WESTERN POND TURTLE
Emys [=Actinemys] marmorata Baird and Girard 1852

Western pond turtle, Solano County, California. Courtesy of Adam Clause.

Western pond turtle, Santa Barbara County, California. Courtesy of Robert Hansen.

Status Summary
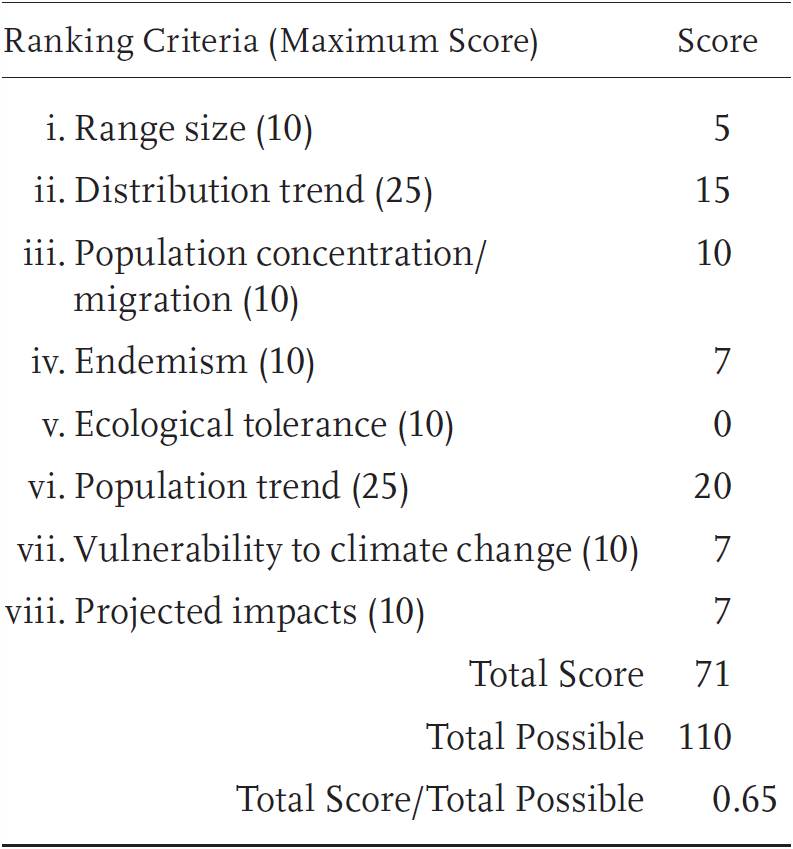
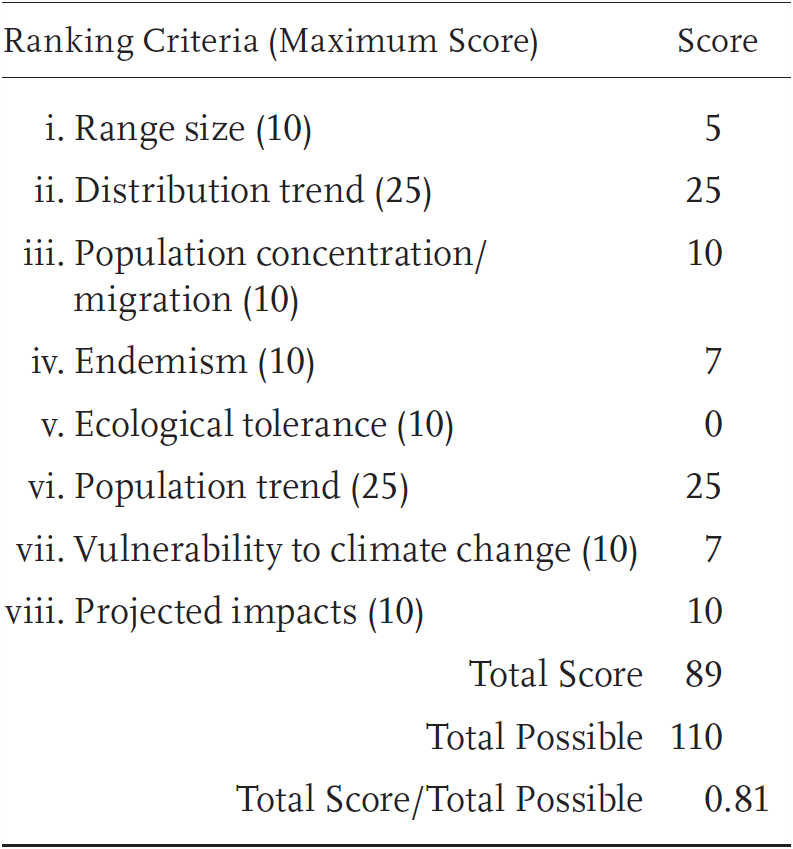
Emys marmorata is a Priority 1 Species of Special Concern in the southern part of the range (roughly corresponding to the range of the southwestern pond turtle, E. m. pallida) and a Priority 3 Species of Special Concern elsewhere (roughly corresponding to the range of the northwestern pond turtle, E. m. marmorata; see below for additional detail). These two populations received a Total Score/Total Possible of 81% (89/110) and 65% (71/110), respectively. During the previous evaluation, both populations were considered Species of Special Concern, also with different overall levels of threat (Jennings and Hayes 1994a).
Northern Western Pond Turtle: Risk Factors
Southern Western Pond Turtle: Risk Factors
Identification
Emys marmorata is a small to medium-sized (generally 17–18 cm, rarely to 24 cm, straight carapace length) brown, tan, or olive turtle (Stebbins 2003). The carapace is low, keelless, and often marked with a pattern of dark lines and/or dots, sometimes forming a pattern that radiates from the centers of each scute. The posterior edge of the carapace forms a smooth, non-serrated rim. In some individuals, the carapace has no patterning. The plastron is lighter tan or beige, hingeless, and often marked with dark blotches (Stebbins 2003). The shell shape varies among habitat types, with turtles from foothill streams being flatter and narrower than individuals occurring at lower elevations in canals and sloughs (Lubcke and Wilson 2007).
This species is unlikely to be confused with other turtles within its range with the possible exception of melanistic individuals of the nonnative red-eared slider (Trachemys scripta elegans). This latter species has a much shorter tail, attains larger overall body sizes, and has a serrated rim around the posterior edge of the carapace. Most individuals of this species also have prominent yellow stripes on the neck and shell and a broad red stripe over the temporal region of the head, although older individuals often develop an overall dark melanistic coloration.
Taxonomic Relationships
Emys marmorata is a member of the family Emydidae, which encompasses the majority of North American turtle species. The relationships within this group have undergone extensive revision in recent years, leading to many taxonomic changes and some instability. Formerly, this species was included in the genus Clemmys along with the bog turtle (now Glyptemys muhlenbergii), the wood turtle (now G. insculpta), and the spotted turtle (now C. guttata). Recent molecular analyses have suggested a close relationship between E. marmorata, Blanding’s turtle (Emys [=Emydoidea] blandingii), and the European pond turtles (E. orbicularis and E. trinacris) (Bickham et al. 1996, Burke et al. 1996, Feldman and Parham 2002, Spinks and Shaffer 2009, Spinks et al. 2009). This species is now generally placed in either the monotypic genus Actinemys (Holman and Fritz 2001) or the genus Emys (the arrangement that we follow here).
Intraspecific variation within E. marmorata is also undergoing intensive study. Two subspecies have traditionally been recognized, E. m. marmorata (Baird and Girard 1852) and E. m. pallida (Seeliger 1945). These subspecies were initially distinguished by the presence or absence of inguinal scutes in the shell and coloration of the throat and neck. Subsequent studies also detected substantial morphological variation present across the range (Holland 1992a). Genetic analyses of intraspecific variation suggest that substantial variation is present, which is generally, but not precisely, concordant with the traditionally defined subspecies (Spinks and Shaffer 2005, Spinks et al. 2010). Spinks et al. (2014) analyzed a large panel of SNPs and concluded that E. m. sensu lato should be divided into two species. Because this arrangement is very recent, here we follow the earlier arrangement (of a single species) but consider threats separately for southern and northern populations as was done by Jennings and Hayes (1994a).
Life History
Emys marmorata is a highly aquatic species and basks frequently. In the northern part of the range (particularly at higher elevations), this species enters a period of dormancy throughout much of the winter. It is one of relatively few emydid turtles that regularly overwinter on land (Ultsch 2006), perhaps as a mechanism to avoid mortality from increased winter water flows in the Mediterranean climate. Where it overwinters terrestrially, the species uses a variety of habitat types but chooses sites above the normal high water mark and burrows into loose soils and leaf litter (Reese 1996). In aquatic habitats that experience little change in water level (lakes, ponds, and reservoirs), pond turtles are known to overwinter in the water and will choose undercut banks, bottom mud, “snags” of downed wood, or rocks (Nussbaum et al. 1983, Ernst and Lovich 2009). Movement to overwintering sites occurs at the end of summer, most often in September, although the timing varies with the particular habitat and area (Reese 1996, Reese and Welsh 1997). In warmer areas, particularly in the southern part of the range, this species may remain active year-round.
Western pond turtles are known to mate throughout the spring, summer, and fall. Nesting usually occurs in the spring or early summer, although double clutching has been reported from several parts of the range (Goodman 1997, Germano and Bury 2001, Germano and Rathbun 2008, Scott et al. 2008). Females usually select nest sites within 100 m of a water body, although nests as far away as 500 m have occasionally been reported (Storer 1930, Holland 1994, Reese 1996, Holte 1998, Lovich and Meyer 2002). Clutch sizes vary from 1 to 13 eggs and vary depending on local conditions (Holland 1994, Lovich and Meyer 2002, Germano and Rathbun 2008). The eggs hatch in the fall and, at least in the northern part of the range, hatchlings often remain in the nest through the first winter, emerging the following spring (Holland 1994).
The diet is generalized and consists of a variety of small aquatic invertebrates (including insects, crustaceans, and mollusks) and a wide variety of algae and other plant material (Bury 1986). Carrion and small vertebrates are also occasionally consumed (Bury 1986). Growth rates vary widely depending on local conditions but appear to be highest in hatchlings and then gradually slow in adults. Reproductive maturity is widely variable and appears to be linked to size. Females generally mature at slightly over 13 cm SCL as young as 4–5 years of age, while males mature at about 12.5 cm SCL at 6–8 years of age (Holland 1994, Reese 1996, Germano and Bury 2001, Germano and Rathbun 2008, Germano and Bury 2009; T. Engstrom, pers. comm.), although maturation can happen more quickly depending on local conditions in some areas (e.g., Germano 2010).
Habitat Requirements
Emys marmorata is generalized in its habitat requirements, occurring in a broad range of aquatic water bodies including flowing rivers and streams, permanent lakes, ponds, reservoirs, settling ponds, marshes, and other wetlands. This species will also temporarily use semipermanent or ephemeral water bodies, including stock ponds, vernal pools, and seasonal wetlands (Stebbins 2003, Bury and Germano 2008). This species will also at least occasionally enter sea water (Stebbins 1954, Holland 1989). Pond turtles require upland habitat that is suitable for nesting and overwintering use. Localized soil conditions, as well as the frequency and degree of disturbance in the upland habitat, probably limit their distribution. Soils need to be loose enough to allow nest excavation, while disturbance needs to be infrequent enough or of sufficiently low intensity that nests are not disturbed (Ernst and Lovich 2009).
This species is most frequently found in quiet reaches that experience little human impact and have abundant basking substrate in the form of downed wood and large rocks (Bury and Germano 2008, Thomson et al. 2010). The species can persist, at least over moderate periods of time, in highly modified habitats with high human traffic and/or little basking substrate (Spinks et al. 2003, Germano 2010).
Distribution (Past and Present)
Emys marmorata ranges widely along the Pacific coast from western Washington to the northern part of the Baja California Peninsula in Mexico. Within California, the species ranges from the Pacific coast inland to the Sierra Nevada foothills up to elevations of 2048 m (Ernst and Lovich 2009). Further south, it ranges from the coast inland to the peninsular ranges. Scattered populations exist in the Mojave River (e.g., Victorville, Camp Cady, and Afton Canyon, San Bernardino County, California) and in some Great Basin drainages including the Susan River (Lassen County, California), and the Truckee and Carson Rivers (Nevada, possibly extending into Nevada County, California, although this has not been documented) (Holland 1992b, Lovich and Meyer 2002). Additional scattered populations are known from the Klamath Basin (R. Bury, pers. comm.). Some or all of these populations could represent introductions. One hundred and eighty individuals of this species were introduced in the state of Nevada in 1887, and these may be the source of the population in the Truckee and/or Carson Rivers (Cary 1889).
Within E. marmorata, the southern subspecies (E. m. pallida) extends from the southern range edge in Baja California, Mexico, northward in the Coast Range to San Francisco Bay, while the northern subspecies (E. m. marmorata) extends from San Francisco Bay north through the Sacramento Valley and Coast Range to the northern range limit in Washington. A large intergrade zone between the two subspecies has been hypothesized to exist in the San Joaquin Valley (Seeliger 1945), although recent work has shown that this area is genetically a member of the northern subspecies (Spinks et al. 2014). The populations that we recognize correspond to these subspecies distributions.
In the north, large and relatively intact populations still exist through large areas of the Coast Range and Sierra foothills, although agriculture and habitat modification have destroyed large areas of riparian and wetland habitat in the Sacramento Valley that almost certainly supported large populations of this species in the past. Scattered populations remain throughout the Sacramento Valley, but the extensive marsh habitat that dominated much of the valley floor has been largely drained and converted to agriculture. Kelly et al. (2005) estimated that the extent of wetland habitat in the Central Valley has declined by ∼80% since the 1860s when large-scale land conversion began, and this undoubtedly eliminated many E. marmorata populations. Holland (1992b) argued that the San Joaquin River drainage formerly represented the stronghold of this species, supporting vast numbers of individuals, and that the species has been lost from >99% of its range in the region. Overall, the number of viable populations in this area has clearly decreased, but some do remain (Holland 1992a, Jennings and Hayes 1994a, Germano 2010, Bury et al. 2012).
In the south, extensive urbanization and land conversion have caused precipitous population declines. A large fraction of remaining habitat in southern California exists as patches surrounded by large tracts of unsuitable habitat that have little suitable upland nesting habitat. Dispersal corridors between adjacent habitats have also been mostly severed by intervening urban development and heavily used roadways, resulting in heavy mortality on females searching out nest sites (R. Fisher, pers. comm.).
Trends in Abundance
Emys marmorata was formerly abundant throughout much of California. Bogert (1930) reported that E. marmorata was “common in larger streams along the coast and in many of the marshes adjacent to the coast,” and many of these habitats still support relatively large populations (Jennings and Hayes 1994a, Germano and Rathbun 2008, Thomson et al. 2010). Elsewhere declines have occurred, particularly in southern California. Van Denburgh (1922) reported that the species was “abundant on the west fork of the San Gabriel River,” but recent reports suggest that the species has declined precipitously in this area and in the Los Angeles Basin in general (Brattstrom 1988, Jennings and Hayes 1994a). Large, relatively intact populations remain through much of the northern Coast Ranges, although areas in the Central Valley and southern California that still support the species have severely declined (Holland 1992b, Jennings and Hayes 1994a). Populations that remain in the Central Valley are undoubtedly smaller and more fragmented than they once were due to the large-scale land conversion that occurred in this area beginning in the 1860s. Further, E. marmorata were harvested commercially for many years, selling for 3–6 dollars per dozen in San Francisco markets during the 1920s and 1930s (Pope 1939, Nussbaum et al. 1983). The overall extent of declines in abundance caused by market collection is poorly understood. However, localized declines due to market collection were noted as early as 1879 in Sacramento (Lockington 1879), and the species’ life history would make it particularly susceptible to declines from intense adult mortality.
Some published and ongoing surveys suggest that population sizes are stable in several remaining populations in the southern part of the range. In particular, southern populations near Gorman, Fresno, and along the central coast of California appear to be stable in abundance with a population structure that indicates continued breeding (Germano 2010; D. Germano, pers. comm.). Unpublished field data also indicate that the species persists in some numbers throughout Merced (particularly east of Gustine) and Fresno Counties, as well as some areas of Kern County (S. Barry, pers. comm.). At least in some areas, ongoing declines in abundance may have slowed or stopped. If additional data corroborate these observations, a decrease in the population trend scores may be warranted during the next Species of Special Concern evaluation.
Nature and Degree of Threat
The largest threats currently facing Emys marmorata are land use changes and fragmentation of existing habitat, as well as possible impacts via competition and predation by introduced species.
Throughout the range of E. marmorata, extensive wetland habitats that once supported large numbers of this species have declined in extent and quality. Ongoing land use conversion to agriculture as well as urban development have degraded and fragmented habitat throughout virtually all of this taxon’s range. These effects are most pronounced in southern California, where relatively few viable populations of this species now remain. Even in northern California, land use changes are having impacts. Reese and Welsh (1998) documented changes in the age structure of E. marmorata populations as a result of damming in the Trinity River drainage, suggesting negative impacts on juvenile turtles and therefore recruitment in populations affected by dams.
The impact of introduced species is largely unknown but could potentially be detrimental in several ways. The red-eared slider is widely established throughout the range of E. marmorata and may serve as a disease vector and competitor (Bury 2008a). The spiny softshell turtle (Apalone spinifera) is a more recent introduction to the Central Valley of California and is now breeding in at least one site in the Sacramento Valley (L. Patterson, pers. comm.). If this species becomes invasive on a larger scale, it is also likely to compete with and possibly prey on small E. marmorata. In Southern California, the range of these two species appears not to overlap, suggesting that softshells may have strong impacts on pond turtles (R. Fisher, pers. comm.). Additional introduced species that may affect E. marmorata are bullfrogs, crayfish, and introduced centrarchids. In the Salinas River, E. marmorata declined following the invasion of bullfrogs in the 1970s (B. Hubbs, pers. comm.). The strength and mechanism (predation or competition) of their impact is not currently clear, and further studies are needed. Ravens, crows, raccoons, and opossums are all known predators of E. marmorata adults and nests. The population sizes of these human commensal species have increased through time and may also be having impacts on E. marmorata populations via increased predation pressure. A very important source of this decline may operate through nest predation that leads to reduced or failed recruitment year to year (S. Sweet, pers. comm.).
The impacts of climate change on E. marmorata are still poorly understood but are likely to be significant. Climate simulation models project strong changes to river hydrology in California. In particular, decreasing snowpacks and a shift to earlier and stronger river flows (and increased frequency and strength of scouring floods) are likely to negatively affect habitat and could cause local extirpations (Cayan et al. 2008b). Because the habitat is now fragmented, recolonization of these areas following localized extirpations is unlikely, particularly in southern California where the habitat is the most fragmented. Importantly, the genetic data indicate that most of the genetic diversity within this species resides in southern California. Because of this, declines in this area could result in the extirpation of much of the genetic diversity that is currently present (Spinks et al. 2010, Spinks et al. 2014; R. Fisher, unpublished data).
Status Determination
Priority 1 Species of Special Concern status is justified for Emys marmorata in the southern portion of the range because these populations are experiencing ongoing and strong declines in distribution and abundance (although, as noted above, some evidence indicates these declines may be slowing in some areas). Further, this area contains most of the genetic diversity that has been identified within this taxon, so entire genetic lineages are at risk. In the north, populations are experiencing declines, although to date they are less severe than in the southern portion of the range. Many of the remaining populations in the north occur in habitats that are unlikely to experience land use changes on a scale that will threaten long-term survival, so we consider this segment of the range a Priority 3 Species of Special Concern.
Management Recommendations
Our recommendations follow those of Bury et al. (2012). We outline these recommendations below and refer readers to that document for additional discussion. Protecting habitat from further degradation and fragmentation is the highest priority for this species. Following this, habitat restoration, particularly that which increases connectivity between currently isolated habitats and increases the extent of setback or buffer habitat around wetlands that is suitable for nesting, is an important management priority. Efforts to reduce or control the impact of predators (especially on nests) are also an important way to maintain current populations and increase recruitment of juveniles. Formal headstarting programs may be a useful tool for repopulating areas where local extirpations have occurred but only as a last resort and if the habitat can be restored to an extent that a population can survive with little intervention. One encouraging observation is that Emys marmorata can live in close proximity to human disturbance, provided that they have adequate suitable basking and nesting sites.
Monitoring, Research, and Survey Needs
Further research on the impact of invasive species is needed. In particular, the impact of red-eared sliders, bullfrogs, and centrarchids needs to be further characterized, to understand both to what extent these species can coexist and the effects these species have on the native populations. Both nest and hatchling habitat requirements are relatively poorly characterized, and need to be clarified if the species is to persist and thrive in human-modified habitats. The effectiveness of headstarting efforts needs to be evaluated in various habitats and predation situations. Because a large amount of life history variation is present in this taxon (particularly relating to time to maturity, body size, and clutch size; e.g., Germano 2010), researchers and managers should be cautious when applying life history data collected in one population to a different population, particularly those occurring at widely different elevations, water temperatures, or habitat types.