SONORA MUD TURTLE
Kinosternon sonoriense Le Conte 1854

Sonora mud turtle, Santa Cruz County, Arizona. Courtesy of Jeff Lemm.

Status Summary
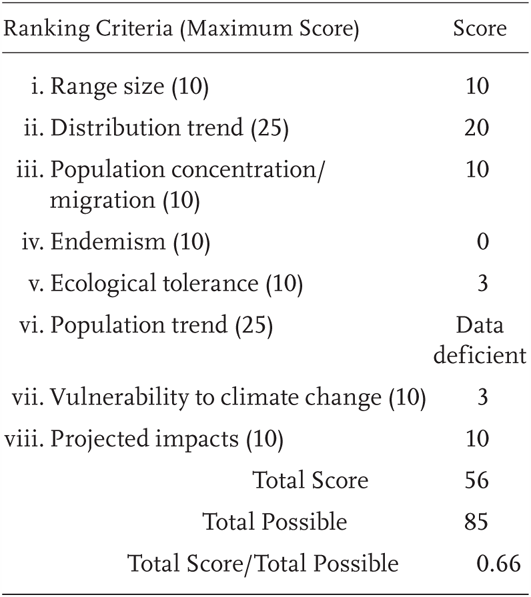
Kinosternon sonoriense is a Priority 1 Species of Special Concern, receiving a Total Score/Total Possible of 66% (56/85). During the previous evaluation, it was also considered a Species of Special Concern (Jennings and Hayes 1994a). It has not been recorded from its historic range along the California–Arizona border since 1962.
Sonora Mud Turtle: Risk Factors
Identification
Kinosternon sonoriense is a small (maximum size ∼17 cm SCL) black or brown turtle, with prominent barbels on the chin and neck and usually with yellow or cream mottling on the sides of the head that form broken stripes (Ernst and Lovich 2009). The plastron is lighter than the carapace, usually pale brown to yellow, with dark pigmentation along the scute seams and well-developed anterior and posterior hinges (Ernst and Lovich 2009). The overall carapace shape is oval and moderately domed. This species is unlikely to be confused with other native California turtles, since it is the only California native that possesses barbels and the only native aquatic turtle that occurs within its range. However, many kinosternid turtle species are difficult to distinguish, and some of these have been sporadically introduced around the state. The most common introduced kinosternid is likely the common musk (or stinkpot) turtle (Sternotherus odoratus). This species has two broken light stripes on each side of the head and has only a single, anterior hinge on the plastron. Other species in the genus Kinosternon have also been introduced (K. flavescens in particular; S. Sweet, pers. comm.) but are not common and will often require expert identification (Spinks et al. 2003, Spinks et al., pers. obs.). See Stebbins (2003) for additional details.
Taxonomic Relationships
Two subspecies have been described, one of which historically occurred in California. The Sonora mud turtle (Kinosternon sonoriense sonoriense) includes California as well as the majority of the species’ range in the southwestern United States and northern Mexico. The Sonoyta mud turtle (K. s. longifemorales) is restricted to the Rio Sonoyta drainage in Mexico and southern Arizona (Iverson 1976). Intraspecific, including subspecific, variation has not yet been investigated genetically.
Life History
The life history of this species has not been studied in California. Life history studies in Arizona and New Mexico suggest that there is some interpopulation variation in basic life history parameters of this species. We base our life history description on work conducted primarily in Arizona and New Mexico but recognize that these data should be regarded as tentative for California populations.
Kinosternon sonoriense is active throughout the year as long as water is present, though in warmer months it may become active primarily at night (Hulse 1974, Hulse 1982). Hibernation is known to occur in high-elevation populations in New Mexico (Degenhardt et al. 1996), although it is unlikely that this occurs in California populations, which were exclusively low elevation. Kinosternon sonoriense aestivates terrestrially in response to seasonal drying in several populations (Ligon and Stone 2003, Hall and Steidl 2007, Hensley et al. 2010) but elsewhere may be more closely tied to permanent water (Ligon and Peterson 2002). In Arizona, females come into reproductive condition after a minimum of 5 years or with a carapace length between 115 and 125 mm, after which they produce one to four clutches per year although this varies depending on location (Van Loben Sels et al. 1997, Ernst and Lovich 2009, Lovich et al. 2012). Females become gravid between April and September, although most frequently in June and July (Lovich et al. 2012). The developing embryos apparently require a period of cooling before development restarts in the spring (Hulse 1982, Ewert 1991, Ernst and Lovich 2009). In Arizona, hatching may be associated with the summer monsoon in late summer (van Loben Sels et al. 1997).
Kinosternon sonoriense can attain high local population densities. One population in Hidalgo County, New Mexico, contained 212 turtles (Stone 2001). Another population in Yavapai County, Arizona, reached 750 individuals/ha of aquatic habitat (Hulse 1982). Individuals are known to undertake long terrestrial movements (>1 km) when water becomes limiting (Stone 2001, Hall and Steidl 2007), and Stone (2001) found that 26% of recaptured individuals had moved overland between aquatic capture sites. In the Santa Catalina Mountains (Pima County, Arizona), where the aquatic habitat consists of small and discrete pools, the presence of two or more adult turtles of the same sex within single pools was rare, suggesting that the species may be territorial where resources are limiting (Hall and Steidl 2007).
Kinosternon sonoriense is primarily carnivorous, feeding on a variety of invertebrates. It is known to shift to omnivory in suboptimal habitat (Hulse 1974) and to feed on or scavenge small vertebrates (Stone et al. 2005, Lovich et al. 2010).
Habitat Requirements
Habitat requirements for Kinosternon sonoriense in California are unknown but are likely tied to the presence of a reliable water source and a suitable prey base. Elsewhere in its range, it inhabits a wide variety of both permanent and temporary aquatic habitats including streams, creeks, stock ponds, and natural ponds (van Loben Sels et al. 1997, Ernst and Lovich 2009, Stanila 2009, Hensley et al. 2010, Stone et al. 2011). In California, it was known to enter artificial water bodies, although the long-term suitability of this habitat is unknown. Optimal habitat appears to be slow-moving, permanent water with a high density of aquatic invertebrates and a muddy bottom (Jennings and Hayes 1994a).
Distribution (Past and Present)
Historically, this species occurred in California along the Lower Colorado River drainage (Van Denburgh and Slevin 1913, Grinnell and Camp 1917, Dill 1944). La Rivers (1942) reported the northernmost record for the species in the Colorado River drainage from Clark County, Nevada. Cooper (1870) mentioned a specimen from an unspecified locality in the Colorado River Valley, collected while he was stationed at Fort Mohave, Arizona. Several more individuals were collected from the vicinity of Yuma, Arizona, and Palo Verde, California, in the early 1900s (Van Denburgh and Slevin 1913, Van Denburgh 1922). A 1942 record (SDNHM 17897) extended the western range in California to within ∼20 km of Calexico, suggesting that this taxon was present in ditches and canals in the Imperial Valley for at least some period of time. Klauber (1934) indicates that it was not “yet” present in the Imperial Valley, though by 1942 it clearly was. The overall extent and timing of its expansion into the Imperial Valley is essentially unknown. In the Lower Colorado River Valley, the species was present at least until 1941 near Bard, Imperial County (SDNHM 33866).
The last published record of Kinosternon sonoriense in the Lower Colorado River drainage occurred on the Arizona side of the river ∼1.6 km southwest of Laguna Dam on 31 March 1962 (Funk 1974, Lovich and Beaman 2008). Turtle trapping surveys were conducted in April of 1991 throughout much of the historic California range and failed to detect the species (King and Robbins 1991). The presence of “small black turtles along the Coachella Canal” was rumored in the 1990s, but these reports were never verified and could have been misidentified Trachemys scripta or Apalone spinifera (J. Lovich, pers. comm.).
Outside of California, K. sonoriense ranges through much of southern Arizona, into the southwestern corner of New Mexico and south into northern Sonora and Chihuahua, Mexico, from sea level to 2040 m (Stebbins 2003, Lovich and Beaman 2008, Ernst and Lovich 2009).
Trends in Abundance
There is no information concerning historical abundance of this species in California. Only five reliable localities have been recorded in California, and historical accounts from the early twentieth century contain few data on abundance. Van Denburgh and Slevin (1913) reported that “six or eight” specimens were collected near Yuma before 1906, and stated that “whether it ascends the Colorado River above the Gila is not known.” Van Denburgh (1922) stated that the species occurred in the Lower Colorado River drainage but was aware of records only near Yuma and at Palo Verde in Imperial County. The Clark County Nevada record had not yet been reported at this time (La Rivers 1942). Dill (1944) mentioned only that this taxon occasionally stole bait from fishermen (presumably implying that it was fairly well known to fishermen). The paucity of records from California suggests that populations here may not have occurred in the high densities documented elsewhere, although this species is difficult to detect without specific trapping efforts, and it is not clear that these efforts were ever made while the species was known to be present. Thus, the historical data on abundance are inconclusive. Kinosternon sonoriense has not been collected in or near California in nearly 50 years, despite extensive surveys (King and Robbins 1991). It is clear that declines, and possibly extirpation, have occurred during the last century.
Nature and Degree of Threat
The causes of decline of Kinosternon sonoriense in California are poorly understood, but may be associated with habitat modification and water diversion along the Colorado River and the Imperial Valley (Ohmart et al. 1988). Increased use of pesticides may have modified the available prey base, forcing the species to shift to a suboptimal herbivorous diet, which has been suggested as a factor in other K. sonoriense declines (King et al. 1996). The impact of introduced exotic crayfish, bullfrogs, warm water fishes, and softshelled turtles, all of which were well established around the time of K. sonoriense declines (Dill 1944, Lovich and Beaman 2008), is unknown, but they could plausibly have had a negative impact on K. sonoriense. At one site in Arizona, reduced K. sonoriense densities appear to be associated with the presence of introduced crayfish (Lazaroff et al. 2006).
Between 1941 and 1943, the Imperial Irrigation District burned and sprayed oil on 13,000 km of ditches and canals in the Imperial Valley in an effort to control the damage being done by spreading muskrat populations (Twining and Hensley 1943). These efforts certainly destroyed a great deal of aquatic habitat in the region, and the effect of the oil residues may have also had strong impacts on K. sonoriense and other taxa that disappeared from this area during the same time period (e.g., Rana yavapaiensis, Bufo alvarius).
Status Determination
A Priority 1 Species of Special Concern designation is justified by the complete absence of records for this species since the 1960s. This is the primary cause for concern. Little understanding of Kinosternon sonoriense’s habitat requirements or factors leading to decline in California currently exists. However, given the survey efforts that have been conducted to date, we assume that any remaining California populations are small, fragmentary, and vulnerable to extirpation. The species may also be vulnerable to increasing temperatures and changing hydrology due to climate change.
Management Recommendations
If future surveys detect any remaining populations, initial management efforts should focus on protecting those populations while research is performed that focuses on expanding suitable habitat and rebuilding local populations. If initial estimates of population structure indicate that reproduction and/or recruitment is not occurring, a headstarting program could be effective as a stopgap measure to prevent local extirpation. Many aquatic turtles have very different habitat requirements for hatchlings and adults, and ecological studies of both age classes will almost certainly be necessary to ensure the survival of remnant native populations.
Monitoring, Research, and Survey Needs
Although surveys have been performed for Kinosternon sonoriense in California, these efforts are not yet comprehensive. As this species is generally easily captured using submersible turtle traps, more complete survey efforts will help to clarify the species’ status in California. Areas that have not yet been systematically surveyed include the backwaters of the Colorado River below Needles and along Lake Havasu (R. Fisher, pers. comm.); Haughtelin, Ferguson, Taylor, Draper, and Walker Lakes (King and Robbins 1991); the Coachella Canal; and any riparian habitat remaining in the area of Laguna Dam, as well as at Topock Marsh in the Havasu National Wildlife Refuge. Because the Lower Colorado River segment of the species’ range spanned both California and Arizona, additional surveys should be coordinated with wildlife managers in Arizona to search potential habitat on the eastern side of the Colorado River.
If surveys do detect any individuals, managers should immediately initiate a monitoring program to determine the size and stability of the population, as well as an ecological study of population structure and life history. This will almost certainly involve individually marking turtles with shell notches and/or PIT tags and performing mark–recapture surveys to estimate population size and individual growth rate. In particular, whether, and how much, reproduction is taking place in existing populations will be critical to determine. Juvenile turtles rarely enter submersible traps; thus, alternative methods should be employed to search for them (such as seining or snorkeling). Female turtles should also be checked for eggs using either palpation or radiographs, preferably with portable field-capable digital X-ray units.
Genetic samples from the Lower Colorado River do not exist and should be collected, should remaining populations be found. These samples will be valuable to researchers working on Kinosternon phylogenetics and phylogeography and will also be critical in assessing the existing diversity within remaining populations and the divergence between these and more abundant populations to the east in Arizona.
Finally, researchers should attempt to characterize differences between habitat that supports this species and nearby habitats that do not. The causes of decline are still poorly understood, so management efforts that focus on rebuilding populations must be informed with strong data on the impact of introduced predators, pesticide, and herbicide drift, introduced aquatic plants, and habitat modification on K. sonoriense population persistence.