MOJAVE FRINGE-TOED LIZARD
Uma scoparia Cope 1894

Mojave fringe-toed lizard, San Bernardino County, California. Courtesy of Luke Mahler.

Status Summary
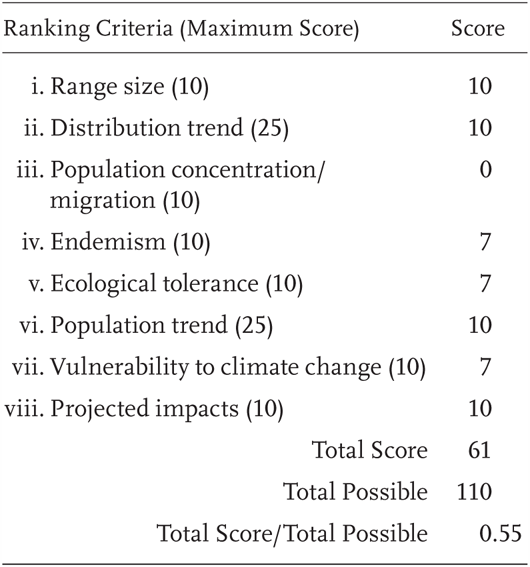
Uma scoparia is a Priority 3 Species of Special Concern, receiving a Total Score/Total Possible of 55% (61/110). During the previous evaluation, it was also considered a Species of Special Concern (Jennings and Hayes 1994a).
Mojave Fringe-Toed Lizard: Risk Factors
Identification
Uma scoparia is a medium-sized lizard (7.0–11.4 cm SVL) with a moderately flattened body, a countersunk lower jaw, keeled labial scales, a projecting row of pointed scales on the toes, eyelids, and ear openings that form a fringe (Cope 1894, Stebbins 1954). The dorsal ground coloration is black and is heavily covered, with a pattern of white or tan ocelli with blackish to reddish centers that do not form lines over the shoulders (Cope 1894, Heifetz 1941, Jennings and Hayes 1994a, Stebbins 2003). This dark coloration fades to brown or tan on the head, limbs, and tail. The light dorsal coloration tends to vary among populations and usually matches the color of the sand in the vicinity (Miller and Stebbins 1964). The ventral surface is white, with two prominent black spots on either side of the body (some populations have an additional set of preanal spots) and black bars along the underside of the tail (Heifetz 1941). The throat is marked with narrow crescent-shaped black bars (Cope 1895b, Heifetz 1941, Stebbins 2003). During the breeding season, a yellow-green wash may develop on the ventral surface and fade into pink on the sides (Stebbins 2003).
This species could be confused with its congeners, the Coachella Valley fringe-toed lizard (U. inornata) and the Colorado Desert fringetoed lizard (U. notata). Uma inornata has greatly reduced, or lacks altogether, the conspicuous black spots on the sides of the belly and has ocelli that tend to form lines over the shoulders. Uma notata usually has diagonal lines on the throat rather than crescent-shaped lines and has ocelli that tend to form lines over the shoulders (Stebbins 2003). These three species do not overlap in range, although U. scoparia is broadly sympatric with the zebratailed lizard (C. draconoides), with which it also might be confused. Callisaurus draconoides lacks fringe scales on the ear openings and toes, has an overall slimmer body shape, and has black bands that form rings around the distal portion of the tail rather than only being on the tail underside (Stebbins 2003).
Taxonomic Relationships
Uma scoparia was initially described on the basis of femoral pore counts and several scalation characters (Cope 1894, Cope 1895b). It was later placed in synonymy with U. notata when several of Cope’s diagnostic characters were reinterpreted as representing individual variation rather than species differences (Camp 1916b, Van Denburgh 1922). The taxon was later resurrected to full species status based on a larger series of specimens that identified diagnostic morphological differences among the taxa (Heifetz 1941). Several different authors have noted external morphological, osteological, and genetic similarity among members of the genus and have variously treated U. scoparia as a full species or subspecies of U. notata (Stebbins 1954, Norris 1958, Mayhew 1964a, Mayhew 1964b, Adest 1977, Zalusky et al. 1980). Carpenter (1963) showed that the pattern of push-up behavior used in territorial displays was distinct in U. scoparia, compared to U. inornata and U. notata, and suggested that this may serve as an isolating mechanism.
Phylogenetic analyses of mitochondrial data suggested that U. scoparia is monophyletic (Trépanier and Murphy 2001, Murphy et al. 2006) and forms a clade with the other Mojave and Sonoran Desert taxa (U. inornata and U. notata) (Wilgenbusch and De Queiroz 2000). Mitochondrial data also suggest that some haplotype diversity occurs within the U. scoparia (Murphy et al. 2006), although divergences are low and additional, multigene nuclear data are needed to clarify intraspecific variation. Populations occurring in the northern part of the range have been proposed as a distinct population segment based on mitochondrial phylogeography and presumed isolation (Murphy et al. 2006).
Life History
Uma scoparia is an active, wary, diurnal lizard that specializes on fine windblown sand habitat. It is extremely similar in most aspects of life history to other species in the genus (Stebbins 1944), and here we make use of life history information from these other species when it is not available for U. scoparia. Species in the genus Uma all possess a number of morphological, behavioral, and physiological adaptations that allow them to persist in arid habitats. Specifically, a countersunk lower jaw, nasal valves, and fringes on the eyes and ear openings allow U. scoparia to prevent sand from entering the body (Norris 1958). The nasal passages have a complex convoluted shape that reduces moisture loss and excludes sand from inhalation (Stebbins 1943, Stebbins 1948). Enlarged fringes on the toes have been experimentally shown to increase both maximum velocity and acceleration on fine sand, particularly on steeply sloped landscapes such as are often found in sand dunes (Carothers 1986). The flattened body form, wedge-shaped head, enlarged, keeled scales on the head, limbs and toes, and the smooth granular scales over the rest of the body aid in burrowing and “sand-swimming” behavior (Stebbins 1944). Uma scoparia employs this behavior both to escape from predators and to take refuge from extremely hot surface conditions (typically when surface temperature exceeds 43°C; Norris 1958). Uma scoparia possesses both acute vision and hearing, which aid in predator avoidance and prey capture (Stebbins 1944).
Adult U. scoparia overwinter in the sand between November and February, then become surface-active throughout the day as temperatures allow. The species maintains a mean body temperature of 36–37.5°C, often becoming inactive during the hottest part of the day during midsummer (Mayhew 1964b, Miller and Stebbins 1964). Breeding occurs throughout the spring and summer between April and July, and females lay clutches of 1–5 eggs (usually 2 or 3); more than one clutch may be produced in optimal years (Stebbins 1954, Mayhew 1966, Fromer et al. 1983, Stebbins 2003). Young begin to appear on the surface in September (Miller and Stebbins 1964).
Uma scoparia has a generalized diet that includes a variety of beetles, ants, wasps, flies, and other small arthropods, as well as plant leaves and seeds (Stebbins 1944). At Dale Dry Lake, San Bernardino County, the diet of adult U. scoparia consisted of approximately 60% plant material (mainly in the form of small seeds) and 40% small arthropods (Minnich and Shoemaker 1972). The juvenile diet, conversely, was composed of over 90% arthropods (Minnich and Shoemaker 1972). In low rainfall years, adults may be forced to switch to a diet composed mostly of arthropods due to lack of vegetation, and this may be suboptimal (Barrows 2006). The quality of available food is probably dependent on the local rainfall, which varies widely from year to year throughout the species’ range. Barrows (2006) found that a regression model including rainfall and diet explained 92% of the variation in U. inornata density and that population sizes could approach zero during multiyear droughts and then quickly rebound when average rainfall resumed.
Habitat Requirements
Uma scoparia lives exclusively on fine windblown sand (Stebbins 1944). Habitat where lizards are found in the highest abundances generally consists of relatively sparse creosote scrub on loose sand dunes. The diameter of individual sand grains in these areas is usually <0.5 mm. Areas with large sand grains (>2 mm in diameter) appear to be avoided, presumably because this impedes sand swimming and burying behavior (Stebbins 1944, Norris 1958, Fromer et al. 1983). Within appropriate habitat, individuals select areas with the finest sand available (often the downwind side of vegetation and slopes) (Stebbins 1944, Norris 1958). Some vegetation is probably required for food and shade (Miller and Stebbins 1964). The species is not present in areas where the sand becomes too firmly packed to allow for sand swimming, and washes and desert flats are generally unsuitable (Miller and Stebbins 1964). No evidence exists that Uma will enter these areas to migrate between adjacent areas of suitable habitat, although additional study of this question would be valuable.
Uma scoparia may require relatively large habitat patches for long-term persistence. Population modeling in the ecologically similar U. inornata suggests that plot sizes smaller than 100–200 ha are unlikely to allow long-term persistence of isolated populations (Chen et al. 2006).
Distribution (Past and Present)
Uma scoparia is patchily distributed throughout much of the Mojave Desert in California. The range extends from near the southern end of Death Valley at the Inyo–San Bernadino County line south through San Bernardino and Riverside Counties, extending west narrowly into Los Angeles County (Van Denburgh 1922, Norris 1958, Miller and Stebbins 1964, Pough 1974, Jennings and Hayes 1994a, Stebbins 2003). Norris (1958) reports a record from Inyo County, which has often been repeated in the literature. However, the stated locality “one and one-half miles southeast of Saratoga Springs” places this record in San Bernardino County, and we know of no other confirmed records from Inyo County. This species is nearly endemic to California, extending into Arizona in one small area near Parker, Yuma County (Pough 1974). A single report of possible Uma tracks reported from the Eureka Sand Dunes, Inyo County, California, would extend the known range ∼175 km to the northwest and requires verification (Bolster et al. 2000). The known elevational range extends from below sea level to nearly 1000 m (Jennings and Hayes 1994a).
Extirpations have been documented at El Mirage and Harper Dry Lakes, San Bernardino County, and at Lovejoy Buttes and Piute Butte, Los Angeles County (Murphy et al. 2006). Additional extirpations may have occurred at Rogers Dry Lake, Kern County, California, and Saddleback Butte, Los Angeles County, California (CBD 2006).
Trends in Abundance
No quantitative data are available regarding historical abundance, though the lizard was, and is, common at many isolated localities. Some data suggest that this species has become uncommon in areas where habitat degradation due to off-highway vehicle use has occurred (Bolster et al. 2000, CBD 2006).
Nature and Degree of Threat
The most important threats facing Uma scoparia are habitat loss and fragmentation due to human activities and off-highway vehicle use, which negatively impacts loose sand habitat. Other activities, including the development of renewable energy facilities, may also negatively impact the structure of essential windblown sand habitat patches. The species is only found in loose sand areas, and experimental work in the closely related and ecologically similar species U. inornata suggests that these lizards are highly sensitive to stabilization of their sand habitat (Turner et al. 1984). Habitat fragmentation is also an important threat. Even where patches of intact habitat remain, fragmentation and small patch sizes have been shown to be associated with declines and extirpations in U. inornata (Barrows and Allen 2007). In addition, surveys for the ecologically similar U. notata that compared lizard abundances in areas that experienced off-highway vehicle use to areas that do not, found much higher densities in the less-impacted habitat (Luckenbach and Bury 1983). Off-highway vehicles impact this species through direct mortality, destruction of vegetation (which is correlated with lizard abundance), and increased rates of tail loss (Luckenbach and Bury 1983, Ouren et al. 2007). Further, U. scoparia has sensitive hearing that is easily damaged by even moderate and short duration off-highway vehicle activity (Brattstrom and Bondello 1983). Hearing loss likely harms this lizard’s efficiency at capturing prey and its ability to avoid predation (Brattstrom and Bondello 1983). Increasing predator densities (e.g., common ravens) in certain areas, often in association with human development and the presence of garbage dumps, may also be causing declines in lizard abundance in localized areas (Bolster et al. 2000).
Uma scoparia is likely sensitive to the effects of climate change. Climate change models for this region predict relatively sharp increases in mean temperature of up to 2°C (PRBO 2011). The impact of such increases on U. scoparia and on critical plant species is not known but could be large and should be a high priority for future research. The distribution of U. inornata is associated with an east-to-west drought gradient in the Coachella Valley (Barrows and Allen 2007). Like off-highway vehicle use, drought decreases the amount and quality of vegetation present, which limits both food and cover for this species (Barrows et al. 2010). Because Uma specializes on relatively isolated patches of habitat, it is probably unable to track available habitat with changing climatic conditions. Climate change modeling studies on other Uma species (U. inornata; the Coahuila fringe-toed lizard, U. exsul; and the Chihuahuan fringe-toed lizard, U. paraphygas) predict significant habitat loss under a relatively wide range of climate change scenarios (Ballesteros-Barrera et al. 2007, Barrows et al. 2010) and these results are also likely to apply to U. scoparia.
Status Determination
Uma’s specialized habitat is relatively uncommon and undergoing significant degradation, and this is the primary justification for Priority 3 designation. While some populations have been extirpated, several populations of this species are still common, and some habitat occurs on protected land that is not subject to off-highway vehicle use, precluding the need for a higher-priority designation.
On 10 April 2006, the Center for Biological Diversity and Sylvia Papadakos-Morafka petitioned the US Department of the Interior to list the northern population segment identified by Murphy et al. (2006) under the Federal Endangered Species Act (CBD 2006). The USFWS issued a 90-day finding that substantial evidence for listing need had been presented and initiated a 12-month status review for the taxon (USFWS 2008). This review concluded that the Amargosa River populations of U. scoparia do not constitute a distinct population segment and are therefore ineligible for listing under the US Endangered Species Act (USFWS 2011).
Management Recommendations
Effective management of this taxon over the short term can likely be accomplished by protecting habitat from development and degradation from off-highway vehicles and other human impacts. Over longer time periods, climate change could begin to have a larger impact, and this may require additional management efforts. Such efforts could range from human-assisted translocation to planting drought-resistant vegetation, depending on local conditions and the extent of temperature and precipitation changes. If restoration occurs in areas where extirpation has occurred or if development activities further isolate occupied habitat patches, human-assisted translocation, potentially in association with captive breeding programs, may be a key strategy for this species.
Monitoring, Research and Survey Needs
Two key research efforts for Uma scoparia should focus on the effects of human activities (including off-highway vehicles, solar and wind energy development, and roads) and the genetic effects of both natural and anthropogenic habitat fragmentation. The effects of off-highway vehicles are particularly important, and monitoring efforts should be initiated in areas that experience off-highway vehicle use compared to more pristine, adjacent areas. In particular, these efforts should focus on comparing the effect of varying intensity of anthropogenic disturbance on populations, with the aim of establishing what intensity of off-highway vehicle use can be tolerated. These efforts should also attempt to disentangle the effects of habitat destruction, noise pollution, and direct mortality on populations, since each can in principle be managed independently. For example, if off-highway vehicle use primarily affects these lizards through reductions in vegetation, habitat restoration coupled with restricting off-highway vehicles to certain trails or corridors could constitute a reasonable management strategy. Alternatively, noise pollution effects may require eliminating off-highway vehicle access in areas where the lizards are present. Because population sizes naturally fluctuate with rainfall in this species (Barrows 2006), and in some cases can approach zero before rebounding, monitoring this species is inherently difficult, and multiyear surveys spanning several drought and non-drought years are essential. The frequency of lizard detection and the accuracy of population size estimates can be increased with repeated sampling and specific detection methods (Turner et al. 1984, Bolster et al. 2000), and these should form the foundation of monitoring protocols.
Genetic studies are critical at two distinct levels of resolution that require different datasets and analytical approaches. Species boundaries across Uma and large-scale phylogeographic patterns within U. scoparia remain poorly resolved (see also the species account for U. notata), and both are critical for effective management. For species boundary work, the resolution of the number and identity of species contained within the genus requires a multi-locus nuclear dataset to complement initial work using mitochondrial DNA (Trépanier and Murphy 2001). Within U. scoparia, phylogeographic studies using multiple nuclear markers are also needed in order to quantify the intraspecific diversity present within the species. At a finer scale, landscape and population genetic studies are also badly needed to establish natural levels of gene flow, including movement across seemingly inhospitable habitat patches, for this windblown sand habitat specialist. These data can advise and guide plans for habitat acquisition both now and in the face of climate change, and may be a critical element in establishing appropriate habitat corridors and supplementing ecological survey data to guide potential human-assisted translocation. Finally, these multi-locus microsatellite or SNP-based studies can help clarify the amount of migration (if any) between adjacent populations and effective population sizes of existing local populations.