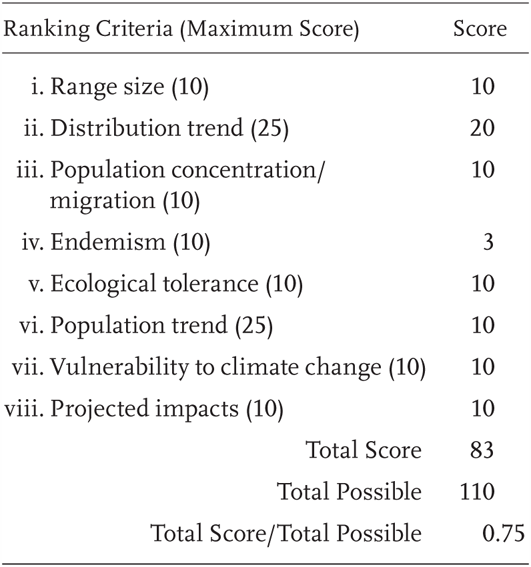
Rhyacotriton variegatus is a Priority 1 Species of Special Concern, receiving a Total Score/Total Possible of 75% (83/110). Previously it was also considered a Species of Special Concern, although at a lower priority level. Additional research on ecology and phylogeography since Jennings and Hayes (1994a) supports this change in status.
Southern Torrent Salamander: Risk Factors
Identification
Rhyacotriton variegatus is a small to medium-sized salamander (5 cm SVL) (Welsh and Lind 1992, Tait and Diller 2006), with a small head and a short, laterally compressed tail (Stebbins 2003). Expanded square-shaped glands lateral and posterior to the vent in adult males distinguish this genus from all other North American salamanders (Petranka 1998). Rhyacotriton has large bulging eyes, with eye diameter roughly equal to the distance between the anterior edge of the eye and the tip of the snout (Stebbins 2003). The dorsal ground color is brownish to olive, and the venter is yellow to yellowish green with a sharp, abrupt demarcation between the dorsal and ventral coloration (Petranka 1998). California R. variegatus are heavily speckled with small dark spots on the dorsum and venter (Good and Wake 1992).
Larvae are of the stream type and have morphological adaptations unique to headwater specialists (Valentine and Dennis 1964). Larvae have short stubby gills and a tail fin that does not extend anteriorly onto the trunk. The dorsum is light brown above, the venter is cream to yellow, and the body is sprinkled with dark speckling above and below except on the tail fin. The eyes are prominent and dorsally positioned (Petranka 1998).
Taxonomic Relationships
Rhyacotriton variegatus has been recognized as a species since 1992 based on protein variation (Good and Wake 1992). Miller et al. (2006) identified three mitochondrial DNA clades within R. variegatus. The California clade/southern Oregon clade split occurs at the Smith River in California, a common biogeographic boundary. Miller et al. (2006) concluded that the California clade constitutes an evolutionarily significant unit (sensu Moritz 1994). The California clade is endemic to the state with a ∼50% smaller range than the species as a whole, and the southern Oregon clade animals in California have an extremely small range. Although Miller et al. (2006) recognized these clades as potential management units, we consider them as a single taxon here pending additional research on their geographic ranges and genetic distinctiveness using additional molecular markers.
Life History
Breeding may occur throughout much of the year. Males produce sperm year-round, with peak production from February through April (Humboldt County; Tait and Diller 2006). California females have been found carrying spermatophores from February through June (Stebbins and Lowe 1951, Tait and Diller 2006), and females from an Oregon population had cloacal spermatophores as late as October (Nussbaum and Tait 1977).
Females produce smaller clutches than most similarly sized stream-breeding salamanders (Petranka 1998), with gravid females carrying from 4 to 16 ovarian eggs (Nussbaum and Tait 1977, Good and Wake 1992, Tait and Diller 2006). Karraker (1999) found a nest with 11 cream-colored eggs deposited singly beneath a small boulder in a first-order stream channel in Humboldt County.
Developmental times are slow, with oviposition to sexual maturity taking approximately 4.5 years (Nussbaum and Tait 1977, Tait and Diller 2006). Time from oviposition to hatching is roughly 8 months (Karraker 1999), with time from oviposition to absorption of yolk probably closer to a year (Tait and Diller 2006). Peak oviposition is in August and September in California, with peak hatching occurring in the spring (Humboldt County; Tait and Diller 2006). Larval development from hatching to metamorphosis takes 2–2.5 years (Nussbaum and Tait 1977, Tait and Diller 2006). After metamorphosis, an additional 1–1.5 years of growth is required before sexual maturity is attained (Nussbaum and Tait 1977, Tait and Diller 2006).
The extended reproductive period and overwintering of larvae result in overlapping size cohorts in streams (Welsh and Lind 1992, Tait and Diller 2006). Hatchlings are 14–16 mm SVL (Tait and Diller 2006), and size at metamorphosis is around 35 mm SVL (Nussbaum and Tait 1977, Good and Wake 1992, Tait and Diller 2006). In Humboldt County, larval growth rates were recorded as 2.3 mm/year in Six Rivers National Forest (Welsh and Lind 1992) and 8.9 mm/year in a more coastal site in the Mad River drainage (Tait and Diller 2006). Larvae and adults weighed more in the spring than fall at one site, suggesting active foraging and growth over the winter months (Welsh and Lind 1992).
Adults are active at air and water temperatures of 5–10°C, lower than those known for any other aquatic salamander (Stebbins and Lowe 1951, Stebbins 1955, Brattstrom 1963). The average critical thermal maximum for adults and larvae are also lower than reported for other salamanders (larvae: 26.7°C; adults: 27.9°C; Bury 2008b). Welsh and Lind (1996) observed signs of stress in adults at 17.2°C. Thermal tolerances of eggs are unknown (Bury 2008b).
Very few data are available on movement or diet in this species. One mark–recapture study at a single headwater stream/seep site in Humboldt County found extremely low levels of movement, with approximately 1 m/year of movement for adults and 2 m/year for larvae on average (Welsh and Lind 1992). However, unrecaptured animals may have moved longer distances (20% of originally marked animals were recaptured). The diet of Rhyacotriton variegatus appears to be generalized on aquatic and semiaquatic invertebrates, with amphipods and collembolans the most abundant prey (Bury and Martin 1967).
Habitat Requirements
Rhyacotriton variegatus occurs within a relatively narrow range of ecological conditions that are typical of late-seral forests. These conditions include cold, clear, flowing permanent seeps and headwater to low-order streams with coarse, rocky substrates in mesic to moist forests (Welsh and Lind 1988, Welsh 1990, Welsh and Lind 1991, Welsh and Lind 1996, Vesely and McComb 2002, Welsh et al. 2005, Ashton et al. 2006, Welsh and Hodgson 2011). Key habitat requirements are the maintenance of cold water temperatures (6.5–15°C) and presence of loose substrates composed of gravel and cobble (Diller and Wallace 1996, Welsh and Lind 1996, Stoddard and Hayes 2005, Welsh et al. 2005, Bury 2008b, Welsh and Hodgson 2008). In the Mattole Watershed, R. variegatus occurred primarily in undisturbed headwater channels and was never detected in streams where canopy closure was less than 91% or water temperatures were warmer than 13.5°C (Welsh and Hodgson 2011). Rhyacotriton variegatus is extremely desiccation intolerant (Ray 1958), although it will occasionally venture away from the stream channel and use riparian and forest habitat in the wet season (Vesely and McComb 2002; Vesely and McComb, pers. obs., in Welsh and Lind 1996).
Rhyacotriton variegatus is sensitive to fine sediment load and embeddedness (Welsh and Lind 1996, Welsh and Ollivier 1998) and has been found to be positively associated with high-gradient streams, particularly in areas with timber harvesting. This may be due to stream network processes that flush fine sediments out of high-gradient reaches (Corn and Bury 1989, Diller and Wallace 1996, Stoddard and Hayes 2005, Ashton et al. 2006). In a review of seven studies of R. variegatus habitat associations, Welsh and Hodgson (2008) found that the species occurred at sites where fine sediment ranged from 2% to 40%, and zero detections occurred when more than 65% of the coarse substrate was embedded with fine sediment.
Distribution (Past and Present)
Rhyacotriton variegatus occurs patchily at elevations below 1469 m throughout the Pacific Coast Ranges of Oregon and California, from the Little Nestucca River and Grande Ronde Valley in Oregon to near Alder Creek in Mendocino County in California (Good and Wake 1992). Populations also occur in the Cascade Range in Oregon (Good and Wake 1992, Miller et al. 2006). A previously reported disjunct population in the McCloud River, Siskiyou County, appears to be based on incorrectly identified museum specimens of the southern long-toed salamander (Ambystoma macrodactylum sigillatum) in the California State University, Chico collection.
Suitable microhabitat is patchily distributed in California, and R. variegatus is only found in suitable sites about half of the time. Random stratified sampling of 117 sites throughout the geographic range in California found that 45% of sites contained suitable microhabitat, but only 62% of those sites were occupied (Welsh and Lind 1992). Sampling of 38 different sites in the same region selected for the US Forest Service “Old-growth Wildlife Project” found suitable microhabitat in 79% of sites, with R. variegatus present in 47% of suitable sites (Welsh and Lind 1992). Systematic stratified sampling of 53 mixed conifer–hardwood stands on public lands in northern California found R. variegatus at 62% of sites (Welsh and Lind 1996).
Some of the variation in distribution can be explained by forest age and timber harvest histories, with R. variegatus more often found in older, unharvested stands. Welsh (1990) surveyed spring and seep habitats in 34 forest stands in the Coast Ranges in California and southern Oregon ranging from 30 to 560 years old and at elevations of 150–1500 m. Rhyacotriton variegatus was found in 70% of old-growth stands, 50% of mature stands, and 11% of young stands. Recent surveys of the Mattole Watershed in northern California (Humboldt and Mendocino Counties) found R. variegatus mostly in late-seral headwater tributaries, habitats that are now rare in the watershed (Welsh et al. 2005, Welsh and Hodgson 2011). However, occupancy rates were higher in young forests along the coast where temperatures are mediated by the maritime climate: R. variegatus was found in 48% of 30 m sampling reaches and 80% of entire stream reaches in stands less than 80 years old (Diller and Wallace 1996).
Exact figures are difficult to come by, but most of the historical coastal old-growth habitat in California is now gone (85–96.5% gone; references in USFWS 1997). In addition to habitat modification, several investigators have hypothesized that Dicamptodon predation may restrict Rhyacotriton distribution to small headwater streams (e.g., Stebbins 1955, Nussbaum 1969, Welsh and Lind 1996, Welsh and Ollivier 1998). However, Rundio and Olson (2001) found that R. variegatus larvae were unpalatable to D. tenebrosus larvae, surviving 90% of encounters in experimental trials.
Trends in Abundance
Estimates of abundance are not available for time periods before timber harvesting became a prominent factor in landscape management, but the highest documented abundances over the last several decades have been in late-seral sites, supporting the idea that abundances are reduced in response to disturbances such as timber harvest and road building. Rhyacotriton variegatus can be locally abundant, with densities of up to 22 salamanders/m2 recorded in suitable streamside habitat at an old-growth site in Six Rivers National Forest, Humboldt County (Welsh and Lind 1992). However, most sites in that study yielded 1–5 captures/10 m2 (Welsh and Lind 1992). By sampling across the range of R. variegatus in California and across stands of different ages, Welsh and Lind (1996) documented a much lower mean density of 0.68 salamanders/m2. In young stands in coastal northern California (<80 years old), Diller and Wallace (1996) found that densities were 0.18–5.5 salamanders/m2. Welsh et al. (2000) reanalyzed Welsh and Ollivier’s (1998) data from sites in Prairie Creek Redwoods State Park in Humboldt County for comparison to encounter rate data reported by Wroble and Waters (1989) from timber company lands in the same county. Rhyacotriton variegatus was found at the rate of 0.72 salamanders/hour on parkland compared to 0.05 salamanders/hour on harvested lands (Welsh et al. 2000). In Oregon, densities averaged 0.29 salamanders/m2 on forested lands versus 0.04 salamanders/m2 on logged habitat (Corn and Bury 1989).
Nature and Degree of Threat
Major threats to this species include timber harvesting, road building, rural development, marijuana cultivation, and climate change. Rhyacotriton variegatus is sensitive to the impacts of timber harvesting and roadbuilding due to direct impacts of heavy equipment and indirect effects on temperature, humidity, and sediment load (Welsh et al. 2000, Welsh and Hodgson 2008). Several researchers have argued that declines and extirpations will continue due to timber harvesting and related land management practices (e.g., Welsh et al. 2000, Ashton et al. 2006, Olson et al. 2007, Welsh and Hodgson 2008). While R. variegatus can persist in some harvested areas, particularly in coastal forests where the effects of logging may be ameliorated by the milder climate (e.g., Welsh 1990, Diller and Wallace 1996; S. Barry, unpublished data), it occurs in more sites and with higher density in older stands.
Habitat loss and degradation due to rural residential development and marijuana cultivation is a growing concern for this species in California. Every new house built in forested lands requires a source of water, which is often provided by diverting headwater streams. In some cases, R. variegatus has been observed to occur above but not below such diversions (M. van Hattem, pers. comm.). This threat is likely to increase in the near future. For example, the Humboldt County General Plan is currently being updated, with some proposals considering a doubling or tripling of rural development. Marijuana cultivation also presents a water diversion threat to this species, as well as potential negative impacts due to grading, roadbuilding, and the application of herbicides and pesticides (e.g., Thompson et al. 2014).
Rhyacotriton variegatus has slow developmental times and low vagility, leading to potentially high susceptibility to rapidly changing environmental conditions. Expected climate changes within its range over the next 100 years include increased temperatures, changes in hydrology, changes in fire regime, and vegetation shifts. Mean annual temperatures are expected to increase throughout the range of R. variegatus in California (reviewed in PRBO 2011). The frequency of extremely hot days is projected to increase, with roughly 9 additional days over 32.2°C (Bell et al. 2004). Such temperatures exceed the critical thermal maxima for adults and larvae of R. variegatus, although water temperatures, microhabitat structure, and behavioral thermoregulation may ameliorate these effects. For coastal populations, upwelling is expected to intensify, which may increase fog development and contribute to cooler, moister conditions (Snyder et al. 2003, Lebassi et al. 2009). Coastal populations may therefore continue to provide more favorable climatic conditions than areas farther inland. Potential changes in precipitation are less clear, with some models predicting modest increases, others modest decreases, and others reductions in rainfall of up to 28% (reviewed in PRBO 2011). Warmer temperatures will result in less precipitation stored as snow, and reductions of 30–80% are predicted for snowpack accumulation in northwestern California (Snyder et al. 2004, Cayan et al. 2008b). The timing of spring snowmelt has shifted later in the spring in this region over the last 50 years (Stewart et al. 2005), though the timing of future shifts is unknown. Reductions in water availability due to reduced snowpack and possibly reduced precipitation will affect the timing and magnitude of stream flows. This may negatively affect habitat quality and availability for all life stages of this highly aquatic salamander. How fire regime will be affected by climate change in northwestern California is not well understood. Some models predict little change in fire regime or even decreases in area burned along the northern coast (Fried et al. 2004, Lenihan et al. 2008), while increases in area burned have been predicted for the southern coast of northwestern California (Lenihan et al. 2008). Westerling et al. (2011) projected a 100% increase in area burned in northwestern California under some scenarios. How fire affects R. variegatus needs further study, although direct mortality and habitat degradation due to fire have been documented in other stream-breeding amphibians (e.g., Gamradt and Kats 1997, Pilliod et al. 2003). Vegetation communities are expected to shift from moist conifer to drier mixed evergreen forest, with reductions in Douglas fir and redwood forest in particular (Lenihan et al. 2008, PRBO 2011). It is unclear what effect these shifts may have on R. variegatus because stream conditions and forest age seem to be more important indicators of habitat quality than forest type.
Status Determination
Rhyacotriton variegatus is a Priority 1 Species of Special Concern due to its high degree of habitat specificity resulting in a patchy distribution in isolated habitat islands, high degree of genetic variation among management units, and association with late-seral forests that are now rare and often ecologically compromised by timber harvesting (Good and Wake 1992, Welsh and Lind 1996).
Management Recommendations
Rhyacotriton variegatus populations would benefit from forest management activities that maintain cold water temperatures and low sedimentation levels such as decreasing the use and building of roads, decreasing timber harvest, and leaving riparian vegetation intact in harvested areas. Suitable microhabitats should be surveyed for R. variegatus presence during the wet season when salamanders are more likely to be detected before such areas are disturbed (Tait and Diller 2006, Olson et al. 2007). Monitoring activities themselves can damage sensitive microhabitats (L. Diller, pers. comm.), and personnel should be well trained in techniques to minimize such negative effects. Occupied microhabitats in particular should be protected from direct impacts of heavy equipment. In areas where timber harvest occurs, vegetation should be left intact around R. variegatus habitat, particularly to maintain canopy cover, though the width and configuration of such buffers is an important research need detailed below. In the absence of more detailed research, Olson et al. (2007) recommend using relatively wide buffers on the order of 40–150 m to maintain obligate riparian species. In addition to buffers along streams, habitat should be left intact around seeps (“leave islands”; reviewed in Olson et al. 2007). Marijuana cultivation appears to pose a growing threat to maintenance of high-quality habitat for this species. Enforcement and regulation of marijuana cultivation is an ongoing issue in California and we suggest that the environmental impact of such activities be considered. Little is known about use of upland habitats, but protection of large channel networks and associated seeps and springs to maintain aquatic and upland connectivity would likely help maintain populations of R. variegatus (Welsh and Lind 1992, Vesely and McComb 2002, Olson et al. 2007, Welsh 2011).
Monitoring, Research, and Survey Needs
Several studies have been conducted to determine the presence/absence of Rhyacotriton variegatus across the landscape, and such surveys should continue. A critical research need is studies that monitor population abundance over time, particularly under different timber harvesting regimes. Given the long life span and slow development time of this species, such long-term studies might provide insights that shorter, single-season analyses would miss. When possible, population estimates in managed forests should be compared to R. variegatus abundance in nearby undisturbed mature forest stands (i.e., reference populations) to assess the impacts of disturbance (Welsh 2011). Additional studies on movement ecology and dispersal beyond localized movements would aid in designing management strategies to promote habitat connectivity. The extent to which upland versus aquatic habitats are used for dispersal is unknown and is crucial for determining whether buffers should be focused around continuous waterways, upland linkages between waterways, or both (Welsh and Lind 1992, Olson et al. 2007, Welsh 2011).
Experiments that test the efficacy of buffer strips for maintaining favorable habitat conditions in harvested areas would also be valuable. Buffer strips from 6 to over 90 m wide have been proposed for maintaining riparian fauna under a range of management scenarios (reviewed in Olson et al. 2007). Stoddard and Hayes (2005) recommended buffer strips >46 m wide for Rhyacotriton. Similarly, riparian buffer strips 40 m wide around first through third-order streams in Oregon supported similar salamander abundance (including R. variegatus) as unharvested stands (Vesely and McComb 2002). Welsh and Hodgson (2008) recommend stream temperatures <15°C to maintain populations. The relationship between the size and aspect of a subbasin, the amount of the surrounding area harvested, the resulting maximum stream temperature, and how much buffer would be required to ameliorate any critical biological temperature thresholds are important research needs (Welsh et al. 2005). Temperature is not the only factor that can be influenced by management activities however, and other indicators of habitat quality such as embeddedness should be measured as well (Olson et al. 2007).
Because R. variegatus is patchily distributed, monitoring studies should first identify areas with suitable habitat. In surveys for R. variegatus in Douglas fir/hardwood forests in the Klamath region, Welsh and Lind (1992, 1996) defined minimum essential microhabitat for R. variegatus as an area of at least 10 m2 of flowing water (e.g., a patch of spring seep or first- or second-order streams) at least 75 m away from a forest edge. Within these sites, aquatic searches seemed most effective at detecting R. variegatus, as they are rarely encountered using techniques such as terrestrial pitfall trapping (e.g., Welsh 1990). Sampling should be done in the spring when R. variegatus are most abundant (Welsh and Lind 1992, Ashton et al. 2006, Tait and Diller 2006).
Landscape genetic studies that quantify levels of connectivity within and across stream systems would help to better delimit local management units as well as important dispersal corridors for this species. Studies similar to recent analyses on another western stream salamander, the Idaho giant salamander (Dicamptodon aterrimus) (Mullen et al. 2010), would be particularly instructive as a way to examine the relationship between stream connectivity and salamander gene flow.