Seizures
Perspective
Seizures are episodes of abnormal neurologic functioning caused by pathologically excessive activation of neurons, either in the cerebral cortex or in the deep limbic system. Epilepsy is defined as recurrent unprovoked seizures caused by a genetically determined or acquired brain disorder1; it is not an appropriate term for seizures that occur intermittently or predictably after a known insult, such as alcohol intoxication and withdrawal.
Presentation to the emergency department (ED) with a generalized convulsive seizure prompts immediate concern for airway protection and treatment to stop the ictal activity, followed by a focused search for the cause. Nonconvulsive seizures, which are much less common, may be relatively obscure in their presentation, are more diverse in their etiology, and are sometimes more difficult to recognize and acutely control.
Epidemiology and Classification
More than 10% of the United States population will experience at least one seizure during their lifetime; however, only 3% will be diagnosed with epilepsy. Seizures account for 3% of transports by emergency medical services, 2% of pediatric ED visits, and 1% of all ED visits. Alcohol and central nervous system pathologies (tumor, stroke, trauma, infection) are common causes in adults. Metabolic derangements and infection more frequently cause seizures in infants than adults, but febrile seizures are the most common type of seizure in infants and young children. Approximately 7% of patients who come to the ED with seizures are in status epilepticus.2
Seizures can be classified based on cause (primary or secondary), effect on mentation (generalized or focal), and motor activity (convulsive or nonconvulsive). Primary seizures are unprovoked and not linked to an inciting event. Secondary seizures may be caused by trauma, illness, intoxications and poisonings, organ failure, other metabolic disturbances, cerebral tumors, pregnancy, and, paradoxically, supratherapeutic levels of some anticonvulsants.3,4
A newer terminology scheme has been developed and may affect communications between emergency physicians and epileptologists or patients. The “partial” classification is now solely “focal,” and the distinctions between “complex partial” and “simple partial” have been eliminated. The terms genetic, structural-metabolic, and unknown have replaced idiopathic, symptomatic, and cryptogenic.3
A generalized seizure is defined as abnormal neuronal activity in both cerebral hemispheres causing an alteration in the level of consciousness. Generalized seizures may be further divided into tonic-clonic, absence, atonic, and myoclonic; some patients may experience only the tonic or clonic phase. Focal seizures usually involve one cerebral hemisphere, thereby preserving consciousness, though these seizures may progress and cause an altered sensorium. Some seizures are impossible to classify because of inadequate or inaccurate description of the ictal activity.3,4
Convulsive seizures are characterized by uncontrolled, rhythmic motor movements and can affect part or all of the body. Nonconvulsive seizures do not result in abnormal motor activity; patients may display confusion, altered mental status, abnormal behavior, or coma.
Status epilepticus has been classically defined as at least 30 minutes of persistent seizures or a series of recurrent seizures without intervening return to full consciousness.5 The time criterion has been shortened to 5 minutes with recognition that the duration of seizure activity is related to outcome and that the likelihood of achieving seizure cessation with typical treatments decreases with ictal duration.4,6 Common causes of status epilepticus in adults are shown in Table 18-1.7
Table 18-1
Etiology of Status Epilepticus in Adults
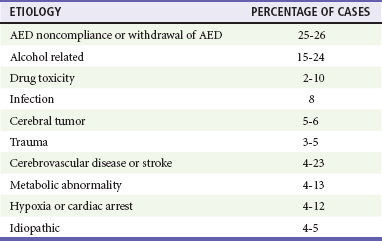
Adapted from Bassin S, Smith TL, Bleck TP: Clinical review: Status epilepticus. Crit Care 6:137-142, 2010.
Seizures in children follow a different distribution, primarily because of the relatively high incidence of febrile seizures and the inherent limitations of the observational history of possible ictal activity. Febrile seizure is the most common pediatric seizure, occurring in 2 to 5% of children 6 months to 5 years of age; 20 to 30% of those children have at least one recurrence. It is important to differentiate between the febrile seizure, which is typically of short duration, has a limited postictal phase, and is related to the slope of the body temperature rise, and the seizure with fever, which is often more serious and prolonged and indicates more significant underlying infection.8 First-time seizures in infants younger than 6 months may indicate important underlying pathology and necessitate a full assessment.9
Pathophysiology
Seizures occur when the abnormal increased electrical activity of initiating neurons activates adjacent neurons and propagates until the thalamus and other subcortical structures are similarly stimulated. At a cellular level, the pathophysiology is not well understood, although research in specific epilepsy syndromes is elucidating possible mechanisms. Investigation of rare inherited epilepsy syndromes has identified mutations in neuronal ion channel proteins, limiting intracellular passage of potassium. Given that the potassium current is the primary force behind repolarization of membranes, depolarization is prolonged in these patients, leading to an increase in neuronal hyperexcitability.1 Other studies have found that malformations of cortical development and glial cells may play a role in epileptogenesis.1
Clinical seizure activity typically, but not always, reflects the initiating focus. When the ictal discharge extends below the cortex to deeper structures, the reticular activating system in the brainstem may be affected, altering consciousness. In generalized seizures, the focus is often deep and midline, which explains the prompt loss of consciousness and bilateral involvement. Seizures are typically self-limited; at some point the hyperpolarization subsides and the bursts of electrical discharges from the focus terminate. This cessation may be related to reflex inhibition, neuronal exhaustion, or alteration of the local balance of neurotransmitters.
Focal seizures may represent a similar pathophysiologic process in which less recruitment occurs and the ictal activity does not cross the midline. Because of the more limited focus of abnormal activity, convulsive motor activity may not be the predominant clinical manifestation.10
Diagnostic Approach
Because a diagnosis of seizure has major consequences for the patient in the ED—including loss of driving privileges and exposure to potentially toxic medicines—the first diagnostic task is to determine whether the patient actually experienced a seizure.11,12 Ictal activity can be irrefutably verified only by electroencephalography. Other abnormal movements and states of consciousness, including nonepileptic convulsions (pseudoseizures), can be confused with ictal activity. The differential diagnoses to consider when evaluating for seizure are listed in Table 18-2.12
Table 18-2
Differential Considerations for the Diagnosis of Seizure
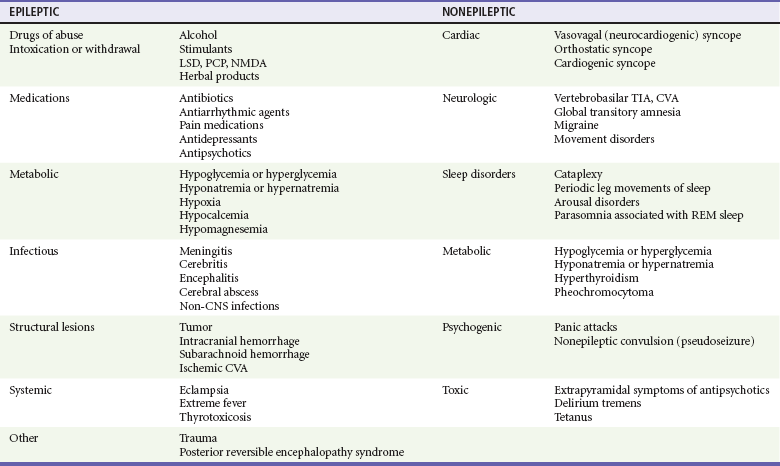
CNS, central nervous system; CVA, cerebrovascular accident; LSD, lysergic acid diethylamide; NMDA, N-methyl-D-aspartate; PCP, phencyclidine; REM, rapid eye movement; TIA, transient ischemic attack.
Adapted from Carreno M: Recognition of nonepileptic events. Semin Neurol 28:297-304, 2008; and Slattery DE, Pollack CV Jr: Seizures as a cause of altered mental status. Emerg Med Clin North Am 28:517-534, 2010.
Syncope, whether vasodepressive (vagal syncope), orthostatic, or dysrhythmia related, can be confused with seizures by observers. A sudden loss of consciousness followed by abnormal movements can be ictal or syncopal in origin, hence the consideration “fit versus faint.” One video analysis of 56 brief syncopal episodes showed myoclonic activity in 90% of patients, together with frequent head turns, upward gaze, oral automatisms, and righting movements. These are likely a transient response by the brain to sudden deprivation of blood flow. In general, ictal tonic-clonic movements are more forceful and prolonged than the brief convulsive activity sometimes associated with fainting. In addition, most generalized seizures are characterized by a postictal state that syncope patients do not manifest.13 An important exception to this is an atonic seizure, or “drop attack,” in which the patient experiences a sudden and complete loss of muscle tone associated with altered mentation; in this type of generalized seizure, motor activity is not displayed and a postictal state may not occur.4
The cause of an unwitnessed, unprovoked loss of consciousness with a fall, after which the patient visits the ED, may be difficult to determine. Suggestions of seizure as the cause include retrograde amnesia, loss of continence (which also can occur with vasogenic and other forms of syncope), and evidence of oral trauma such as maceration of the buccal mucosa or bite lacerations of the tongue. Blood drawn soon after a true seizure may demonstrate a lactic acidosis that may resolve by the time a repeat analysis is performed in the ED.14
Rapid Assessment and Stabilization
The patient who arrives with a history of possible seizure activity should be placed in a monitored area of the ED and prepared for prompt physician examination.5 An intravenous line or saline lock catheter should be placed in case anticonvulsants are emergently indicated. Blood glucose is checked at the bedside, and a thorough list of all medications currently being used by the patient is obtained. Hypoglycemia is the most common metabolic cause of seizure activity. The only treatment required for the patient may be administration of intravenous glucose. Prolonged seizure activity may also cause hypoglycemia, so the cause-and-effect relationship may sometimes be reversed; but in either case, glucose therapy is required. If the patient stopped seizing before arrival in the ED, the history, physical examination, and diagnostic course should focus on possible precipitants of the seizure. The patient should be protected from self-injury during this time.
If the patient is seizing in the ED, the first step is to confirm that a pulse is present and that the convulsions are not the result of cerebral hypoxia from lack of blood flow. After this, attention is paid to protecting and maintaining the airway, although airway obstruction during ictus usually is transient. Nasopharyngeal airways and positioning the patient on the side generally are all that is required for airway management. Oropharyngeal airways are less useful because the mouth often is clenched, and damage to teeth or tongue may ensue during attempts to insert an oral airway. Oxygen may be administered by nasal cannula if the saturation is below 90% or the patient appears cyanotic (when saturation cannot be measured.) Suction should be available but not used during seizure activity because of risk of injury to teeth, tongue, or lips. Vascular access should be established, but benzodiazepine treatment can be initiated before placement of an intravenous line.
If the patient is immobilized on a spine board after trauma, the entire board can be tipped to one side to place the patient in the semiprone position for protection against aspiration. If the seizure persists after administration of anticonvulsant medication, preparation is made for endotracheal intubation.
Most seizures will terminate without intervention. If a patient begins to seize while under ED observation, it is prudent to administer low-dose benzodiazepines (approximately half of the doses for status epilepticus, Table 18-3). Patients who arrive at the ED with continued seizure activity, experience recurrent seizure without recovering from the postictal period, or experience witnessed convulsions lasting longer than 5 minutes are considered in status epilepticus and treated as indicated in Table 18-3.
Table 18-3
Medications and Dosages for Emergency Treatment of Prolonged Seizures and Status Epilepticus
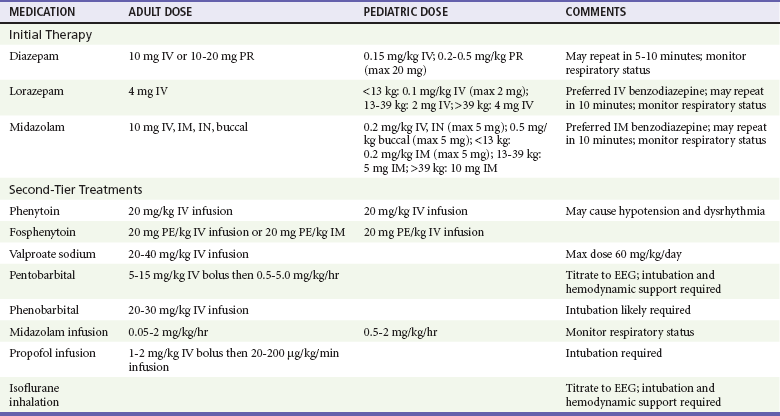
EEG, electroencephalogram; ET, endotracheally; IM, intramuscular; IN, intranasally; IV, intravenous; PR, per rectum.
Intraosseous administration is an acceptable alternative for intravenous medications.
Adapted from Brophy GM, et al: Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 17:3-23, 2012.
Benzodiazepines are the optimal first-line agents for stopping seizure activity in patients of all ages. Available agents include lorazepam, diazepam, and midazolam; all three are effective in terminating seizure activity. If intravenous access is established, lorazepam is the preferred first agent for the management of status epilepticus, particularly because of a long half-life, which decreases risk of seizure recurrence.15 For the patient without vascular access, immediate intramuscular midazolam is preferred over delayed intravenous lorazepam.16 Diazepam and midazolam can both be administered via alternate routes, and buccal midazolam is more effective than rectal diazepam in achieving seizure cessation.17
If repeated doses of benzodiazepines do not abort seizure activity, or if the patient's oxygen saturation remains persistently below 90%, emergent intubation is indicated. (See Chapter 1.) If possible, the patient should not receive long-acting neuromuscular blockade, so that recurrence or persistence of seizure activity can be monitored.
Medications for the treatment of status epilepticus are shown in Table 18-3. There is little evidence on which to base the choice and timing of second-line medications in the treatment of benzodiazepine-refractory status epilepticus; most recommendations are supported by expert consensus.6,15,18 However, moving quickly to a second-line agent is supported, and phenytoin is the most recommended second-line therapy for adults with persistent seizure activity.6,18 The prodrug, fosphenytoin, can be administered more quickly, can be given intramuscularly, and has less tendency to cause hypotension, but it is significantly more expensive.19 Second-line therapy for children is intravenous phenobarbital. Third-line therapy is phenytoin for children and phenobarbital for adults.20–22 Intravenous valproate sodium is safe and is an alternative for patients who are on chronic valproic therapy and whose levels are subtherapeutic.23,24 The role of newer antiepileptic drugs such as levetiracetam and lacosamide in the management of status epilepticus has not yet been established.
Seizures refractory to these initial treatments prompt an evaluation for nonepileptic causes of the convulsions. Isoniazid overdose can cause prolonged seizures refractory to benzodiazepines. Pyridoxine is the only fully effective pharmacologic treatment for toxic isoniazid seizures, although benzodiazepines have been shown to suppress seizure activity in some cases.25 In seizing female patients of childbearing age, eclampsia may be the cause, and intravenous magnesium is the initial treatment (see Chapter 177). Eclamptic seizures refractory to magnesium may respond to benzodiazepines or barbiturates with or without phenytoin. Children and psychiatric patients at risk for water intoxication may be potential candidates for hypertonic saline therapy, after laboratory confirmation of hyponatremia.
Patients who remain unresponsive to the third-level choice of pharmacologic intervention are by definition in refractory status epilepticus. Further choices for therapy are general anesthetic doses of midazolam or propofol, barbiturate coma, and isoflurane anesthesia; all of these treatments necessitate endotracheal intubation.21,23A nondepolarizing neuromuscular blocking agent such as rocuronium or vecuronium is administered concomitantly to reduce the metabolic burden and potential hyperthermia that can ensue from prolonged status seizures; continuous electroencephalographic monitoring should be used, if possible, in these cases.
Pivotal Findings
When the patient is stabilized with a secure airway and ictal activity has been controlled, attention is turned to gathering more complete data.
History
History taking in the patient with seizure is directed by two main questions. First, “Was the incident truly a seizure?” This is important because of the broad differential diagnosis for seizures (see Table 18-2) and the frequency of inaccurate descriptions of seizure-like activity from laypersons. In general, ictal events have six properties:
1. Abrupt onset: Generalized seizures typically occur without an aura.
2. Brief duration: Seizures rarely last longer than 90 to 120 seconds, although bystanders may overestimate the duration.
3. Loss of consciousness: Present by definition, except for focal seizures.
4. Purposeless activity: For example, automatisms and undirected tonic-clonic movements.
5. Unprovoked: Especially with regard to emotional stimuli; fever in children and substance withdrawal in adults are notable exceptions.
6. Postictal state: An acute confusion state that typically occurs with all seizure types except focal and absence.
Information regarding focality of onset, loss of bowel or bladder control, or tongue biting should also be elicited. Presence or absence of these findings, however, does not confirm or exclude the possibility that a seizure occurred.
The second question to direct the history is, “Does this patient have a history of seizures?” If there is a documented history of seizures, ED evaluation may be limited to a thorough history and consideration of measurement of anticonvulsant drug levels. History should focus on recent illness or trauma, drug or alcohol use, potential adverse drug-drug interactions with anticonvulsants, medication compliance, a recent change in anticonvulsant dosing regimens, or a change in ictal pattern or characteristics. Evaluation of injuries sustained during the seizure is also warranted.
Supratherapeutic and toxic levels of some anticonvulsants such as phenytoin and carbamazepine, whether attained chronically or after acute overdose, may cause seizures. If empirical anticonvulsant therapy is indicated before the serum level is available, only 50% of a full loading dose is given unless the patient is known reliably not to be taking anticonvulsant medication.
If the patient does not have a history of seizures and the description of the event is truly consistent with a seizure, the history should focus on potential underlying medical, toxicologic, or neurologic causes.
A personal history from the patient, close friend, relative, or medical record may reveal potential ictogenic factors such as recent or remote head trauma, developmental abnormalities, metabolic diseases, drug or alcohol abuse, sleep deprivation, pregnancy, recent travel, previous seizures, or use of herbal supplements. When no witness or family member is available, extensive questioning awaits clearance of the postictal state.
Physical Examination
The physical manifestations of convulsive ictal activity include hypertension, tachycardia, and tachypnea from sympathetic stimulation. These signs typically resolve quickly after the seizure activity ceases. With more prolonged convulsions, skeletal muscle damage, lactic acidosis, and, rarely, frank rhabdomyolysis may ensue. Autonomic discharges and bulbar muscle involvement may result in urinary or fecal incontinence, vomiting (with significant aspiration risk), tongue biting, and airway impairment. All of these signs are helpful discriminators in the differential evaluation of seizure-like spells, although presence or absence of these findings neither confirms nor excludes seizure occurrence.
After the seizure activity has ceased, resting vital signs are evaluated. Fever and underlying infection can cause seizures, although there may be a low-grade temperature elevation immediately after a convulsive generalized seizure. Tachypnea, tachycardia, or an abnormal blood pressure that persists beyond the immediate postictal period may indicate toxic exposure, hypoxia, or a central nervous system lesion. Pertinent physical findings may include nuchal rigidity, stigmata of substance abuse, lymphadenopathy suggestive of human immunodeficiency virus (HIV) disease or malignancy, dysmorphic features, or skin lesions. The examination should also focus on potential adverse sequelae of convulsive seizures, such as head trauma, oral and tongue injury, posterior shoulder dislocation, or back pain.
Finally, a complete neurologic examination is performed. A persistent focal deficit after a seizure (e.g., Todd's paralysis) often indicates the focal origin of the event but also can be evidence of an underlying stroke. The patient should be carefully examined for papilledema; elevated intracranial pressure can both cause and result from ictal activity. Failure to note steady improvement of postictal depression of consciousness suggests the possibility of an underlying encephalopathy or nonconvulsive status epilepticus.
Ancillary Testing
Laboratory.: Routine screening studies such as a complete blood count and chemistry profile have little use in the neurologically normal, otherwise healthy, postictal patient with a known seizure disorder for whom a reliable history can be obtained. In patients with new onset of seizure or those with a known seizure disorder but with a significant change in seizure pattern (e.g., a substantial increase in seizure frequency despite medication compliance), limited blood testing is indicated.5
Bedside blood glucose levels should be measured in every patient, whether he or she is known to have a seizure disorder or not. Serum sodium is the most important electrolyte to assess, particularly if mental status remains altered after apparent recovery from the postictal state. Anticonvulsant levels are appropriate in patients known or thought to be taking anticonvulsant medication. Febrile patients are evaluated for the source of the fever, including consideration of lumbar puncture. Leukocytosis, with or without bandemia, however, is a common finding after a seizure and is not a reliable indicator of infection. For medically ill adults (e.g., those with diabetes, cancer, or liver disease or those taking medications that can affect serum electrolyte levels) and in patients with a first-time seizure or substantial change in seizure pattern, serum electrolytes, including calcium and magnesium, are indicated. Liver function tests may be helpful if physical examination reveals liver disease or if there is history of liver infection or alcoholism.11 Directed toxicology screens are performed if substance abuse is suspected, particularly cocaine, amphetamines, and other sympathomimetic agents. Eclampsia occurs late in pregnancy or in the early postpartum period, so pregnancy usually is clinically evident in such cases. Headache may be a feature of the patient's postictal state, but otherwise, presence of fever and headache or sudden onset of headache is an indication for computed tomography (CT) scan, lumbar puncture, or both.26
Imaging.: When feasible, cranial CT is recommended for patients in the ED with new-onset seizures and is mandatory if neurologic examination identifies focal abnormalities postseizure.5 Cranial CT is indicated in any age group when there is a possibility of head trauma, elevated intracranial pressure, intracranial mass, persistently abnormal mental status or focal neurologic abnormality, or HIV disease.
In the fully recovered patient without headache and with fully normal mental status and neurologic examination findings who has had a single, brief seizure, a cranial CT scan can be performed in the ED or at a follow-up visit at the discretion of the treating physician.5 Box 18-1 lists the circumstances under which a head CT is recommended in the ED because of a higher likelihood of discovering an acute abnormality.5
The literature on head CT imaging for first-time, nonfebrile seizures in children is inconclusive.27
Electroencephalography.: Electroencephalography is not consistently available in the ED. It may be particularly useful in specific cases, such as the diagnosis of nonconvulsive status epilepticus, to monitor seizure activity after intubation and neuromuscular blockade, and to help differentiate seizures from other similar presentations. In general, electroencephalograms (EEGs) are most appropriate for the follow-up evaluation of patients with first-time seizures without clear cause after a complete ED evaluation.28
Management
Usually an acute seizure self-terminates or can be pharmacologically terminated before a need arises for active airway management. Rapidly reversible disorders (e.g., hypoglycemia, hypoxemia, isoniazid ingestion) should be considered and, if found, treated. Primary abortive therapy in the ED is accomplished as described earlier. Although a number of newer antiepileptic medications have become available, their therapeutic purpose is directed toward chronic rather than acute seizures.29
Identifying a new-onset seizure in the ED generates consideration for further management. The choice to initiate anticonvulsant therapy depends on the risk of seizure recurrence, any underlying predisposing disease, and the risk of initiating anticonvulsant therapy. Chronic anticonvulsant medication may be advisable, even in the short term, pending further outpatient evaluation, and the decision to initiate this is made in consultation with the neurologist who will be following the patient after discharge from the ED.30,31 Prompt treatment of any apparent causative disorder discovered in the ED, however, is appropriate.
Disposition
Disposition plans are individualized according to the findings of the ED evaluation and the presence or absence of underlying disease. One quarter of adult patients with seizure-related complaints have new-onset seizures.10,16 Almost half of them require admission, often because of abnormal CT scans or persistent focal abnormalities; 95% of those who retrospectively required admission are correctly identified through use of an ED evaluation consistent with that recommended previously. Patients may be discharged home with early referral to a neurologist if they have normal neurologic examination findings, no significant medical comorbidities, and no known structural brain disease; do not require the use of an antiepileptic drug or multiple doses of benzodiazepines in the ED; and are felt to have sufficient resources to reliably comply with follow-up instructions.5 Patients discharged home from the ED should receive appropriate state-specific guidance regarding driver's license privileges, warning about potentially dangerous activities (e.g., swimming, climbing ladders and heights, operating machinery), and information for prompt follow-up with a neurologist.
References
1. Chang, BS, Lowenstein, DH. Epilepsy. N Engl J Med. 2003;349:1257–1266.
2. Martindale, JL, Goldstein, JN, Pallin, DJ. Emergency department seizure epidemiology. Emerg Med Clin North Am. 2011;29:15–27.
3. Berg, AT, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–685.
4. Huff, JS, Fountain, NB. Pathophysiology and definitions of seizures and status epilepticus. Emerg Med Clin North Am. 2011;29:1–13.
5. ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: Critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2004;43:605–625.
6. Millikan, D, Rice, B, Silbergleit, R. Emergency treatment of status epilepticus: Current thinking. Emerg Med Clin North Am. 2009;27:101–113.
7. Bassin, S, Smith, TL, Bleck, TP. Clinical review: Status epilepticus. Crit Care. 2002;6:137–142.
8. Hirtz, D, et al. Practice parameter: Treatment of the child with a first unprovoked seizure: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166–175.
9. Bui, TT, Delgado, CA, Simon, HK. Infant seizures not so infantile: First-time seizures in children under six months of age presenting to the ED. Am J Emerg Med. 2002;20:518–520.
10. Huff, JS, Morris, DL, Kothari, RU, Gibbs, MA. Emergency department management of patients with seizures: A multicenter study. Acad Emerg Med. 2001;8:622–628.
11. Browne, TR, Holmes, GL. Epilepsy. N Engl J Med. 2001;344:1145–1151.
12. Carreno, M. Recognition of nonepileptic events. Semin Neurol. 2008;28:297–304.
13. Schmidt, D. Syncopes and seizures. Curr Opin Neurol. 1996;9:78–81.
14. Bakes, KM, Faragher, J, Markovchick, VJ, Donahoe, K, Haukoos, JS. The Denver Seizure Score: Anion gap metabolic acidosis predicts generalized seizure. Am J Emerg Med. 2011;29:1097–1102.
15. Treiman, DM. Treatment of convulsive status epilepticus. Int Rev Neurobiol. 2007;81:273–285.
16. Silbergleit, R, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600.
17. McMullan, J, Sasson, C, Pancioli, A, Silbergleit, R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: A meta-analysis. Acad Emerg Med. 2010;17:575–582.
18. Manno, EM. New management strategies in the treatment of status epilepticus. Mayo Clin Proc. 2003;78:508–518.
19. Pryor, FM, Gidal, B, Ramsay, RE, DeToledo, J, Morgan, RO. Fosphenytoin: Pharmacokinetics and tolerance of intramuscular loading doses. Epilepsia. 2001;42:245–250.
20. Hanhan, UA, Fiallos, MR, Orlowski, JP. Status epilepticus. Pediatr Clin North Am. 2001;48:683–694.
21. Smith, BJ. Treatment of status epilepticus. Neurol Clin. 2001;19:347–369.
22. Prasad, AN, Seshia, SS. Status epilepticus in pediatric practice: Neonate to adolescent. Adv Neurol. 2006;97:229–243.
23. Lowenstein, DH. The management of refractory status epilepticus: An update. Epilepsia. 2006;47(Suppl 1):35–40.
24. Sinha, S, Naritoku, DK. Intravenous valproate is well tolerated in unstable patients with status epilepticus. Neurology. 2000;55:722–724.
25. Minns, AB, Ghafouri, N, Clark, RF. Isoniazid-induced status epilepticus in a pediatric patient after inadequate pyridoxine therapy. Pediatr Emerg Care. 2010;26:380–381.
26. Hasbun, R, Abrahams, J, Jekel, J, Quagliarello, VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727–1733.
27. Sharma, S, Riviello, JJ, Harper, MB, Baskin, MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics. 2003;111:1–5.
28. Quigg, M, Shneker, B, Domer, P. Current practice in administration and clinical criteria of emergent EEG. J Clin Neurophysiol. 2001;18:162–165.
29. French, JA, et al. Efficacy and tolerability of the new antiepileptic drugs I: Treatment of new onset epilepsy: Report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1252–1260.
30. Jacoby, A, Gamble, C, Doughty, J, Marson, A, Chadwick, D. Quality of life outcomes of immediate or delayed treatment of early epilepsy and single seizures. Neurology. 2007;68:1188–1196.
31. Marson, A, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: A randomised controlled trial. Lancet. 2005;365:2007–2013.