SANDSTONE NIGHT LIZARD
Xantusia gracilis Grismer and Galvan 1986

Sandstone night lizard, San Diego County, California. Courtesy of Jeff Lemm.
Status Summary
Xantusia gracilis is a Priority 3 Species of Special Concern, receiving a Total Score/Total Possible of 38% (42/110). During the previous evaluation, it was also designated as a Species of Special Concern (as Xantusia henshawi gracilis; Jennings and Hayes 1994a).
Sandstone Night Lizard: Risk Factors

Identification
Xantusia gracilis is a medium-sized (5.1–7 cm SVL) lizard with soft skin and granular scales on the dorsal surface, enlarged plates on the ventral surface, and a prominent gular fold (Grismer and Galvan 1986, Lovich and Grismer 2001, Stebbins 2003, Lovich 2009b). The dorsal coloration is pale tan/brown, with many round dark-brown spots, while the ventral surface is clean white or white, with a very small amount of black speckling on the front limbs and throat (Grismer and Galvan 1986). The head is flattened, and the eyes have vertically oriented pupils (Stebbins 2003). The overall body shape is relatively slender compared to its closest (and most similar) relative the granite night lizard (X. henshawi) (Grismer and Galvan 1986).
Within its range, X. gracilis is only likely to be confused with its sister species X. henshawi. The two species do not overlap in range but occur within 32 km of each other. Xantusia henshawi has larger dark spots on the dorsal surface, more extensive speckling on the ventral surface, and an overall more robust body shape (Grismer and Galvan 1986). Xantusia gracilis also has an enlarged temporal scale (about half the size of the postparietal) compared to X. henshawi (typically less than one-quarter the size of the postparietal; Grismer and Galvan 1986). The peninsular leaf-toed gecko (Phyllodactylus nocticolus) also occurs in the vicinity of X. gracilis, but this lizard lacks the dark-brown dorsal spots and has prominent, expanded toe tips.
Taxonomic Relationships
Xantusia gracilis was initially described as a subspecies of X. henshawi on the basis of color, scalation, allozyme variation, and behavior (Grismer and Galvan 1986). The taxon was elevated to species status because it is diagnosable, geographically isolated, and forms a monophyletic clade nested within X. henshawi for a single mitochondrial locus (Lovich 2001). This arrangement is now widely accepted.
Life History
The life history of Xantusia gracilis is poorly studied, particularly so in wild populations. Given the species’ overall similarity in most respects to X. henshawi, we expect that life history information from X. henshawi is a reasonably good predictor for X. gracilis (Lee 1975). However, the two taxa live in distinct habitats and show some behavioral differences in captivity, so some life history differences probably exist in the wild. Xantusia gracilis is likely active from spring through fall (Lemm 2006). In captivity, it has been shown to be more strongly nocturnal than X. henshawi, more frequently found on the sandy substrate on the bottom of the enclosure and does not seem to be limited to rock faces (Lee 1975, Grismer and Galvan 1986). Based on what is known about X. henshawi, we expect that X. gracilis has a low metabolic rate and is quite sedentary, feeding primarily ants, beetles, and spiders (Brattstrom 1952, Lee 1975, Mautz 1979). In captivity, X. gracilis are also known to feed on the eggs of Phyllodactylus nocticolus, a behavior that captive X. henshawi in the same enclosure did not exhibit (Grismer and Galvan 1986). In X. henshawi, mating occurs in June and July, with one or two live young born in September or October (Brattstrom 1951, Lee 1975), and this may also be the case for X. gracilis. Individuals probably do not become reproductively mature until 2.5–3.5 years of age and are likely long-lived, although field data are lacking (Lee 1975).
Habitat Requirements
Xantusia gracilis lives in eroding sandstone and mudstone habitat where it utilizes crevices, rodent burrows, and the undersides of exfoliating rock flakes as shelter (Grismer and Galvan 1986). At night, it emerges from its shelters and can be found moving about on the surface (Grismer and Galvan 1986). This species is less dependent on exfoliating rock habitat than X. henshawi (Grismer and Galvan 1986).
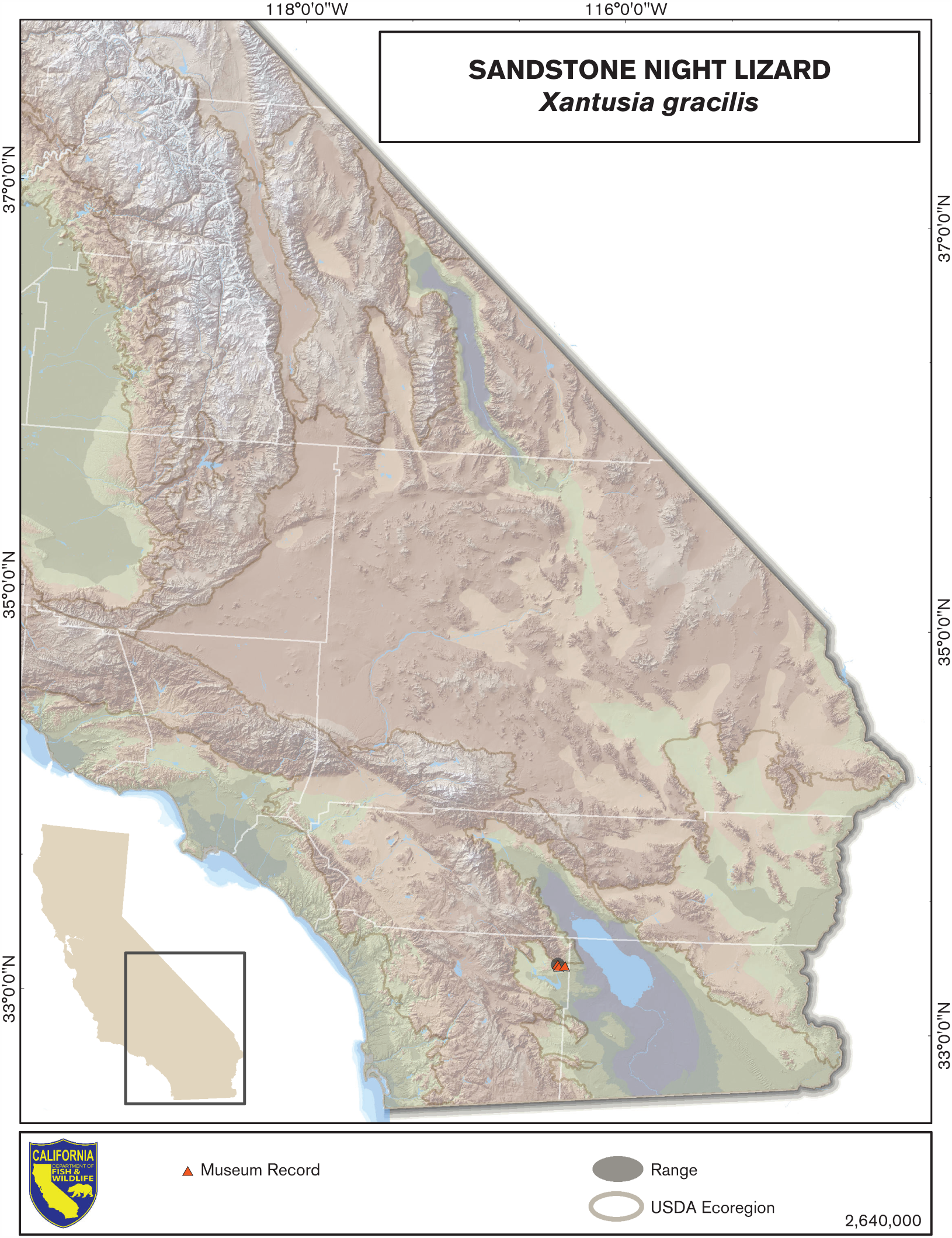
Distribution (Past and Present)
Xantusia gracilis is restricted to one small area, approximately 3.9 km2 in total area, on the southeastern flank of the Santa Rosa Mountains, entirely within Anza Borrego Desert State Park (Grismer and Galvan 1986). The known elevational range extends from approximately 240 to 305 m. Within this small region the species is patchily distributed, common in some areas and apparently absent in others (Grismer and Galvan 1986). Xantusia henshawi occurs approximately 32 km to the north and west, and no xantusiid lizards are known from the intervening area. No historical distribution data are available for this taxon, although we have no reason to think that the distribution has declined recently.
Trends in Abundance
No data on historical or current abundance have been published, although some have suggested that habitat quality has declined due to collection activity (R. Lovich, pers. comm.). The extent and severity of such impact has not been quantified (R. Fisher, pers. comm.). Some amount of illegal collection occurs for this species, which may be driving small declines (M. Jorgensen, pers. comm.).
Nature and Degree of Threat
Xantusia gracilis lives in a fragile habitat in an extremely localized area. Damage to this small patch of habitat, be it from habitat destruction, invasive species, collecting, or climate change, is the largest risk facing the species (Lovich 2009b). It is also likely long-lived and late maturing with a low reproductive potential, and populations are likely to be slow to recover from declines. Some amount of illegal collecting occurs, which could be contributing to such declines, particularly in areas that are most easily accessible by road.
Status Determination
The extremely localized range and relative fragility of Xantusia gracilis’ habitat are significant risk factors. The species’ life history also predisposes it to decline in the face of any increased adult mortality. Although data are almost entirely lacking, X. gracilis appears to be relatively stable at the present time; thus, we designate it as a Priority 3 Species of Special Concern.
Management Recommendations
Limiting access and minimizing disturbance to Xantusia gracilis’ habitat is currently the most important component of effective conservation. This management strategy should be reviewed as needed depending on the results of the surveys outlined below. All collecting should be restricted or eliminated unless it is absolutely necessary for scientific purposes that further conservation of this species.
Monitoring, Research, and Survey Needs
As published historical or current abundances of Xantusia gracilis are lacking, publication of any existing data is a priority. Formal monitoring should be initiated to establish and publish baseline population data. These surveys should be performed at night, and it is essential not to disturb the fragile microhabitat (e.g., moving rocks or rock flakes, excavating rodent burrows). Aside from estimating population size, these surveys should also quantify and document any observed habitat disturbance. Year-to-year fluctuations in population size occur in other xantusiid lizards (Lee 1975) and are to be expected in X. gracilis as well. Establishing a long-term monitoring program is a critical objective. Additional surveys to establish the precise limits of the range of X. gracilis will help determine best practices for managing its fragile habitat in the heavily used Anza-Borrego Desert State Park.
The life history of this species has not been studied and an autecological study is badly needed to provide basic information on habitat suitability and reproduction. These data will be urgently needed should more extensive management efforts become necessary.
Finally, multi-locus microsatellite or SNP data should be collected to provide genetic estimates of effective population size, and potentially levels of gene flow, even for this restricted species. A key issue for this species is to sample individuals without invasive tissue-removal techniques, and it would probably be best to work out such protocols on X. henshawi before applying them to X. gracilis.