PANAMINT ALLIGATOR LIZARD
Elgaria panamintina (Stebbins 1958)

Panamint alligator lizard, Inyo County, California. Courtesy of Adam Clause.

Status Summary
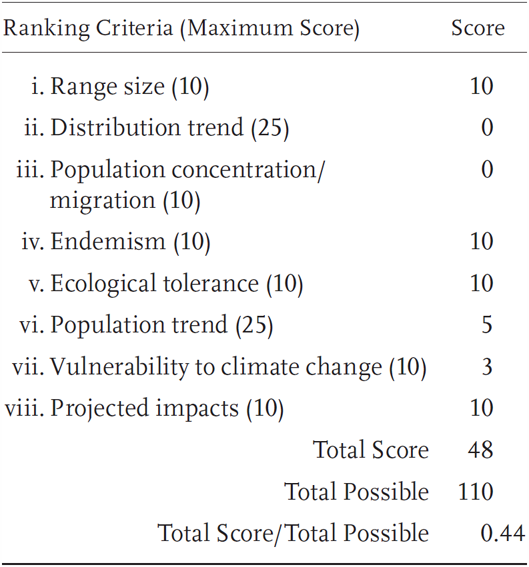
Elgaria panamintina is a Priority 3 Species of Special Concern, receiving a Total Score/Total Possible of 44% (48/110). During the previous evaluation, it was also designated as a Species of Special Concern (Jennings and Hayes 1994a).
Panamint Alligator Lizard: Risk Factors
Identification
Elgaria panamintina is a large (9.2–15.2 cm SVL), slender, elongate lizard with a light yellow-brown or beige dorsum and a series of contrasting brown crossbands extending from the neck down the length of the body and tail (Stebbins 1958, Banta et al. 1996, Stebbins 2003). The ventral surface is light gray or cream, with small dusky markings forming continuous or broken longitudinal lines that run down the center of each scale row (Jennings and Hayes 1994a, Stebbins 2003). The iris is pale yellow (Stebbins 2003). The contrast between the dark crossbands and lighter dorsal coloration is usually more pronounced in juveniles than in adults. The tail, when intact, is up to twice the length of the body, although shorter broken/regenerated tails are common (Stebbins 2003).
This lizard is unlikely to be confused with any other species within its range. However, two similar congeners occur in much of California: the northern alligator lizard (E. coerulea) and the southern alligator lizard (E. multicarinata). Neither of these species has the pattern of broad strongly contrasting cross-bands down the length of the body. The cross-bands are usually interrupted by a longitudinal, middorsal stripe in E. coerulea and are much narrower in E. multicarinata (Stebbins 2003).
Taxonomic Relationships
Different studies have recovered discordant phylogenetic placements of Elgaria panamintina. Good (1988) recovered a sister relationship between E. panamintina and the Madrean alligator lizard (E. kingii) from Arizona, using a dataset composed of 34 allozyme loci. More recent studies find that E. panamintina is nested within E. multicarinata, a placement that was supported by both mitochondrial sequence data (Feldman and Spicer 2006) and nuclear sequence data (D. Leavitt et al., unpublished data).
Leavitt et al. (unpublished data) found low levels of variation among populations of E. panamintina and no evidence for recent or ongoing gene flow between this species and other Elgaria in western North America. The discordance of the allozyme and nuclear sequence data, and therefore the monophyly of E. multicarinata with respect to E. panamintina, awaits further investigation.
Life History
The life history of E. panamintina is poorly understood. The species spends a large amount of time in rock piles and deep vegetation or brush, so it is not commonly observed (Stebbins 1958, Macey and Papenfuss 1991b). We presume that many aspects of E. panamintina’s life history are similar to that found in the better-studied E. multicarinata, particularly given the recent molecular evidence of their very close relationship.
Elgaria panamintina emerges from hibernation in late winter or spring, with higher-elevation populations becoming active later in the year. The species is generally diurnal in the spring through midsummer, when it may switch to nocturnal activity or aestivation, presumably as a response to increasing daytime temperatures (Stebbins 1958, Banta 1963, Dixon 1975, Stebbins 2003). Reproduction has not been documented in the wild, although captive animals have been observed copulating in mid-May (Banta and Leviton 1961). Elgaria multicarinata enters reproductive condition at this time of year as well (Goldberg 1972), so we assume that reproduction occurs in midspring, although the precise timing likely depends on elevation. Goldberg and Beaman (2003) examined sperm formation in museum specimens and concluded that reproduction takes place during the spring. Like E. multicarinata (and unlike E. coerulea), E. panamintina is oviparous. Elgaria multicarinata typically lays eggs in early summer, and we assume that E. panamintina does as well (Goldberg 1972). The timing of reproductive events in E. multicarinata varies among areas, with some populations producing only one clutch a year and others up to three (Burrage 1965, Goldberg 1972). No data on the number of clutches produced per year or incubation times exist for E. panamintina, although Goldberg and Beaman (2003) report a clutch size of four eggs from a single museum specimen.
Dietary data are lacking. We presume that E. panamintina is likely a generalist predator like E. multicarinata. The latter feeds on a wide variety of insects and other small arthropods, including spiders, centipedes, and scorpions, as well as on small vertebrates, including mice, birds, and lizards (including conspecifics) (Cunningham 1956). Observations in captivity found no obvious differences in feeding behavior between E. panamintina, E. multicarinata, and E. kingii, and we tentatively assume that feeding behavior is also similar in the wild (Stebbins 1958).
Elgaria species have a lower thermal tolerance than most sympatric lizards, which may allow them to maintain higher activity levels in the shaded moist habitats in which they are most commonly found (Cunningham 1956, Stebbins 1958). Predation on E. panamintina has not been recorded, though we assume that they are preyed upon by co-distributed lizard-eating snakes (e.g., coachwhips [Masticophis] and patch-nosed snakes [Salvadora]) and birds (e.g., raptors and roadrunners [Geococcyx]).
Habitat Requirements
Elgaria panamintina are most frequently found in rocky canyons in the immediate vicinity of permanent springs and seeps that are patchily distributed across their limited range (Stebbins 1958, Macey and Papenfuss 1991b). The species usually occurs in or adjacent to narrow strips of riparian vegetation immediately below springs and in deep leaf litter and rock piles along the margins of riparian habitat (Stebbins 1958, Macey and Papenfuss 1991b, Jennings and Hayes 1994a). Elgaria panamintina was initially thought to be restricted to these areas, but pitfall trapping surveys have documented their presence in arid areas well away from water (Banta 1963). Few quantitative data are available on the relative frequency of arid versus mesic habitat use, and it seems likely that populations require permanent water for persistence.
Distribution (Past and Present)
Elgaria panamintina occurs in relatively remote regions of the Great Basin in California. Given the difficulty of accessing much of its potential habitat and the limited work on the species to date, it may occur more widely than has so far been recorded. The known range encompasses many of the desert mountain ranges of Inyo and southern Mono Counties, including the Panamint, Inyo, Nelson, Argus, and Coso Mountains, as well as the western slopes of the White Mountains (Macey and Papenfuss 1991b, Banta et al. 1996, La Berteaux and Garlinger 1998). The known elevational range extends from 760 to 2290 m (Dixon 1975, Macey and Papenfuss 1991b, Stebbins 2003).
The species’ present-day distribution is likely relictual, resulting from gradual drying of the Great Basin throughout the Pliocene and Pleistocene. This general drying has presumably isolated the remaining populations around the few remaining water sources (Stebbins 1958, Good 1988).
Trends in Abundance
No data are available regarding current or historical abundance, although habitat degradation due to mining, livestock grazing, and off-highway vehicle use has likely resulted in population declines (Jennings and Hayes 1994a). Given the very sensitive nature of the remaining islands of mesic habitat in the region, surveys of both population size and connectivity via arid habitat occupancy are needed to provide baseline information on current status.
Nature and Degree of Threat
The primary threat to this species is habitat loss or alteration in its already small range. Many of the known localities occur on private land and are vulnerable to mining, livestock grazing, off-highway vehicle use, and/or diversion of the water sources. Climate change could potentially impact this species if changes in hydrology cause springs to dry up or become less regular in their flow regimes.
Status Determination
Elgaria panamintina is a California endemic with a very small range. It primarily occurs in, and is likely dependent upon, uncommon, small patches of mesic habitat that are scattered widely throughout its range. Each habitat patch is sensitive to several potential disturbances, and if local extirpations occur, natural recolonization seems unlikely. Nearly all known localities occur on unprotected land and are subject to further alteration (Jennings and Hayes 1994a). These factors all contribute to a Priority 3 designation.
Management Recommendations
Terrestrial habitat surrounding permanent springs and seeps should be protected from water diversion and destruction or alteration of riparian vegetation. There may well be conflicts with livestock and large feral mammals since these animals may trample or otherwise disturb the vegetation and leaf litter surrounding desert springs. Elgaria panamintina may also occur at additional springs outside of its currently known range; therefore, riparian areas throughout the area should be preserved to the extent possible, even if E. panamintina has not yet specifically been documented at them.
Monitoring, Research, and Survey Needs
Surveys should be conducted at additional springs surrounding the known distribution of Elgaria pananmintina. These surveys should involve pitfall trapping and/or drift fence arrays, in order to increase detection probabilities. A thorough understanding of E. panamintina’s habitat requirements would be invaluable in determining what habitat modifications can be made to riparian areas without negatively impacting the species, as well as identifying suitable areas to focus survey efforts to look for new populations. A key question is the extent to which the species uses arid habitat away from springs, both as corridors for dispersal among springs and as upland habitat. Both drift fence surveys of this habitat and landscape genetic analyses of known spring populations may contribute to greater understanding of habitat use in this species. The lack of basic life history information on E. panamintina also needs to be addressed. Mark–recapture surveys would yield important information about population sizes and the extent of migration between springs. This basic information is crucial for any kind of active management and is largely lacking at the present time.