CHAPTER 23
Children with Chronic Health Conditions: Implications for Oral Health
Göran Dahllöf, Pernille Endrup Jacobsen, and Luc Martens
Over the past few decades chronic health conditions (CHCs) and disabilities among children and youth have steadily risen primarily for common conditions such as asthma, obesity, mental health conditions, and neurodevelopmental disorders. With new technology, better drugs, and more efficient use of existing treatments an increasing number of children survive their CHCs. In industrialized countries, over 85% of children with CHCs will survive at least to 20 years of age. Other important changes are that children with CHCs are not institutionalized as frequently as in earlier times, they receive early stimulation, and they are usually integrated in the schooling system and attend outpatient medical and dental clinics. Yet, many of these children have not been cured or they will have disabling sequelae of their disease or treatment. Managed care protocols including oral care are at present being developed for several CHCs. For example, in programs for children treated with stem cell transplantation it is recommended that they should be referred to the dentist prior to the start of cytotoxic therapy for oral health information as well as evaluation of the oral health status, particularly the presence of infectious foci. In some instances, oral diseases and also dental care may be life‐threatening to the child, e.g., surgical treatment in children with blood or bleeding disorders. A close collaboration with the medical treatment team is an integral part of the management of oral health care in children with CHC.
General dentists will increasingly meet children with CHCs in their practices and up‐to‐date knowledge of how a CHC affects the oral health of a child is an important aspect of pediatric dental care.
Definition of chronic health conditions
A modern definition of CHC in children focuses on the consequences of the disorder and is independent of diagnosis [1]. Three elements must coexist for a child to be classified as having a CHC (Box 23.1). The duration of a condition can be difficult to predict, particularly when the onset is recent. Disease pattern is defined as the relative consistency or permanence of symptoms or consequences with time. Five patterns of disease have been identified (see Box 23.2 and Figures 23.1 to 23.5).
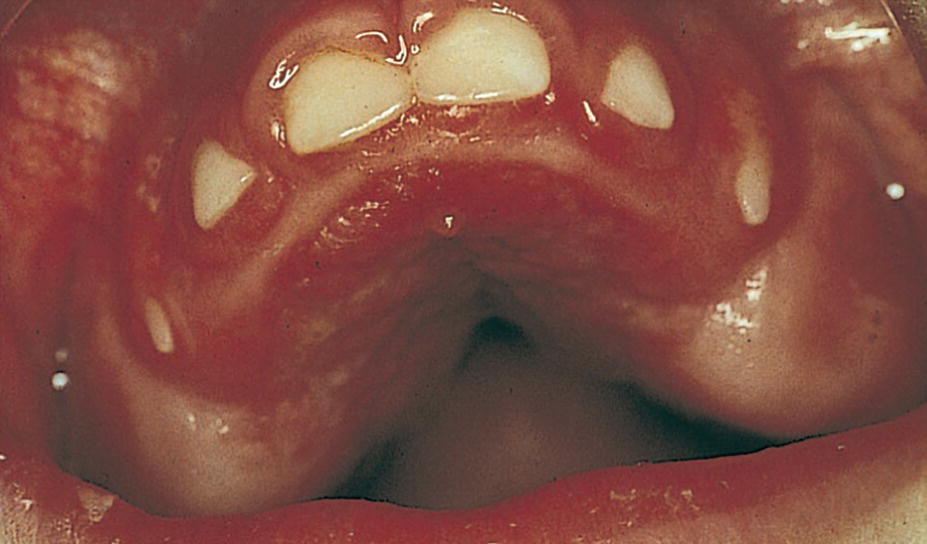
Figure 23.1 A 3‐year‐old boy with cerebral palsy and showing severe gingival overgrowth.
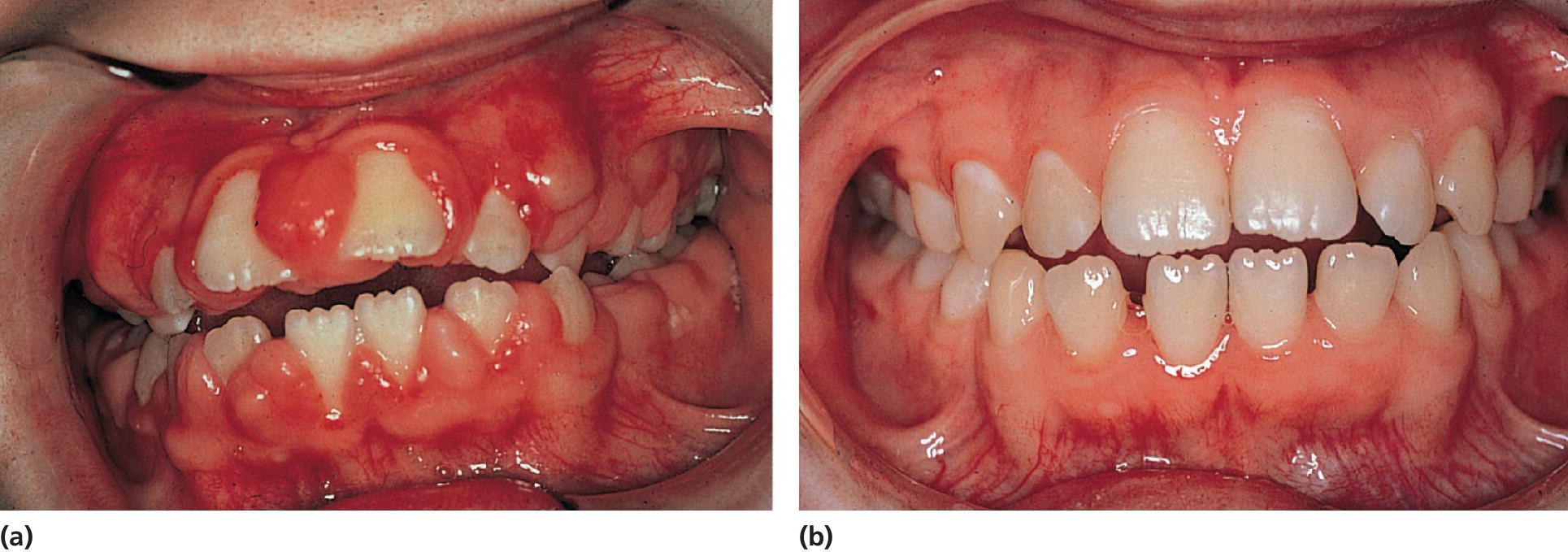
Figure 23.2 (a) A 10‐year‐old girl on phenytoin medication exhibiting severe gingival overgrowth. (b) At 13 years of age, exhibiting a normal gingival 1 year after gingivectomy and discontinuation of phenytoin medication.
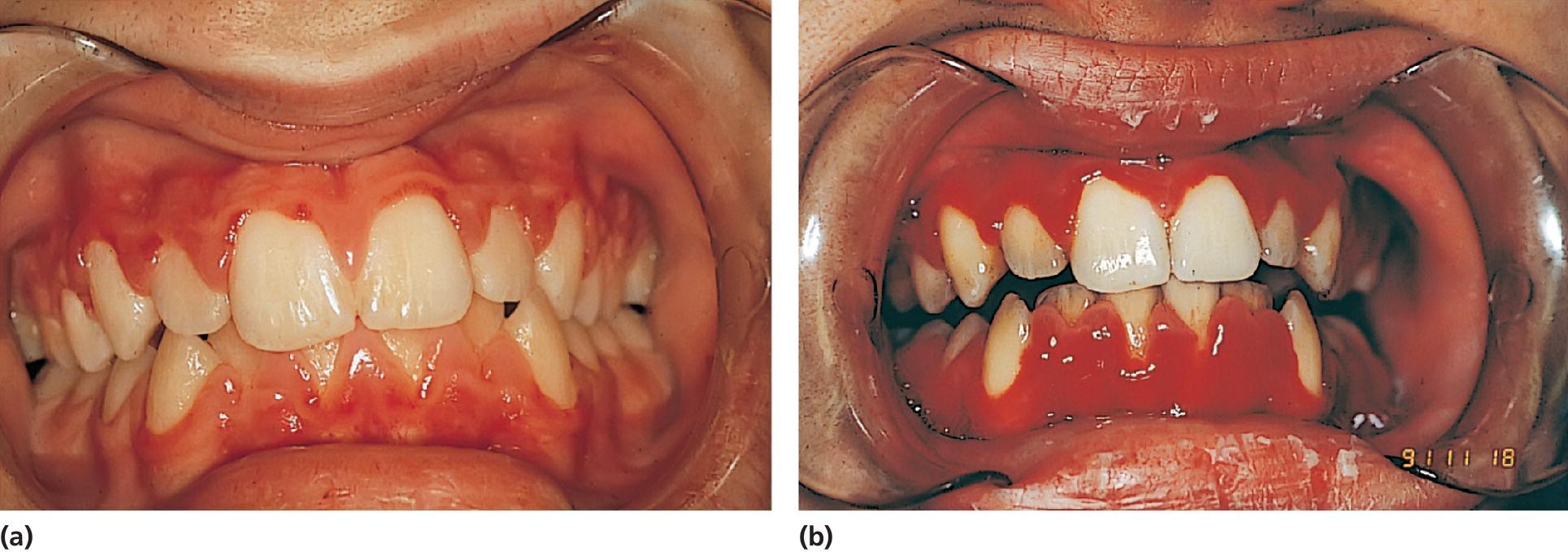
Figure 23.3 (a) A 15‐year‐old boy with aplastic anemia prescribed cyclosporine medication. (b) At 18 years of age, deteriorating gingival health coincidental with a hematological crisis.
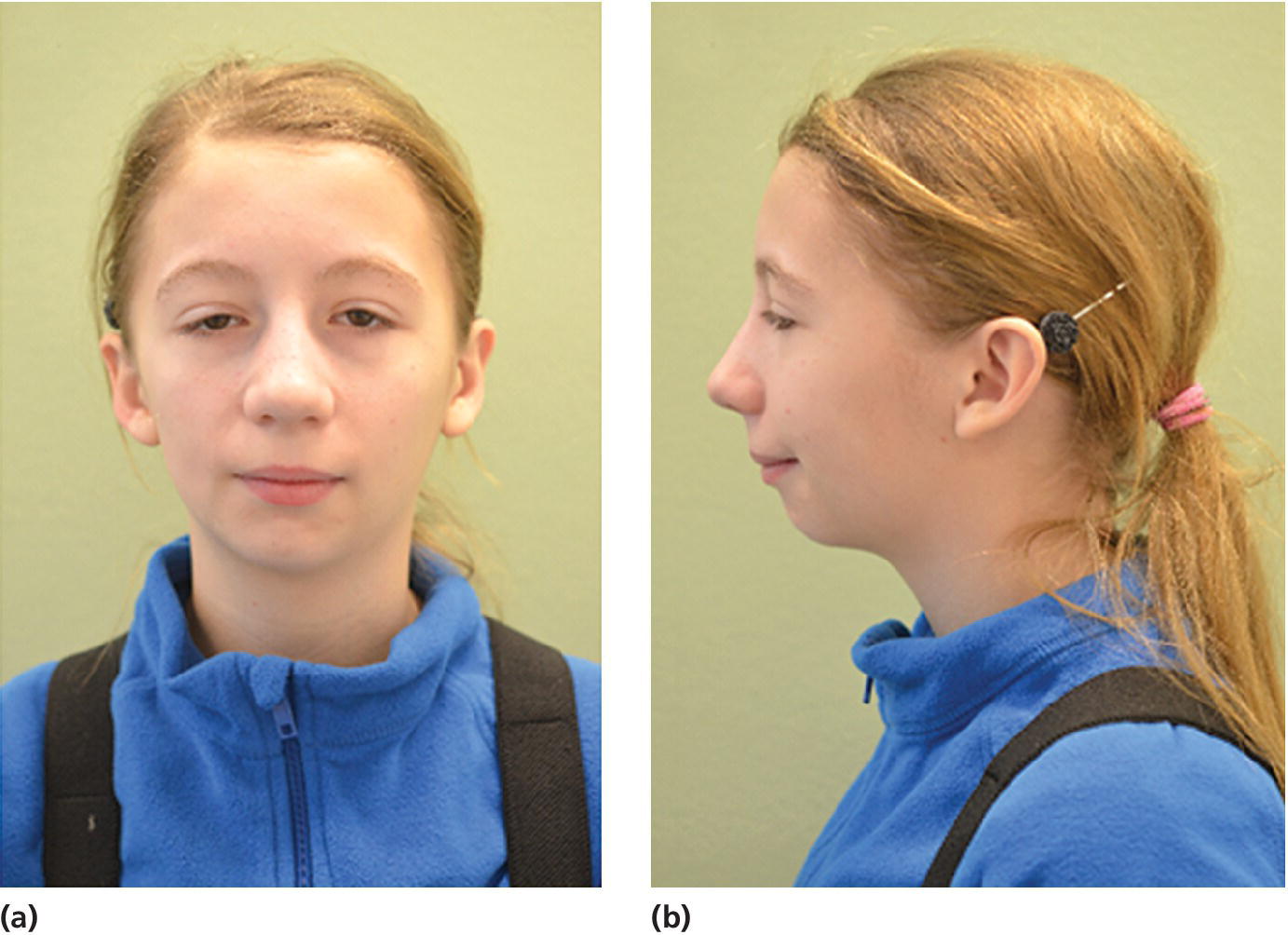
Figure 23.4 A 14‐year‐old girl with restricted and asymmetric growth of the mandible due to unilateral TMJ arthritis in her right side.
(Courtesy of Professor Thomas Klit Pedersen, and Specialist in Orthodontics Anne Agger Mortensen, Division for Dento‐ and Craniofacial anomalies, Section of Orthodontics, Department of Dentistry, Health, University of Aarhus, Denmark.)
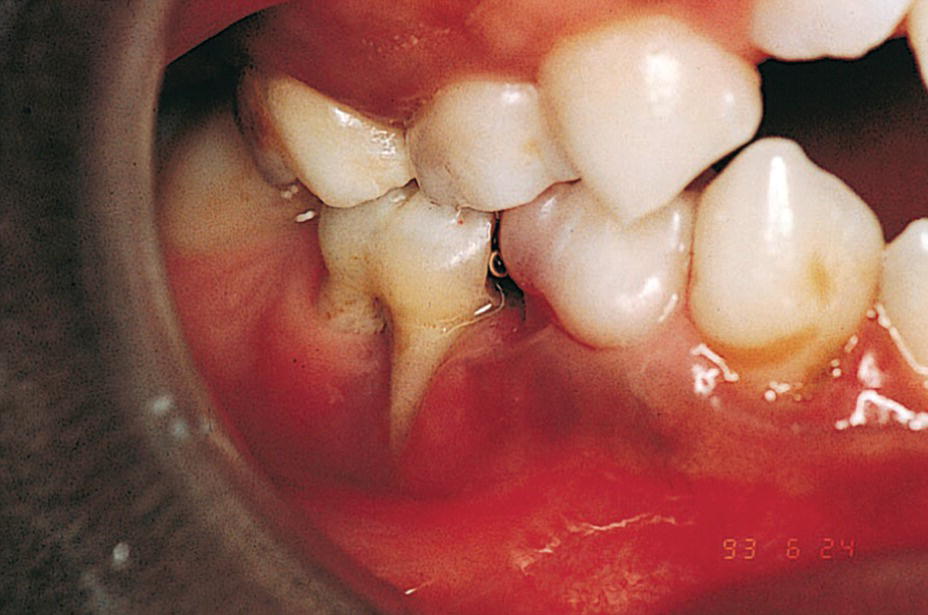
Figure 23.5 A 9‐year‐old boy with a congenital HIV infection exhibiting periodontal disease.
CHCs in children have a variable expression. In order to give the best treatment to a child with such a condition it is important to identify the limitations and also the possibilities of each child. In Box 23.3, 13 different dimensions on a continuous scale used to describe a child with a CHC are presented [2]. It should be observed that the definitions of CHC and impairment, disabilities, and handicap are related. They concentrate on the consequences for the individual rather than on the diagnosis (see Chapter 24, Box 24.1). The international classification of impairments, disabilities, and handicaps model proposes that there are three consequences of disease—impairment, disability, and handicap—‐and that they are sequentially related.
Prevalence
The prevalence of CHCs has been studied in different populations. Depending on the definition, estimates vary from less than 5% to more than 30% of children. The National Health Interview Study [3] used limitations in the kinds or amounts of play activities done by other children (<5 years); needing help with personal care including bathing, dressing, eating, getting in and out of bed and chairs, using the toilet, and getting around at home (3+ years); difficulty walking without equipment (<18 years); difficulty remembering (<18 years); receipt of special education services or early intervention services (<18 years); or any other activity limitation (<18 years). The overall rate of disability for non‐institutionalized children, 18 years old, increased 15.6% between 2001–2002 and 2010–2011 (Box 23.4). The estimated number of children with disabilities increased from 6.9% to 7.9% of the population. In a study of a total population in the south of Sweden in children aged 0–15 years, 8.4% were identified as having a CHC [4]. There is a predominance of boys over girls: 9.2 vs 7.6%. Eight percent of children with CHCs needed extensive help with activities of daily life and/or had a poor prognosis concerning short‐term survival, while 70% had only occasional limitations or needed regular medical treatment which did not interfere with normal activities.
Multi‐handicaps are common, particularly in children with mental retardation who exhibit additional diagnoses such as congenital malformation, epilepsy, vision impairment, and cerebral palsy. Among children with chronic conditions, approximately 70% have one diagnosis, 21% have two, and 9% have three or more. The prevalence of developmental delay, learning disabilities, and emotional and behavioral problems increases sharply with the number of chronic conditions in a child. The number of days in bed, school absences, and activity limitation also increased.
There are strong correlations between social disadvantage and child health. Overall, mortality throughout childhood and adolescence is increased among socially disadvantaged groups. In addition, survival of CHCs is worse in socially disadvantaged children. Factors apart from the material aspects are poorer social support, lifestyle factors such as smoking, poor dietary habits, lack of breastfeeding, and parenting style.
Despite obvious differences among the specific types of health conditions, important commonalties exist in the experience of children and families affected with various conditions. Among them are the need for a wide array of community and professional services, increased challenges to self‐concept and optimal emotional development, extra financial hardship, and disruption of family and social activities (see also Chapter 24).
Increased risk for oral diseases in children with chronic health conditions
Children with CHCs have an increased risk of oral diseases due to consequences of the disease or the medication given. In Box 23.5, groups of children with CHCs who have an increased risk for oral diseases are presented [5]. An important part of the general dentist’s role is to maintain contact with the family and to initiate the necessary preventive measures and to refer the patients if the condition of the patient requires pediatric dentistry specialist care. An early diagnosis of oral diseases is also of major importance as well as knowledge of any changes in health condition and medication that may affect the dental treatment. The medical history should be examined very carefully covering the child’s whole lifespan. It is of particular importance if local anesthesia and sedation are to be used that the child is classified according to anesthetic risk.
Preventive strategies in children with chronic health conditions
There are many CHCs that can directly influence dental care and some are the consequences of dental disease, and also dental treatment may have life‐threatening consequences. Unfortunately, there exist barriers to accessing dental care. Many children and adolescents with CHCs require frequent and sometimes prolonged hospitalizations that separate them from their home environment. This may result in missed appointments. Parents may also be reluctant to once again review the history of the child with the dentist or may not understand the need to share this information. Knowledge and skills among dentists may also not be adequate to treat children with severe CHCs. Early professional intervention is particularly important to provide examination, risk assessment, and information and guidance to parents so that oral diseases can be prevented. Box 23.6 summarizes preventive strategies that can be implemented in children with CHCs.
Chronic health conditions
Prematurity
Preterm is defined by the World Health Organization (WHO) as babies born alive before 37 weeks of pregnancy are completed. Sub‐categories are provided based on the gestational age (GA): moderate to late preterm (32 to < 37 weeks), very preterm (28 to < 32 weeks), and extremely preterm (<28 weeks). Furthermore, preterm children are often categorized according to their birth weight (BW); low birth weight (<2500 g), very low birth weight (<1500 g), and extreme low birth weight (<1000 g). In Western countries, the reduced mortality and morbidity of especially extremely preterm children have increased the prevalence. The survival of preterm birth is, though, not without complications. Immediate complications are, e.g., respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular bleeding, necrotizing enterocolitis, persistent ductus arteriosus, along with increased risk of infections, whereas late complications include cerebral palsy, neuropsychiatric disorders, learning disabilities, altered pain perception, and restricted or delayed growth, among others. Factors that influence the development and growth of a child, prenatally or in the early years of life, are also potential factors to influence the development of the primary and permanent teeth.
Implications for oral health
It is well documented that premature children have an increased risk of developing hypoplasia in the primary dentition. Furthermore, studies suggest an increased risk of enamel opacities in the primary dentition in the case of very low birth weight [6]. Early diagnosis and prevention are therefore important to prevent development of early childhood caries. Furthermore, the extreme preterm children can display behavior management problems, due to hypersensitivity and delayed development, which should be considered when treating these children in the dental clinic.
Asthma
Asthma is one of the CHCs with an increasing prevalence in the Western world. It is a serious global health problem affecting more than 100 million people, and in most countries the prevalence has increased during the past two decades. In Sweden, 5–7% of the population has asthma. Chronic inflammatory mechanisms are important in the development of obstructive symptoms in asthma. Mild symptoms are treated with β2‐agonists and, when symptoms occur more frequently, treatment with inhalation steroids is instituted.
Implications for oral health
Children with asthma have an increased incidence of dental caries, particularly in the permanent dentition, gingivitis, calculus, and dental erosion as well as an altered salivary composition and flow rate [7]. Although there are conflicting results in the literature regarding the effect on caries, most investigators suggest that children are at higher risk due to their pharmacotherapy as well as the duration of the disease. Contributing factors are the medication these children are taking in the form of inhalers and liquid medicine. A large proportion of inhaled drugs, up to 80%, is retained in the oral cavity. Since inhalation powder often contains sugars such as lactose, this may contribute to the increased caries prevalence. It is recommended that children rinse their mouths with water after each steroid inhalation. An increased consumption of sugar‐containing beverages has also been reported.
The use of β2‐agonists is associated with a decreased salivary secretion rate. The mechanism involved is a downregulation of β2‐receptors in the salivary glands, leading to a decreased secretory signal. An increased incidence of gingivitis is also found in asthmatic children. This has been explained by an altered immune response and their tendencies to mouth breathe, particularly during an episode of rhinitis or acute asthmatic attack. Increased levels of calcium and phosphorus in parotid saliva may be associated with higher levels of calculus in asthmatic children. An increased level of erosive tooth loss is also found. A low salivary secretion rate, frequent consumption of acidic drinks, and an increased incidence of gastroesophageal reflux are reportedly associated factors. The dental treatment itself may cause a stress reaction that may precipitate an attack. Children are advised to bring their medication list to the dental office. Nitrous oxide–oxygen sedation should be used in preference to intravenous sedation if sedation is required. Due to increased risk of caries a regular preventive program should be instituted and the salivary secretion rate should be monitored.
Aspirin and other nonsteroidal anti‐inflammatory drugs (NSAIDs) trigger exacerbations in susceptible people with asthma, a condition referred to as NSAID‐exacerbated respiratory disease (NERD). In people with NERD, aspirin inhibits the constitutively active enzyme cyclo‐oxygenase‐1, increasing the levels of arachidonic acid. This in turn increases the levels of proinflammatory cysteinyl leukotrienes and reduces the levels of anti‐inflammatory prostaglandins tipping the balance towards bronchoconstriction in asthma [8].
Cardiovascular disorders
Almost all heart disease in children is congenital in origin, with a birth prevalence of 8 per 1000 live births. The eight most common structural congenital heart diseases (CHDs) account for over 80% of the total; they are ventricular septal defects, patent ductus arteriosus, atrial septal defects, tetralogy of Fallot, pulmonary stenosis, coarctation of the aorta, aortic stenosis, and transposition of the great arteries.
Larger defects are closed surgically in the first years of life, and some defects may require complex surgery and eventually transplantation. Acquired heart diseases such as myocarditis and infective endocarditis (IE) are still a cause of death and disability in children. The incidence of rheumatic fever has fallen sharply. Regarding IE, it is well accepted that there is a real bacteremia association and that oral streptococci cause 50% of all IE. Dental procedures can easily cause bacteremia. It is assumed that after extraction the prevalence is 96.2% after 30 sec, 20% after one hour. Also toothbrushing (40%) and periodontal probing (40%) are possible triggers.
Implications for oral health
Invasive dental procedures such as extractions, scaling, oral surgery, and endodontic treatment are likely to induce a transient bacteremia. Although it has been shown that all procedures involving the gingival tissues, even toothbrushing, induce a bacteremia [9], no evidence was presented in a recent Cochrane review that antibiotic prophylaxis (AP) is either effective or ineffective in preventing IE in people at risk who are about to undergo an invasive dental procedure. It is not clear whether the potential harms and costs of antibiotic administration outweigh any beneficial effect. Ethically, practitioners need to discuss the potential benefits and harms of antibiotic prophylaxis with their patients before a decision is made about administration [10]. Box 23.7 lists conditions where AP is not necessary, if the patient has no additional risk factors, who undergo any dental procedure that involves the gingival tissues and the periapical region of a tooth and for those procedures that perforate the oral mucosa. Consequently invasive dental procedures need AP whereas procedures such as routine anesthetic injections through noninfected tissue, placement of removable prosthetic or orthodontic appliances, shedding of primary teeth and bleeding from trauma to the lips or oral mucosa do not need AP. In a limited patient population, prophylactic antimicrobial therapy should be directed against viridans group streptococci (Box 23.8) [11].
In children with complex heart disease, problems with nutrition and vomiting are common during their first years of life. Frequent meals are necessary to ensure the caloric intake. In addition, some of the medications contain sucrose together with diuretics that can cause salivary dysfunction. Children with CHD are a risk group for caries development, particularly in the primary dentition. Children on digoxin therapy, administered with a sucrose‐containing syrup, have increased caries prevalence. Children with CHD also have an increased prevalence of disturbances in enamel mineralization. As soon as a child is diagnosed with a significant cardiac problem he or she should be referred for dental evaluation. A preventive program including dietary counseling, fluoride therapy, fissure sealants, and oral hygiene instruction should be implemented.
Chronic renal failure
Chronic renal failure (CRF) is the result of progressive, irreversible damage to the kidney, as a consequence of reduction in glomerular filtration speed. Initially, CRF is treated with a protein‐reduced diet supplemented with calories. Carbohydrates will deliver the necessary energy.
Implications for oral health
Often, intake of softened food can lead to excessive calculus formation. Children suffering from CRF at a young age show growth retardation and a delay in the development of the dentition. An early effect is enamel hypoplasia due to deficiencies in enamel development and mineralization. If the glomerular filtration is very low, 50% of the children show mineralization disturbances of the teeth. Despite the frequent intake of carbohydrates and a reduced secretion of saliva, the caries prevalence in children with CRF does not seem to be higher than in healthy counterparts. This can be explained by an increased concentration of urea in saliva and, consequently, a higher saliva pH. Children with CRF experience significantly less gingivitis than healthy children due to immunosuppressive therapy, which leads to a depressed gingival response to inflammation [12].
The production of the vitamin D metabolite that is essential for calcium absorption is inhibited. Consequences include demineralization of the mandible and maxilla, loss of trabeculation, partial or total loss of the lamina dura, giant cell lesions, and metastatic calcification. Socket sclerosis is commonly seen and the prevalence of pulp calcification is very high in patients on hemodialysis, but less so following kidney transplantation. Calcification of the soft tissues and salivary glands and brown tumors have also been reported. On radiographs of children with CRF one can find localized radiolucencies—comparable to cysts—in the jaws, as well as total or partial loss of lamina dura and/or bone demineralization (osteoporosis).
Renal transplant
In the case of complete loss of kidney function, transplantation is the preferred therapy. After kidney transplantation, azathioprine and cyclosporine are used as immunosuppressive agents.
Implications for oral health
In these patients, who already have lowered host resistance, acute or chronic oral infections or bacteremia resulting from dental procedures may cause serious complications. For this reason it is recommended that any necessary dental treatment should be completed before transplantation. About 30% of the patients develop a dose‐correlated gingival overgrowth after the use of cyclosporine. Replacement of cyclosporine with tacrolimus in organ transplant recipients who develop severe gingival enlargement, together with an extensive plaque control program, provides an effective means with which to control gingival hyperplasia, with a minimal risk of graft dysfunction. Concomitant use of calcium channel blockers (nifedipine) and cyclosporine is a significant risk factor for severe gingival overgrowth.
As in all patients on immunosuppressive medication, fungal infections with Candida albicans may occur which require prompt antifungal treatment. There is some evidence that an extensive amount of secondary dentin formation along the pulp chamber walls and the root canal is present in renal transplant patients. Following successful kidney transplantation, the Streptococcus mutans counts are likely to increase compared to those of healthy children, implicating an increased caries risk.
Diabetes mellitus
Diabetes mellitus is a chronic metabolic disturbance characterized by a decreased production of insulin. In children, this is referred to as juvenile diabetes or type 1 diabetes. The lower insulin activity leads to a higher concentration of blood glucose. Diabetes is rare in infancy, but the incidence rises among school‐age children. The treatment is based on the normalization of the blood glucose concentration. This is achieved using insulin treatment and strict dietary advice adapted to the physical activity of the child. A fat‐reduced and fiber‐rich diet is recommended. Three major meals and two/three in‐between meals (snacks) constitute the recommended feeding pattern. Strict patient compliance is essential for effective treatment.
Implications for oral health
Children with diabetes have a lower salivary secretion rate. Moreover, the concentration of glucose in saliva and in crevicular fluid is increased. On the other hand, the lower frequency of carbohydrate intake helps prevent caries. Studies have shown no significant difference in caries experience and dental care level in children with type 1 diabetes mellitus when compared to healthy controls. Only children with poorly controlled diabetes who are not accepting the prescribed diet and who have a high variability of blood glucose can be considered a high caries risk group. In children with diabetes and poor metabolic control, increased levels of gingivitis have been found. The association between diabetes and periodontal disease has been under discussion for many years, with conflicting conclusions. Patients with non‐insulin‐dependent diabetes mellitus (NIDDM) have a higher prevalence and severity of periodontal disease than people without diabetes. Studies of patients with insulin‐dependent diabetes mellitus (IDDM) have found an increased prevalence and severity of periodontal disease compared to controls. For both IDDM and NIDDM, there does not appear to be any correlation between the prevalence and the severity of periodontal disease and the duration of diabetes. Patients with well‐controlled diabetes, as measured by blood glycated hemoglobin levels, have less severe periodontal disease than do patients with poorly controlled diabetes. The principles of treatment of periodontal disease in diabetic patients are the same as in nondiabetic patients. Plaque remains the etiologic factor. Extra periodontal prophylaxis, especially when children reach puberty, is strongly recommended.
Short dental appointments should be made during the morning when the blood glucose level is stable and to reduce stress. Practitioners must be aware of potential hypoglycemia, which includes hunger, mood changes, weakness, and decreased spontaneity leading to tachycardia, sweating, and incoherence. If this occurs, one should stop the treatment immediately and administer 15 grams of fast‐acting carbohydrate (glucose tablets, sugar, juice). After treatment one should be aware of a potential delayed wound healing.
Gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) is the involuntary passage of gastric contents into the esophagus. It occurs where inappropriate relaxation of the lower esophageal sphincter results in reflux of stomach contents back into the esophagus. Long‐term GERD is associated with failure to thrive, feeding problems, esophagitis, and aspiration pneumonia and esophageal stricture. It is particularly frequent in children with cerebral palsy, asthma, bronchitis, and other respiratory disorders.
Implications for oral health
Gastric acidity ranges in pH from 1 to 3, and regurgitation or vomiting of gastric acid into the mouth has been linked to dental erosion. Erosion of the posterior teeth involving lingual and occlusal surfaces may suggest GERD whereas erosion on the lingual surfaces of the anterior teeth has been noted in patients with eating disorders or when erosion is caused by external sources.
In children with GERD the prevalence of erosion is lower than in adults, probably due to the fact that children with GERD have had the disease for a shorter period of time. Furthermore, young children are not exposed to acidic and carbonated foods and beverages as frequently as older individuals, which are major contributing factors to dental erosion. In recent studies, it has been found that erosion occurred in only 17% of children with moderate to severe GERD disease [13]. Tooth surface loss was diagnosed on primary molar teeth as well as on primary incisors (Figure 23.6).
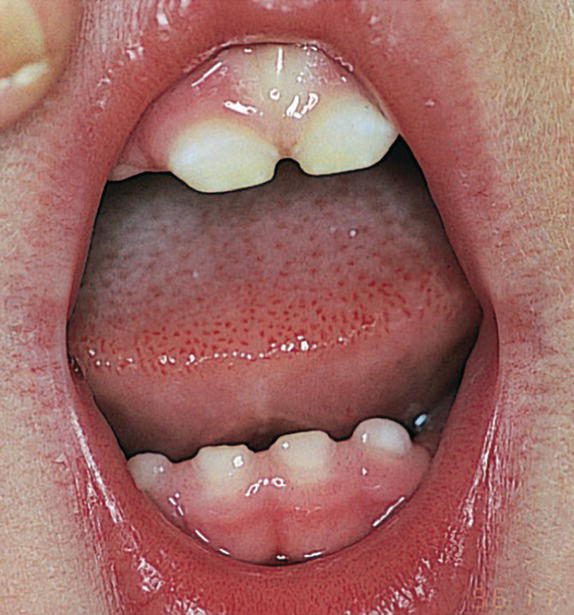
Figure 23.6 A 3‐year‐old boy with a malabsorption syndrome with daily vomiting and regurgitation. Note erosive change on anterior teeth.
Eating disorders
Eating disorders are psychiatric disorders with a common purpose; to maintain control through intake, or lack of intake, of food. The food control is expressed differently but, commonly for all the subtypes of eating disorders are that the adolescents have a feeling of inadequacy and insecurity. The food control is a way to obtain structure in a chaotic mind and coping with emotional pain. Eating disorders occur predominantly in young women with the male‐to‐female ratio being approximately 1:10, and are more prevalent in Western countries. Comorbid conditions such as depression, obsessive‐compulsive disorders, and social phobia are particularly common. The onset of the disorder is approximately 17–20 years of age, but a tendency concerning an earlier debut, as low as 12 years of age, is currently discussed.
Binge eating disorder
Binge eating disorder (BED) is an obsessive and frequent intake of large portions of food, more than twice a week, without compensating actions like vomiting or use of laxative. BED is the most prevalent eating disorder with an estimated prevalence of 4% in the general population. Most people with BED are obese. Obesity is an excess of body fat frequently resulting in a significant impairment of health. About 40–50% of men and 25–40% of women have a body mass index (BMI = weight in kg/height in m2) over 25. Severe obesity (BMI >30) affects up to 10% of the adult population. Childhood obesity is also widespread, affecting as many as 10–15% of children. Obese children who become obese adults are at greater risk of developing heart disease, diabetes, high blood pressure, high cholesterol, and arthritis at a younger age. Weight gain among children is probably due to a combination of factors including family lifestyle, socioeconomic status, and ethnicity, but could also be a result of BED.
Implications for oral health
Dietary habits promoting the development of cardiovascular disease have been documented in studies of children and adolescents with high caries prevalence.
Obese children with high caries prevalence exhibit more risk factors for cardiovascular disease at 15 years of age (Figure 23.7). With regard to periodontal disease, there is increasing evidence the adipose tissue is secreting proinflammatory cytokines such as tumor necrosis factor α and interleukin‐6. An increased prevalence of sites with increased probing depth has been reported with increasing BMI, as well as an increased prevalence of periodontal disease. Among individuals aged 17–21 years, the risk of periodontitis increases by 6% with every kilogram of weight gain [14].
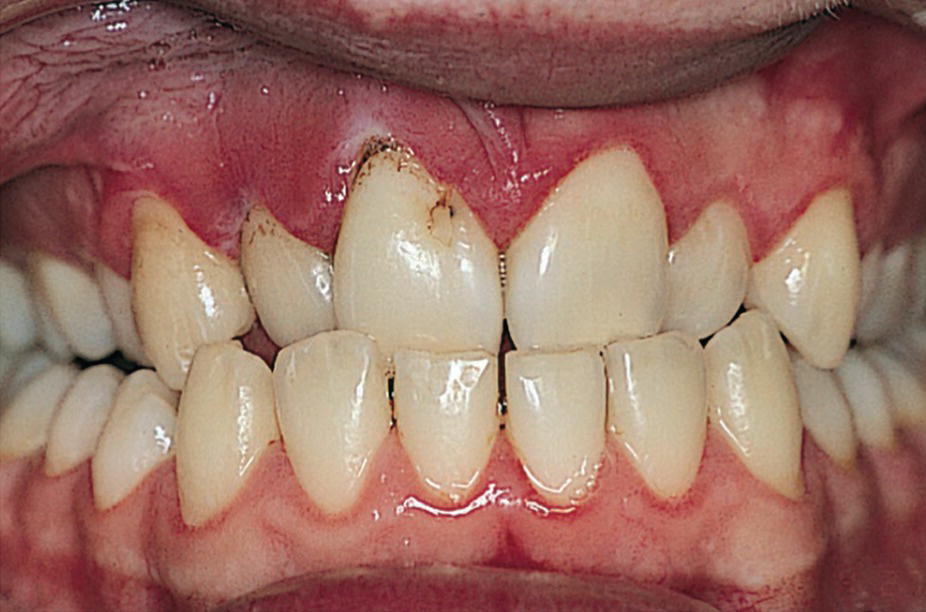
Figure 23.7 A 16‐year‐old boy with severe obesity exhibiting high caries activity and extensive gingivitis.
Dietary counseling to adolescents with a high caries score or periodontal disease in combination with obesity should not be restricted to reduction of fermentable carbohydrates but aim also to reduce risk factors for cardiovascular disease [15].
Bulimia nervosa
Adolescents with bulimia nervosa uses, contrary to BED, compensating means to get relief for their eating disorder. Immediate self‐induced vomiting, intake of laxatives, or excessive exercising are all examples of such compensating means, and should be executed more than twice a week. Adolescents with bulimia are often of normal weight and are therefore capable of hiding their disease for a long time.
Implications for oral health
In general, the adolescents are in risk of malnutrition and low immune response, due of vitamin deficiency. Frequently vomiting will induce acid damage to the teeth, and the dentist is often the first to recognize symptoms of the disorder. The defects will be located at the back of the anterior incisors and the oral surfaces of the lower molars. In case of frequently vomiting the adolescents can develop a very dry mouth due to the dehydration and appear with wounds at the corner of the mouth and the soft palate, due to the impact of the acid [16]. For further instructions, see subsection “Implications for oral health” in the section “Anorexia nervosa.”
Anorexia nervosa
Anorexia nervosa is a serious, potentially life‐threatening eating disorder characterized by self‐starvation and excessive weight loss. The diagnostic criteria for anorexia nervosa are presented in Box 23.9. Two subtypes have been identified: Binge‐eating/purging type (involving binge eating and/or purging behaviors during the last three months), and the restricting type (not involving binge eating or purging). The binge eating and the vomiting are expressed on a minor scale compared with that seen in BED and bulimia. The incidence is 7 per 100,000. The median duration of the illness is up to 6 years, with a high risk of recurrence and with a significant mortality from medical complications and suicide.
Implications for oral health
Erosion is the oral manifestation most often reported in patients with anorexia nervosa, particularly on the palatal surfaces of the maxillary anterior teeth. An increased frequency of erosion is found in subjects with self‐induced vomiting but there is no linear association with the frequency and duration of self‐induced vomiting or the total number of vomiting episodes. Furthermore, the teeth can be mobile, without any clinical signs of periodontal disease. Other factors may be involved, such as oral hygiene practices and the intake of low pH beverages and fresh fruit.
Patients with eating disorders are recommended to reduce the intake of acidic drinks, fresh fruits, especially citrus fruit, and alcohol. They should also rinse their mouths with water or chew gum and, in severe cases, rinse with an antacid preparation in order to increase pH after self‐induced vomiting. Toothbrushing may be performed after self‐induced vomiting, using a gentle brush and bicarbonate‐containing toothpaste. An evaluation of salivary secretion rate should also be performed if the medication list includes medications that cause nausea or dry mouth. As health professionals it is important not to judge the adolescents, but to meet them with understanding and knowledge about the oral consequences.
Substance use disorder
Substance use disorder (SUD) is defined as a use of one or more substances that leads to clinical impairment and distress. Adolescents with SUD display risk‐taking behavior and can exhibit mood swings and challenging behavior. They will not be able to maintain daily life activities, including toothbrushing. The development of SUD can be genetically derived, but social status, male gender, ADHD, and sibling use of tobacco are also predictors.
Implications for oral health
Adults with SUD have more oral diseases than non‐addicted controls [17]). Possible factors for that relation are inadequate diet, dry mouth induced by drugs, tobacco, alcohol, poor oral hygiene, increased acidity from drugs, and sugary drinks among others. If SUD is not detected early and preventive care not carried out, the consequence can be irreversible destruction of the dentition. The choice of treatment will depend on the patient’s state of mind and ability to maintain sufficient oral hygiene. In the extreme an extraction of all teeth will be necessary.
Malabsorption
Malabsorption is the inability to absorb dietary foods. It may be caused by maldigestion (deficiency or inactivation of pancreatic enzymes and bile salts) or by a disease of the small intestine that results in a mucosal barrier to absorption. Patients with the malabsorption syndrome (MAS) may have symptoms of general malabsorption or symptoms caused by the deficiency of a specific substance such as vitamin A, D or K, calcium, magnesium, iron, or folate. The patient with MAS classically has bulky, oily, malodorous stools associated with significant weight loss, muscle wasting, weakness, edema, anemia, ecchymoses, abnormal bleeding, a smooth tongue, hyperkeratosis of the skin, skeletal pain, and even tetanus.
Implications for oral health
The oral manifestations of malabsorption occur when vitamin deficiencies are part of the clinical presentation. These may include reddening and ulceration of the oral mucosa (stomatitis), swelling and burning of the tongue (glossitis), and fissuring of the corner of the mouth (cheilosis), all of which are associated with vitamin B deficiencies. If vitamin B12 deficiency is prominent, atrophy of the tongue may be present.
In patients with MAS or patients receiving long‐term antibiotic therapy, the bacteria in the intestine that produce vitamin K may be affected adversely. The patient’s physician should be consulted regarding the patient’s health and bleeding status before surgery.
Celiac disease
Celiac disease or gluten‐induced enteropathy is a genetic, immunologically mediated disorder of the small intestine caused by intolerance to the gliadin fraction of gluten which is one of the protein fractions of wheat, barley, rye, and oats.
Although celiac disease can occur at any age, it is most obvious in children due to growth retardation as a consequence of malabsorption. Finding linked deficiencies of iron storage (ferritin) and folic acid in a child with aphthous ulceration or other mucosal disease should promptly be investigated for undiagnosed celiac disease [18]. Prevalence data indicate that symptomatic and latent celiac disease is present in one in 300 children. The age of onset ranges from infancy to old age. It is characterized by degenerative changes in the mucosa (villous dystrophy), resulting in a chronic malabsorption with abdominal distension, pale, bulky, foul‐smelling stools, wasting of muscles, anemia and retarded growth, and weight gain. Diagnosis can be confirmed by a biopsy of the jejunum, which shows flattened mucosa with lack of villi, resulting in malabsorption. The villi return to normal following the elimination of gluten from the diet.
Implications for oral health
Hypoplasia of the permanent dentition may occur in those teeth forming at the time solids were first introduced into the diet. Symmetrically and chronologically distributed enamel defects have been reported to be associated with celiac disease. The severity of the hypoplasia probably depends on the severity of the celiac disease and the time that elapsed between the onset of symptoms and the initiation of treatment. Further aphthous ulcers, angular cheilitis or recurrent herpetic lesions are links to the condition. When treating a child with celiac disease, always check that any medication prescribed is gluten free.
Cystic fibrosis
Cystic fibrosis (CF) is an autosomal recessive disorder of the exocrine glands and occurs in approximately one in 2000 births. All the mucus‐producing glands produce thick mucus that causes obstruction of the flow secretions from the ducts; the pancreas as well as the lungs are involved. The lungs are invariably involved and there is a nonproductive cough that leads to acute respiratory infection, bronchopneumonia, bronchiectasis, and lung abscesses.
Implications for oral health
Patients with cystic fibrosis may show occlusion of the nasal cavity and maxillary sinus after recurrent infections. This results in chronic mouth breathing and a higher incidence of open bite and a high palatal vault.
The teeth of patients with cystic fibrosis are often discolored. The discoloration is more pronounced at the cervical and middle third of the clinical crown and is first seen at the cement–enamel junction, where the enamel layer is the thinnest. Tetracycline administration has been implicated as a cause of the staining, since the drug has often been used to combat recurrent pulmonary infections. In addition to a relatively high prevalence of tetracycline discoloration, enamel defects have been found in approximately 10% of these patients (Figure 23.8). The prevalence of dental caries is not significantly elevated. There are indications, however, that adolescents and adults with cystic fibrosis can show high caries rates. In recent studies, it was shown that although cystic fibrosis homozygotes potentially have a high caries risk due to their essential sugar‐rich diet, they do not have a significantly higher caries experience compared to their heterozygote counterparts or healthy controls [19]. The current paradigm that young children with CF may be at lower risk for caries while adolescents with CF are not at lower risk compared to controls without CF was confirmed in a recent qualitative systematic review [19]. As patients with cystic fibrosis nowadays survive into adulthood, assessment of caries risk is important.
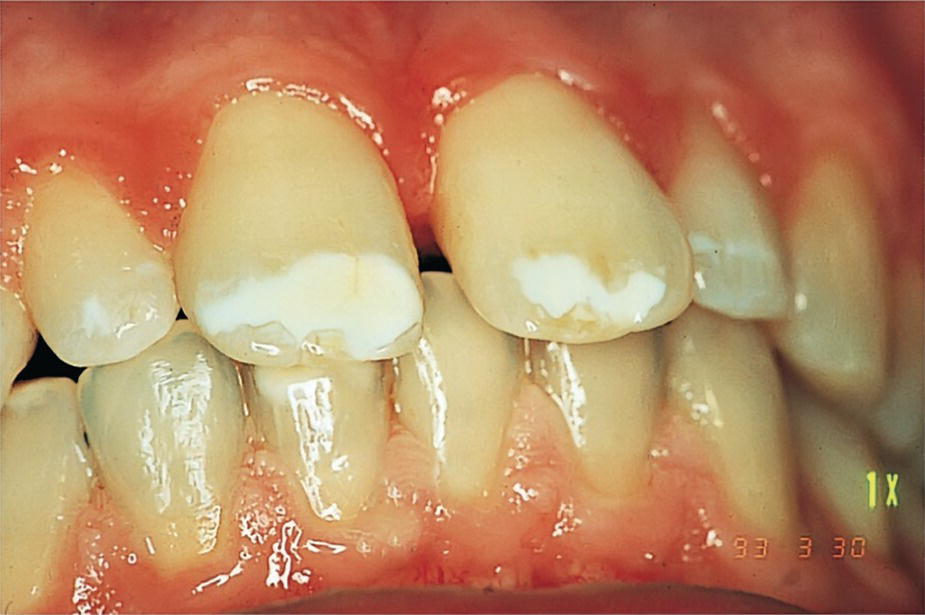
Figure 23.8 Discoloration and enamel hypomineralization in a 14‐year‐old boy with cystic fibrosis.
The dentist must avoid exacerbating the underlying disease in patients with cystic fibrosis. High flow of gases such as oxygen or nitrous oxide can dry out respiratory secretions. This can lead to further plugging and infection. Rubber dam should be used to decrease the possibility of aspiration from dental handpiece aerosols.
Primary and secondary immunodeficiency states
Immunodeficiency states may be primary or secondary (Box 23.10). Primary immunodeficiency syndromes vary in incidence from the relatively common, such as selective immunoglobulin A (IgA) deficiency with a prevalence of about one in 600 of the population, to the extremely rare. Four main components of the immune system may be affected: (a) phagocytosis (both granulocytes and macrophages), (b) immunoglobulins, (c) cellular immunity, and (d) the complement system.
Implications for oral health
Candidiasis and herpetic infections are common in children with T‐cell deficiencies, while children with B‐cell deficiencies are most susceptible to bacterial infections (Figure 23.9). Periodontitis and candidiasis are found in some but not all phagocyte deficiencies [20].
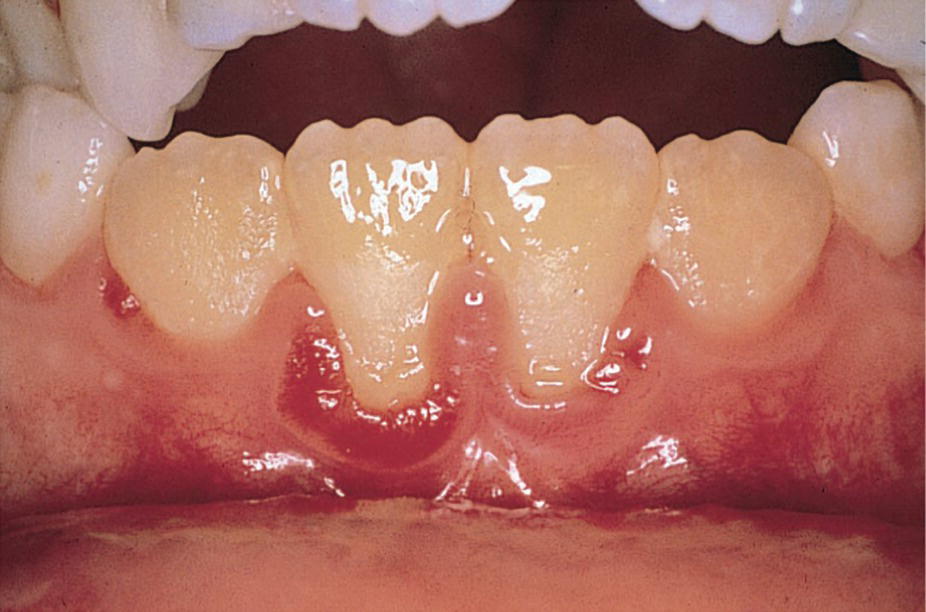
Figure 23.9 A 12‐year‐old boy with immunoglobulin A and G deficiency exhibiting ulceration of the marginal gingiva.
HIV infection/AIDS
Globally, acquired immunodeficiency syndrome (AIDS) is one of the five leading causes of death among children. Antiretroviral drugs reduce viral replication and can reduce mother‐to‐child transmission of human immunodeficiency virus (HIV). In Western countries the vertical transmission rates are around 1–2%, but highly active antiretroviral therapy (HAART) is not yet available in low and middle‐income countries. The use of HAART has reduced the mortality and increased the life expectancy in children with HIV infection.
Implications for oral health
The median age of children at AIDS diagnosis is 12 months, and the oral manifestations are the first sign of infection in about half of all infected children. Even when children with HIV are treated with HAART, oral lesions may still be present. The prevalence depends on type of treatment but about half of children exhibit oral lesions. Antiretroviral therapy has also been associated with oral lesions such as ulcers and xerostomia [21].
Overall, oral candidiasis is the most common oral lesion and often the first clinical manifestation in children with HIV. The incidence of pseudomembranous candidiasis has been reported to be about 10% in a pediatric population. Other common oral lesions are herpes simplex virus infection, linear gingival erythema, parotid enlargement, and recurrent aphthous ulcers. Periodontal disease is a late manifestation of HIV infection. Periodontal disease is the most frequently diagnosed oral lesion in African HIV‐positive children (Figure 23.5). A preventive program for HIV‐infected children should include a careful oral examination at regular intervals to ensure early detection of and intervention for pseudomembranous candidiasis and other oral lesions.
Neutropenia
Blood leukocyte counts normally range between 5000 and 10,000 cells/mm3 with a predominance of neutrophils. Although a reduction in circulating neutrophils usually leads to a decline in the total leukocyte count, such values are not meaningful unless a differential count is performed and the absolute numbers of each cell type are calculated. In healthy individuals, the total neutrophil counts range between 1800 and 7200 cells/mm3. Thus, neutropenia is defined as a decrease in the neutrophil count below 1800 cells/mm3. The disease may occur as an isolated finding or may be associated with a variety of underlying disorders, which may cause anemia and thrombocytopenia.
Implications for oral health
Oral lesions are common and often severe in patients with neutrophil dysfunction syndromes. Three findings repeatedly reported are severe gingivitis, rapidly advancing periodontal disease, and oral ulcers. Since periodontal disease is caused by a chronic bacterial infection, it is not surprising that an increased level of periodontal breakdown accompanies disorders of neutrophils (Figure 23.10). In Kostman’s disease, chronic severe neutropenia, the bacterial infections are treated with granulocyte‐macrophage colony stimulating factor with good results, but this therapy has no effect on periodontal disease progression. It is speculated that deficiencies in the innate immunity contribute to the susceptibility to periodontal disease [22].
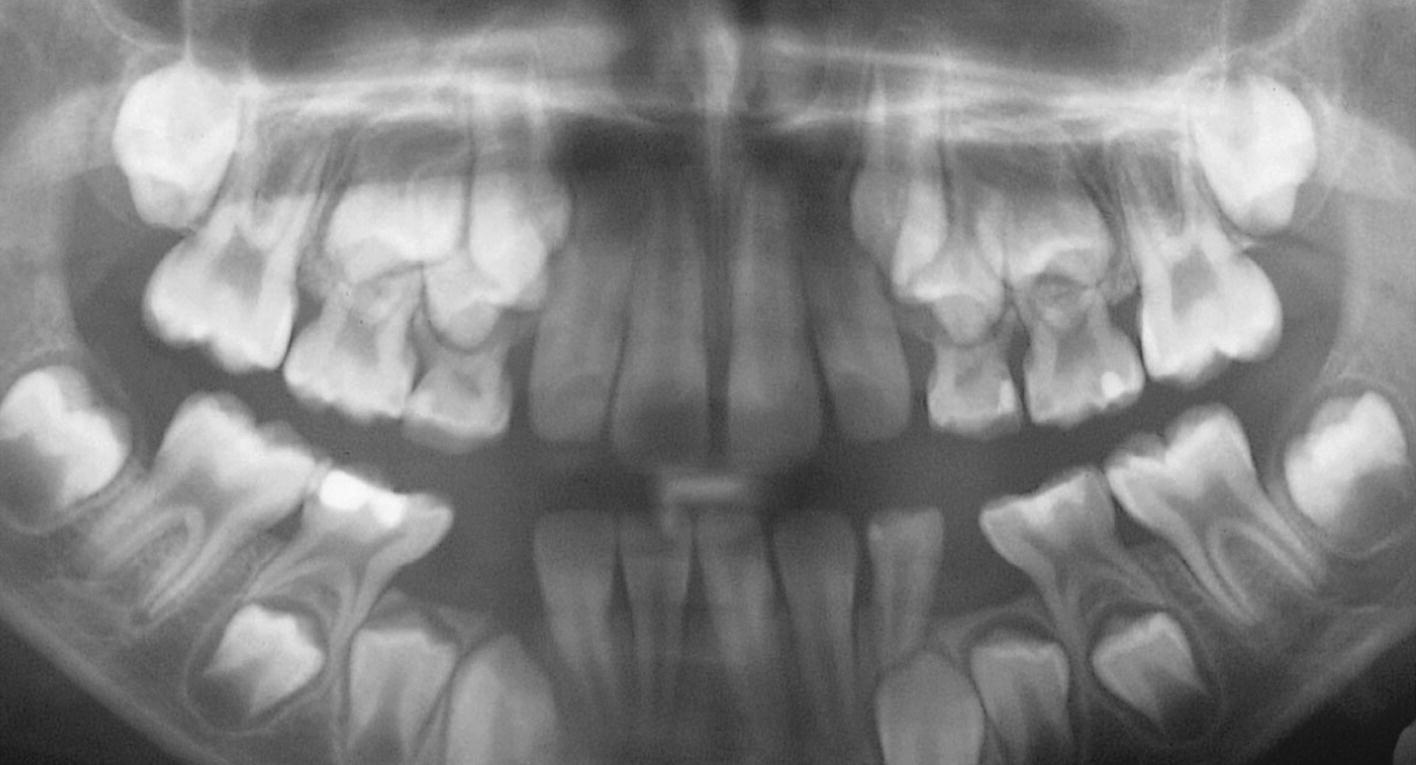
Figure 23.10 Panoramic radiograph showing severe periodontal breakdown in a 6‐year‐old boy with cyclic neutropenia.
Patients with abnormal neutrophil function should be under the close supervision of a dentist to minimize local inflammatory factors causing periodontal disease, dental caries, and oral ulcers. Patients should follow a strict oral hygiene regimen and have periodic dental examinations. When extensive dental treatment is performed in susceptible patients, broad‐spectrum antibiotic coverage should be considered. If surgical procedures are necessary or oral bacterial infection develops, granulocyte transfusions may be necessary.
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis (JIA) is a chronic autoimmune disease defined by arthritis (inflammation of the synovial membrane) of one or more joints in the body, with onset before the age of 16, and persisting for more than 6 weeks, and where other conditions can be excluded. The joints are swollen, warm, and tender on palpation, painful to move and stiff. The incidence of JIA is approximately 15 per 100,000 children/year. JIA is an umbrella diagnosis which includes seven subtypes described in relation to the ILAR criteria. The etiology is basically unknown but genetic predispositions are considered.
Implications for oral health
Estimates of proportion of patients in which the temporomandibular joint (TMJ) is affected varies between 30 and 87%, depending on diagnostic criteria and methodology. The most common radiographic finding is a deformity of the condylar head, the severity of which is related to disease onset, disease duration, and subtype. Deformities can also be seen at the tuberculum of the joint. TMJ arthritis is without radiographic signs in the initial stages, and can only be confirmed by magnetic resonance imaging with contrast. Symptoms can be pain and restricted jaw movement, but a majority of the patients, especially younger patients, will have no or only vague symptoms. TMJ arthritis and the condylar deformity may lead to dentofacial growth disturbances, i.e., restricted growth of the mandible with posterior rotation (Figure 23.4). This is followed by malocclusions, typically frontal open bite and an increased overjet. When the affection is unilateral or has started earlier on one side in bilateral cases, the patient will develop a mandibular asymmetry. This underlines the importance, as part of a routine clinical observation, to monitor craniofacial growth and diagnose asymmetries (see also Chapter 7). The treatment of the developing dentofacial deformity depends on the age and developmental stage of the patient and the extent of the anomaly. Often, a combination of pharmacologic interventions (local or systemic) and orthopedic/orthodontic treatment will be indicated. Physiotherapy can be considered for improvement of oral function. A minor group of patients will develop a need for orthognathic surgery. Effective oral hygiene measures are important, especially in children who are in orthopedic treatment and with orthodontic appliances. Furthermore, attention should be given to children in long‐term corticosteroid treatment, due to the delayed wound healing and increased risk of infections. If the involvement of the TMJ results in restricted opening of the mouth, this can complicate dental treatment.
Juvenile osteoporosis
Juvenile osteoporosis is a rare disease. The disease is characterized by too little bone formation, excessive bone loss, or a combination of both. Sometimes the cause of the disease is unknown, juvenile idiopathic osteoporosis (JIO), but often it is due to an underlying medical condition (secondary osteoporosis). The onset of JIO is prepubertal and is characterized by recurrent bone fractures, bone pain, and kyphosis. Unlike osteogenesis imperfecta, most children with JIO will recover from the disease.
Many diseases can cause secondary osteoporosis. It can either be directly induced by another primary disease such as juvenile rheumatoid arthritis, diabetes, malabsorption syndromes, or anorexia nervosa etc., or indirectly by medications including anticonvulsants and corticosteroids. Behaviors such as physical inactivity, smoking, and inadequate nutrition are also examples of factors associated with secondary osteoporosis.
Implications for oral health
No studies have currently studied the implications of JIO for oral health in children, but altered bone metabolism and treatment with bisphosphonate are both risk factors when undertaking invasive dental treatment and orthodontic treatment, and should be taken into account.
Epidermolysis bullosa
Epidermolysis bullosa comprises a number of genetically determined skin conditions in which the skin and mucous membrane blister as a result of mechanical trauma.
Basement membrane and cellular defects characterize the condition as well as skin fragility and variable extra‐cutaneous involvement. Clinically, two main types were originally described: the simplex type, in which mucous membranes are rarely affected and scarring does not occur, and the dystrophic type, in which scarring occurs (Figure 23.11a). Blistering occurs within the epidermis, within the basement membrane or beneath the basement membrane, respectively, in epidermolysis bullosa simplex, epidermolysis bullosa dystrophica, and junctional epidermolysis bullosa.
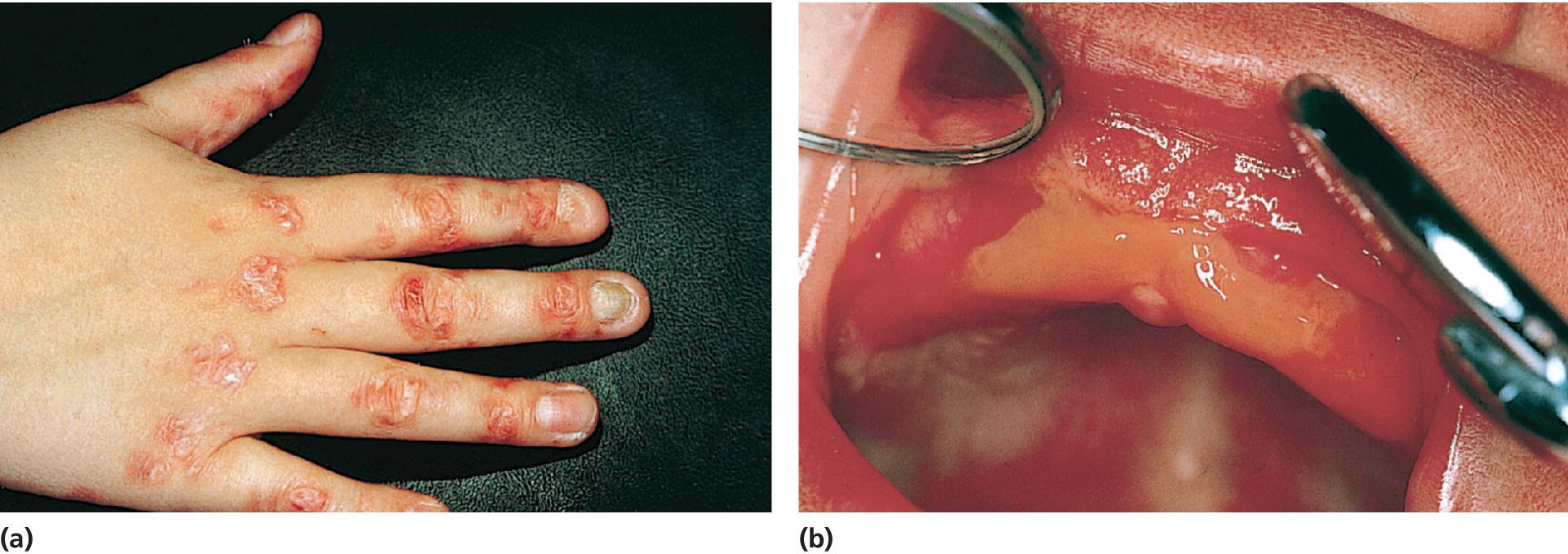
Figure 23.11 (a) Blistering of hand and fingers in a 10‐year old boy with dystrophic epidermolysis bullosa. (b) Oral blistering and scarring in a 1‐month‐old boy with dystrophic epidermolysis bullosa.
Source: Göran Koch, Jönköping, Sweden. Reproduced with permission of G. Koch.
Implications for oral health
The simplex and dominant dystrophic epidermolysis bullosa groups have DMFS levels similar to those in healthy children. Individuals with recessive dystrophic epidermolysis bullosa have the most severe oral blistering and scarring (Figure 23.11b) and do not have generalized enamel hypoplasia. In contrast, junctional epidermolysis bullosa is always associated with generalized enamel hypoplasia, but the intraoral blistering rarely involves scarring. Rampant caries afflicts many individuals with junctional or generalized recessive dystrophic epidermolysis bullosa. Although systemic treatment remains primarily palliative, it is possible to prevent destruction and subsequent loss of the dentition through appropriate interventions and dental therapy. Even the most severely affected individuals with epidermolysis bullosa can retain their dentition through the use of modern dental restorative techniques delivered using general anesthesia. Preventive advice regarding a noncariogenic diet is essential. Advice should be given on the use of a soft toothbrush to maintain oral hygiene and chlorhexidine mouthwashes to prevent the accumulation of plaque. Cleaning with sponges or cotton buds may also be beneficial.
Bleeding disorders
The hemophilias are inherited bleeding disorders caused by low concentrations of specific coagulation factors. The most well‐known are deficiencies of factor VIII, hemophilia A and factor IX, hemophilia “B”, both of which show X‐linked inheritance. Factor XI deficiency (originally called hemophilia C) is a less common and in most cases mild bleeding disorder. The commonest inherited bleeding disorder is von Willebrand’s disease, a defect in the quantity or quality of the von Willebrand factor, present in as many as 1% of the general population. Patients should carry a hemophiliac card, which records details such as factor deficiency and level, the presence or absence of inhibitors, and the telephone number of their hemophiliac center.
Von Willebrand’s disease
Von Willebrand’s disease affects both males and females and is inherited as a dominant trait. Those affected have large tortuous capillaries and low levels of factor VIII and the condition may simulate hemophilia. These patients also have defective platelets, which do not adhere to each other. Bleeding from the gingiva, the mucosa of the nose, and the gastrointestinal tract is common and there is prolonged bleeding following trauma or surgery.
Implications for oral health
Oral health and oral health‐related quality of life is not different in children and adolescents with bleeding disorders compared to controls when dental health protocols are integrated in the comprehensive management of patients with bleeding disorders [23]. About half of the patients have experienced bleeding episodes related to shedding of primary teeth or intraoral trauma such as biting the tongue. In patients with bleeding disorders prevention is the most important aspect both with regard to dental caries and periodontal disease. Each patient should be given an individualized program including oral health education, toothbrushing instruction, fluoride supplements, and topical fluoride applications.
With regard to management, pulp treatment of primary molars is preferred in order to avoid extractions. Dental treatment should be performed at a specialist dental service in contact with the hemophilia treatment center. There must be adequate replacement therapy and careful monitoring of factor VIII levels. Routine therapy with the antifibrinolytic agents, tranexamic acid, or epsilon aminocaproic acid prior to and for a few days following the procedure will significantly reduce the requirement for replacement factor VIII. Local hemostatic measures are required following dental extractions using materials such as oxycellulose or gelatine. Absorbable suture material is preferred to avoid suture removal. For pain control acetaminophen is a safe alternative to prevent postoperative pain. Nonsteroidal anti‐inflammatory drugs and aspirin which affect platelet function must be avoided [24].
Primary teeth when shed in the normal way usually cause little or no hemorrhage. However, if very mobile, extraction may be necessary because constant movement may cause trauma of the soft tissues and bleeding. With regard to local anesthesia, inferior dental nerve blocks should be avoided, since bleeding in the pterygomandibular region may result in asphyxia. Here computerized local anesthesia systems, which allow intra‐ligamental or intra‐osseous injections, are preferred.
Idiopathic thrombocytopenic purpura
Idiopathic thrombocytopenic purpura (ICP) is a relatively common disorder in which isolated thrombocytopenia occurs in otherwise healthy individuals. Two clinical forms of the disease are recognized: acute and chronic. Acute ICP is seen most frequently in children but may occur at any age. The onset is usually sudden, with thrombocytopenia manifested by bruising, bleeding, and petechiae a few days to several weeks after an otherwise uneventful viral illness. Acute ICP is a self‐limiting disease that generally remits permanently without sequelae.
Implications for oral health
Oral manifestations of ICP may represent its initial signs. Purpura, the most common oral sign, is defined as any escape of blood into subcutaneous tissues and includes petechiae, ecchymoses, hemorrhagic vesicles, and hematomas. These may appear on any mucosal surface and are often first seen on the tongue, lips, and occlusal line of the buccal mucosa. They are often secondary to some minor trauma. Other oral signs include spontaneous gingival hemorrhage and prolonged bleeding following trauma, toothbrushing, extractions, or periodontal therapy.
Spontaneous gingival bleeding can usually be managed with oxidizing mouthwashes, but platelet transfusions may be required to stop the bleeding. Good oral hygiene and conservative periodontal therapy help to remove the plaque and the calculus that potentiate the bleeding.
Malignant disease in children
The incidence of cancer during childhood is very low. The incidence rate in the Nordic countries is increasing slowly and is now 17.3 in 100,000 children per year. Acute leukemia, lymphoma, and central nervous system (CNS) tumors represent the three largest diagnostic groups. Unlike the majority of adult cancers, most pediatric tumors are curable. In 1999, the 5‐year survival rate was over 80% among children diagnosed with acute lymphoblastic leukemia. For each diagnosis, a treatment protocol is established according to international guidelines. The treatment is separated into an induction period of 4–5 weeks aiming at achieving remission, followed by a consolidation period, and finally a maintenance period of up to 30 months. The treatment for most hematological malignancies consists of a protocol combining cytotoxic drugs with different modes of action. In children with CNS tumors the therapy comprises radiation therapy, surgery, and cytotoxic drugs. In patients with high‐risk disease and relapse, stem cell transplantation (SCT) may be considered.
Implications for oral health
Oral complications following antineoplastic therapy can be divided into two groups: the acute complications that occur as a direct consequence of the cytotoxic or radiation therapy given and the long‐term complications such as disturbances in dental development, craniofacial growth, and salivary dysfunction [25].
Cytotoxic drugs have effects not only on malignant cells but also on normal cells with rapid turnover such as the hematopoietic cells in the bone marrow and epithelial cells in the gastrointestinal tract and the oral mucosa. The direct effects of cytotoxic therapy are mucositis involving atrophy, desquamation, and ulceration of the mucosa (Figure 23.12), which increase the risk for local and systemic infections, cause pain and discomfort for the patient, and impair oral nutrition. Septicemia with oral streptococci is common during the induction period.
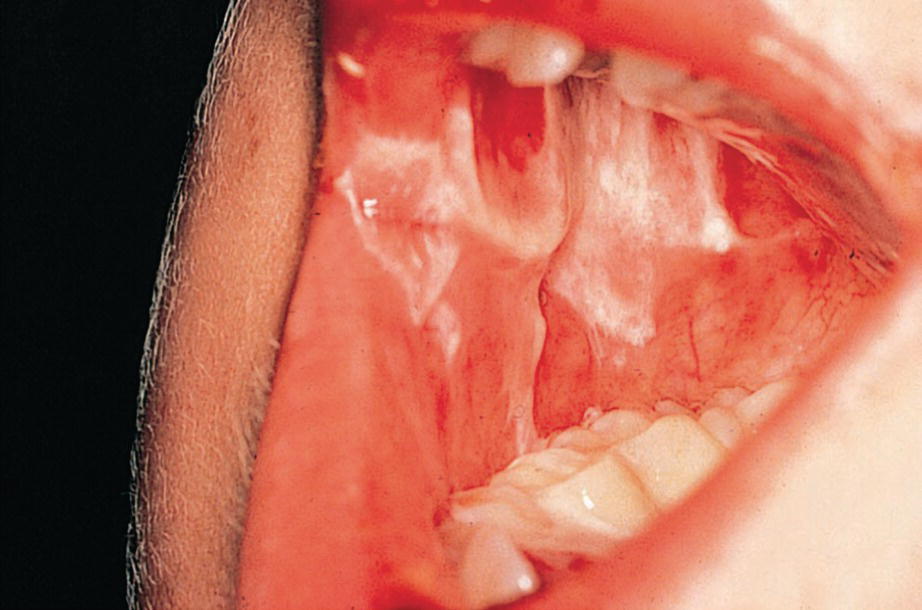
Figure 23.12 Severe oral ulceration in a 5‐year‐old boy during induction chemotherapy for acute lymphoblastic leukemia.
During cytotoxic therapy a transient reduction of salivary secretion is diagnosed, which then attains normal values after completion of therapy. Children treated with cytotoxic drugs only experience a catch‐up growth with no disturbances in dental and craniofacial development.
In stem cell transplantation a combination of cytotoxic drugs is used. When aiming for total myelodepletion, cytotoxic drugs can be combined with 10–12 Gy total body irradiation (TBI). In these children a permanent reduction of salivary secretion rate below 0.5 ml/min is found in approximately 50% of the patients when examined 4 years after BMT. Impaired root development is seen in all children treated with TBI. Other disturbances diagnosed are enamel hypomineralization and hypoplasia, microdontia, and aplasia (Figure 23.13).
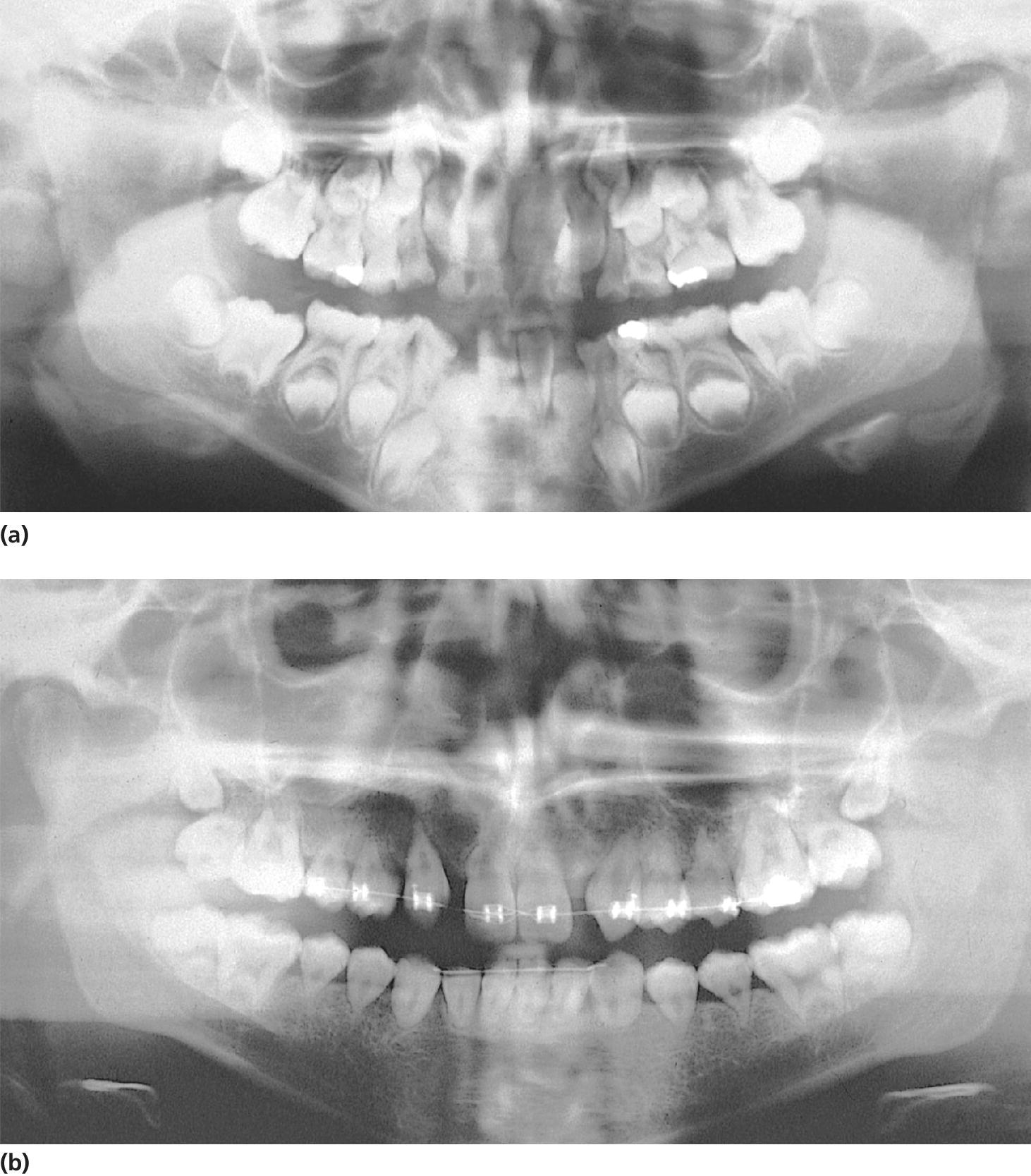
Figure 23.13 (a) Panoramic radiograph showing premature apical closure of permanent first molars in an 8‐year‐old boy treated with 10 Gy total body irradiation at 5 years of age. (b) At 17 years of age, all permanent teeth exhibit short V‐shaped roots.
During antineoplastic therapy, also for extended periods after completion of therapy, children are diagnosed with high counts of mutans streptococci and lactobacilli. There is also a rapid recolonization of oral streptococci after chlorhexidine therapy. Children subjected to oral preventive programs during antineoplastic therapy do not exhibit an increased caries incidence. The preventive program for children can be divided into three phases:
- Before the start of cytotoxic therapy the aim is to treat all existing oral diseases and to educate children and parents on oral complications and how to perform oral hygiene.
- During active cytotoxic therapy the patients are instructed to use a soft toothbrush and toothpaste that does not contain sodium lauryl sulfate. During periods of increased bleeding and infection risk, mouthrinses with chlorhexidine 0.1% are recommended.
- During the long‐term follow‐up, special attention should be paid to patients receiving radiation therapy. Evaluation of salivary secretion rate and levels of oral microorganisms is important. Caries development is extremely rapid in children with poor compliance to preventive measurements. Attention should also be directed towards evaluation of disturbances in dental and craniofacial development.
Epilepsy
Epilepsy is a chronic neurologic disorder, with an abnormal excessive electric activity of the neurotransmitters in the brain and a long‐term risk of recurrent seizures. The major neurotransmitters in the brain are glutamate and gamma‐amino‐butyric acid (GABA), where glutamate has an excitatory effect and GABA has an inhibitory effect on the activity. About 5 children in 1000 have epilepsy. Most of these will have idiopathic epilepsy (i.e., no underlying cause will be evident), but some will have structural/metabolic epilepsy due to a cause such as head injury, meningitis, or birth asphyxia. Third, the epilepsy can have a genetic origin. The international classification of epileptic seizures divides the epilepsies into those that are generalized, where the whole brain is involved, and the partial seizures, where the aberrant activity involves only a part of the brain. The distribution of the activity is important for the severity of the seizures, ranging from few seconds of absence, to several minutes of tonic‐clonic convulsions. Prognosis in the childhood epilepsies is very variable. Most of the refractory epilepsies are found in children who have multiple disabilities.
Seizures are controlled with anti‐epileptic drugs (AED). Many side effects are recorded for AEDs, e.g. xerostomia, and possible medical interactions should always be examined before administering drugs to children in AED treatment. For example, paracetamol interacts with lamotrigine and tegretol, and midazolam has an altered effect in case of concurrent intake of carbamazepine.
Implications for oral health
Children who are given phenytoin medication exhibit gingival overgrowth to a varying degree (see Figure 23.2a). Also sodium valproate, phenobarbital, and carbamazepine lead to symptoms, but less pronounced. The reaction begins as a diffuse swelling of the interdental papillae, which enlarge and coalesce. Clinically significant overgrowth occurs in approximately 50% of patients [4]. The incidence and severity of overgrowth is greatest on the labial surfaces on maxillary and mandibular anterior teeth. The development of phenytoin‐induced gingival overgrowth requires gingival inflammation, and can therefore be minimized by plaque control. A program including frequent sessions of professional tooth cleaning should be instituted before the start of medication. If it is not possible to maintain optimal oral hygiene, a rather conservative approach to surgical removal of gingival overgrowth is recommended because of the high risk of recurrence. Six months after withdrawal of phenytoin medication, the gingival tissues return to a normal state.
Children with seizures have an increased risk of dental trauma, but also an increased risk of caries and poor oral hygiene has been suggested [26]. In case of trauma of the incisors, the treatment planning should always consider the risk of a new trauma. An etch bridge will always be the first choice of treatment, if possible, in order to prevent further damage to the neighboring teeth in case of a new trauma.
Oral health related quality of life
The realization that the biomedical perspective has to be complemented with a broader biopsychosocial model of health has resulted in an increased interest in patient reported outcome measures (PROM). Since many conditions in dentistry have biological, psychological and social consequences, the use of PROM is particularly appropriate. Oral health‐related quality of life (OHRQoL) is defined as “the impact of oral diseases and disorders on aspects of everyday life that a patient or person values, that are of sufficient magnitude, in terms of frequency, severity or duration to affect their experience and perception of their life overall” [27]. The most frequently used measures for self‐completion by children is the Child Perception Questionnaire (CPQ), the C‐OIDP and COHIP [28]. The CPQ measures OHRQoL on four different dimensions: oral symptoms, functional limitations, emotional well‐being, and social wellbeing (CPQ). In children treated for molar–incisor hypomineralization or extensive caries under general anesthesia, an immediate improvement of all dimensions of the CPQ was reported. Children with chronic health conditions are at risk to have their OHRQoL affected by their underlying disease or treatment. OHRQoL was studied in a group of long‐term survivors after childhood cancer [28]. The children had been exposed to treatment with chemotherapy or radiation at an early age and invasive treatments including multiple injections. Contrary to expected, the OHRQoL was not significantly lower compared to controls. In part this can be attributed to a comprehensive oral care program during treatment for childhood cancer.
Oral health problems can generate considerable negative effects on the quality of life of individuals living with HIV. In children with more severe AIDS symptoms OHRQoL was negatively affected in all four domains of the CPQ. For the responsible care provider it is important to identify possible measures to improve the OHRQoL in children with chronic health conditions [29].
Transition to adult care
Successful transition from pediatric to adult‐oriented care has the greatest impact on continuity of care and improved long‐term health outcomes for children with chronic health conditions. Discontinuity of health care, in particular oral health care, is a common outcome following transition from pediatric to adult‐oriented services. Population‐based surveys have suggested inadequate preparation, delayed referral, economic challenges, communication difficulties, and lack of adequately trained adult service providers as the primary barriers to successful transition.
First, awareness and understanding of the eventual need to transition to adult services and also early introduction to the concept of transition has been correlated with successful transition. When transferring patients to adult care, both in general dentistry, special needs clinic or other specialists, it is advised to supplement a written referral including patient history and summary with a verbal contact with the receiving dentist [30].
References
- 1. Stein REK, Bauman LJ, Westbrook LE, Coupey SM, Ireys HT. Framework for identifying children who have chronic conditions: the case for a new definition. J Pediatr 1993;122:342–7.
- 2. Stein RE, Silver EJ. Operationalizing a conceptually based non‐categorical definition: first look at US children with chronic conditions. Arch Pediatr Adolesc Med 1999;153:68–74.
- 3. Houtrow AJ, Larson K, Olson LM, Newacheck PW, Halfon N. Changing trends of childhood disability, 2001–2011. Pediatrics 2014;134:530–8.
- 4. Westbom L, Kornfält R. Chronic illness among children in a total population. Scand J Soc Med 1987;15:87–97.
- 5. Klingberg G, Dahllöf G. Behandling af børn med langvarig sygdom og funktionsnedsættelse i tandplejen. Tandlægebladet 2014;118:878–83.
- 6. Jacobsen PE, Haubek D, Henriksen TB, Ostergaard J, Poulsen S. Evidence for an association between prematurity and enamel defects in permanent teeth is still relatively sparse. Eur J Oral Sci 2014;122(5):361.
- 7. Alavaikko S, Jaakkola MS, Tjäderhane L, Jaakkola JJ. Asthma and caries: a systematic review and meta‐analysis. Am J Epidemiol 2011;174:631–41.
- 8. Morales DR, Guthrie B, Lipworth BJ, Jackson C, Donnan PT, Santiago VH. NSAID‐exacerbated respiratory disease: a meta‐analysis evaluating prevalence, mean provocative dose of aspirin and increased asthma morbidity. Allergy 2015 Apr 8. doi: 10.1111/all.12629.
- 9. Roberts GJ. Dentists are innocent! Everyday bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatr Cardiol 1999;20:317–25.
- 10. Glenny AM, Oliver R, Roberts GJ, Hooper L, Worthington HV. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst Rev 2013;10:CD003813.
- 11. Wilson W, Taubert K, Gewitz M et al., Prevention of infective endocarditis: guidelines from the American Heart Association. J Am Dent Assoc 2007:739–45, 747–60.
- 12. Andrade MR, Antunes LA, Soares RM, Leão AT, Maia LC, Primo LG. Lower dental caries prevalence associated to chronic kidney disease: a systematic review. Pediatr Nephrol 2014;29:771–8.
- 13. Carvalho TS, Lussi A, Jaeggi T, Gambon DL. Erosive tooth wear in children. Monogr Oral Sci 2014;25:262–78.
- 14. Reeves AF, Rees JM, Schiff M, Hujoel P. Total body weight and waist circumference associated with periodontitis among adolescents in the United States. Arch Pediatr Adolesc Med 2006;160:894–9.
- 15. Larsson B, Johansson I, Weinehall L, Hallmans G, Ericson T. Cardiovascular disease risk factors and dental caries in adolescents: effect of a preventive program in Northen Sweden (the Norsjö project). Acta Paediatr 1997;86:63–71.
- 16. Romanos GE, Javed F, Romanos EB, Williams RC. Oro‐facial manifestations in patients with eating disorders. Appetite 2012;59:499–504.
- 17. da Fonseca MA. Substance use disorder in adolescence: a review for the pediatric dentist. J Dent Child (Chic) 2009;76:209–16.
- 18. Crighton A. Paediatric gastrointestinal conditions and their oral implications. Int J Paed Dent 2013:23:338–345.
- 19. Chi DL. Dental caries prevention in children and adolescents with cystic fibrosis: a qualitative systematic review and recommendations for future research. Int J Paed Dent 2013:23:376–86.
- 20. Szczawinska‐Poplonyk A, Gerreth K, Breborowicz A, Borysewicz‐Lewicka M. Oral manifestations of primary immune deficiencies in children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e9–20.
- 21. Ramos‐Gomez FJ, Folayan MO. Oral health considerations in HIV‐infected children. Curr HIV/AIDS Rep 2013;10:283–93.
- 22. Ye Y, Carlsson G, Wondimu B, Fahlén A, Karlsson‐Sjöberg J, Andersson M, Engstrand L, Yucel‐Lindberg T, Modéer T, Pütsep K. Mutations in the ELANE gene are associated with development of periodontitis in patients with severe congenital neutropenia. J Clin Immunol 2011;31:936–45.
- 23. Salem K, Eshghi P. Dental health and oral health‐Dental health and oral health related quality of life in children with congenital bleedingdisorders. Haemophilia 2013;19:65–70.
- 24. Peisker A, Raschke GF, Schultze‐Mosgau S. Management of dental extraction in patients with Haemophilia A and B: a report of 58 extractions. Med Oral Patol Oral Cir Bucal 2014;19(1):e55–60.
- 25. Effinger KE, Migliorati CA, Hudson MM, McMullen KP, Kaste SC, Ruble K, Guilcher GM, Shah AJ, Castellino SM. Oral and dental late effects in survivors of childhood cancer: a Children’s Oncology Group report. Support Care Cancer 2014;22:2009–19.
- 26. Gurbuz T, Tan H. Oral health status in epileptic children. Pediatr Int 2010;52:279–83.
- 27. Gilchrist F, Rodd H, Deery C, Marshman Z. Assessment of the quality of measures of child oral health‐related quality of life. BMC Oral Health 2014;14:40.
- 28. Wogelius P, Rosthøj S, Dahllöf G, Poulsen S. Oral health‐related quality of life among survivors of childhood cancer. Int J Paediatr Dent 2011;21:465–7.
- 29. Soares GB, Garbin CA, Rovida TA, Garbin AJ. Oral health associated with quality of life of people living with HIV/AIDS in Brazil. Health Qual Life Outcomes 2014;12:28.
- 30. Borromeo GL, Bramante G, Betar D, Bhikha C, Cai YY, Cajili C. Transitioning of special needs paediatric patients to adult special needs dental services. Aust Dent J 2014;59:360–5.