Chapter 8
Burning Down the House: Is It Arson?
IN THIS CHAPTER
 Understanding the basics of fire
Understanding the basics of fire
 Identifying why arson happens
Identifying why arson happens
 Using accelerants
Using accelerants
 Investigating the fire scene
Investigating the fire scene
 Wiring explosives
Wiring explosives
Fire is great for roasting marshmallows or cooking a steak, but it wreaks havoc on evidence. Evaluating scenes where fire or explosions have occurred is difficult, at best. This task usually falls to specialized arson investigators, who are trained in suspicious fire analysis. They often face crime scenes where the evidence is severely damaged, if not completely destroyed, by the fire. In addition, the efforts of the firefighters themselves can destroy evidence.
A successful arson investigation overcomes these obstacles to answer two basic questions: Where was the fire’s point of origin, and what was the cause of the fire? The investigator uses physical and chemical evidence to uncover the answers. Based on those findings, the investigator may determine, in general, whether the fire was accidental or incendiary (intentionally set).
Simply bringing together oxygen and a potentially flammable fuel source, such as paper or gasoline, doesn’t produce fire. You need something else — heat. Remove any of the three components — fuel, oxygen, or heat — and no fire occurs.
Striking the Match: Looking into Fire-Starters
Fire is just plain fascinating, and humans have a nearly universal attraction to it. Whether it’s admiration for the beauty of a fireplace or for the practicality and tastiness of food grilled over an open flame, most of us find fire captivating. Maybe it derives from our ancestors’ close relationship with fire, which served as their only source of heat and light. Maybe it’s fire’s ethereal nature that draws us to it. In cases of arson, it may simply be fire’s raw destructive power that fuels the attraction.
The reasons people intentionally set fires are many and varied, but arson invariably has a payoff. The most common reasons criminals set fires include the following:
- Covering their tracks: Arsonists often use fires to cover another crime, such as theft or even murder. An embezzler may use fire to destroy company financial records; an employee who stole goods from a company warehouse may hope that a fire will hide this indiscretion. After all, if you destroy the records or the inventory, how can anyone determine whether anything is missing? Because of this possible motive, arson investigators always search a fire scene for signs of a break-in and theft.
- Insurance fraud: Maybe the arsonist needs quick money, or perhaps the insurance on the home or warehouse is greater than the property’s market value. Arsonists sometimes file insurance claims on valuables they actually removed from the building before setting the fire, hoping to get an insurance settlement for the valuables without actually having to lose them. Greed is a great motivator.
- Psychological reasons: An individual who has a pathological love of fire may start a forest or structure fire simply because he finds it exciting. The resulting destruction and the beauty and power of the fire itself feed some deep-seated psychological need. This kind of fire-starting often becomes a serial offense.
- Revenge: A grudge or deep-seated hatred for another person may drive a hot-headed arsonist to torch that person’s house or business.
- Suicide or murder: Fire rarely is used as a means of committing suicide or murder, because it’s simply too painful for suicide and too unpredictable for murder. Bodies found in fires usually were killed before the fire was started.
- Terrorism: Someone, or some group, may burn structures to create fear or make a political statement.
Determining Where and How the Fire Started
A fire scene is a dangerous place and one fire investigators must approach with great caution. Collapsing floors and falling beams aren’t uncommon. Broken glass, sharp nails, smoldering materials, noxious gases, and asbestos (in older buildings) are other common hazards. Before an investigation can proceed, a structural engineer must give the okay.
The investigation and collection of evidence must begin as soon as possible after the fire has been extinguished and the engineer has declared the structure safe to enter. Time is the enemy: Many of the volatile substances that cause or accelerate a fire rapidly dissipate.
A point of origin near an overloaded wall outlet points toward an accidental fire, but finding a point of origin in a corner of a warehouse, far removed from any electrical source and near a charred gasoline can, suggests the work of an arsonist. Of course, an arsonist may purposely overload a wall outlet in the hopes that a fire would start. But this method is unpredictable, so most arsonists resort to more direct methods for starting fires.
Using other people’s eyes and ears
While members of the arson investigation team inspect and collect samples from the scene, the chief fire investigator or fire marshal interviews witnesses, who can provide many important clues. Someone may have seen the fire in its earliest stages, and that person’s description can lead investigators to the point or points of origin. Many accelerants and combustible materials produce characteristic flame and smoke colors, so witness descriptions of what the fire looked like may help determine its cause. For example, gasoline produces a yellow flame and white smoke.
Investigators may use witnesses’ reports to help
- Locate the point of origin
- Determine whether the fire was accidental or intentional
- Figure out whether the arsonist used an accelerant
Locating the point of origin
Determining a fire’s point of origin requires an understanding of how fire moves through a structure. Fires typically spread out and up from the point of origin, but that pattern can be influenced by structural and decorative elements of the building — stairwells may pull the fire in one direction, and the chemicals in synthetic carpet can cause unusual burn patterns. Usually, however, the largest amount of damage occurs near the point of origin. Investigators often find any igniters or accelerants that were used in this area.
Notice I said that the area of worst damage is usually near the point of origin. That’s not always the case. The worst damage tends to occur where the best combination of fuel, oxygen, and heat is concentrated. It may be that the ventilation is actually poor at the point of origin, but as the fire spreads, it reaches an area with better ventilation and the fire burns more briskly, thus causing more damage. Things are never simple with fires.
After locating the point of origin, an investigator sometimes can retrace the fire’s path even when the structure is heavily damaged. Conversely, backtracking along the fire’s route may lead to the point of origin.
Investigators also can find the point of origin by looking for a V pattern of burned material. Fire tends to rise and spread so that it burns a wall or other vertical surface in a V-shape, with the foot or bottom of the V pointing to the origin of the fire.
Stored fuels and other flammable liquids likewise can interfere not only with locating the true point of origin but also with the search for arson-related accelerants, simply because they too are accelerants. Similarly, an arsonist may have started multiple fires within a building or sloshed a path of gasoline or other accelerant throughout or around the structure, thus creating a fire with multiple or widespread points of origin.
If the building is equipped with a system of smoke detectors, the time at which each alarm was set off can help investigators determine the path that the fire took through the structure and help locate the point of origin.
Liquid and gaseous fuels present special problems for investigators, because they spread more readily and conform to the shapes of their containers. If an arsonist pours gasoline on a floor, the gasoline spreads across the room, runs down the stairs, and seeps into the baseboards. When ignition occurs, the fire follows the liquid and spreads rapidly, making the point of origin widespread. Gaseous fuels, such as natural gas, diffuse in all directions until they fill their containers. When the arsonist adds an ignition source, these fuels can explode. In this example, finding an exact point of origin may be impossible.
Figuring out how it happened
After investigators find the point of origin, they search for potential causes. They examine the circumstances and factors that enabled the fire to start and spread. Human factors, accidental or intentional, top the investigator’s list of potential causes.
Investigators conduct a thorough search of the area near the point of origin for igniters and accelerants. Potential ignition sources include electrical wiring, oil lamps, candles, cigarettes, fireplaces, sophisticated electrical timers, and spontaneous combustion.
Analysis of these possible sources aids fire investigators in categorizing fires as natural, accidental, or intentional. A natural fire results from events like lightning. Accidental sources include frayed wiring or a smoldering cigarette. The presence of an electrical or combustible timing device suggests arson, of course.
Igniting the blaze
Ignition devices range from simple to complex. A match is perhaps the most common ignition device. Arsonists often light fires and toss their matches aside, believing that the resulting fire will completely destroy them. But getting rid of matches isn’t quite so easy.
Another low-tech but effective igniting device can be made by placing a candle on a pile of paper. When the candle burns down, the flame ignites the paper, and the fire takes off. An arsonist may even create a crude timing device by laying a lit cigarette across an open book of matches beneath flammable curtains.
The ignition device also may be a complex electrical timing device. Either commercial timers or a modified clock can be used to time when a circuit closes and switches on an initiator. The construction of these devices is limited only by the creativity of the arsonist.
If a thorough search of the scene reveals candle residue, a cigarette butt, or the remains of an electrical device, investigators have unearthed a possible igniting device.
Heating Things Up: Accelerants
Whenever investigators can’t find a natural or accidental source for a fire, arson must be considered. Like an ignition device, the presence of an accelerant, or something that makes the fire burn faster, suggests an intentional act. Arsonists almost always use accelerants, because simply dropping a match onto a pile of paper or tossing a cigarette on a bed is too unpredictable. Arsonists want to be sure that the fires they start actually burn.
Arsonists most commonly use liquid accelerants, particularly gasoline and kerosene, as fire accelerators; however, even when a structure has been severely damaged, traces of these accelerants often remain. They soak into carpets and brickwork, seep into baseboards and crevices, and settle into areas beneath the fire. Remnants survive most fires, and investigators diligently search for these remnants at the scenes of any suspicious fires.
Collecting samples at the scene
Investigators take samples from the area around the point of origin for chemical analysis. Sometimes, specially trained dogs aid investigators in obtaining the best samples by sniffing out traces of accelerants. Investigators also use chemical detectors, such as a Vapor Trace Analyzer (VTA), which screen materials for accelerant residues (see the nearby sidebar “Sniffing out accelerants with a VTA” to find out more about how it works).
The sooner investigators gather materials for testing, the better. The accelerants of choice for arsonists generally are petroleum-based hydrocarbons, such as gasoline and kerosene, whose vapors dissipate with time. To prevent the loss of this crucial evidence, investigators place any materials they gather in nonporous, sealed containers, such as clean paint cans and glass jars. Plastic bags don’t work because the plastic can react with the hydrocarbons, giving the volatile gases a chance to escape through the damaged material.
In addition to taking samples from the area of the fire, investigators take samples of the same materials from unburned areas. If, for example, the sample suspected of holding an accelerant residue happens to be a section of charred carpet, investigators take a piece of the same carpet from an unburned area, whenever possible, for comparison purposes.
Taking it to the lab
Investigators take the materials they collect to the crime lab for analysis. These materials may include charred wood, pieces of carpet, or even empty bottles. The first step in the analysis is extracting any possible chemical accelerants from the debris.
- Headspace vapor extraction: The material is placed in a closed container. The natural volatility of the hydrocarbons creates a vapor-rich gas in the headspace, or the air-filled area above the material in a closed container. Heating the sample accelerates this process. The vapor is then removed from the container with a syringe and tested.
- Solvent extraction: The sample is placed in a container with a solvent, such as chloroform, carbon tetrachloride, methylene chloride, or carbon disulfide. The solvent dissolves the sample material, separating out the hydrocarbons, which are then analyzed.
- Steam distillation: The charred material is heated, and the steam is collected and condensed. The resulting liquid then is checked for volatile hydrocarbons.
- Vapor concentration: The sample is heated in a closed container that also contains charcoal, which absorbs the volatile hydrocarbons as they leave the sample. Just as an open box of baking soda in your refrigerator absorbs “odor molecules” and freshens your fridge, charcoal readily absorbs hydrocarbon molecules from the air. The charcoal is then removed, and the hydrocarbons are extracted using a solvent, after which the hydrocarbons are analyzed to determine their nature and type.
Digging deeper into iffy samples
After technicians have isolated the suspicious compounds using one of the techniques in the preceding section, they look more closely at them using one or more of the procedures discussed in the following sections.
Gas chromatography
One way to identify a compound is to identify how far each part of it moves through an inert gas, or carrier gas. Gas chromatography (GC) rapidly separates mixtures of compounds into individual components in this manner.
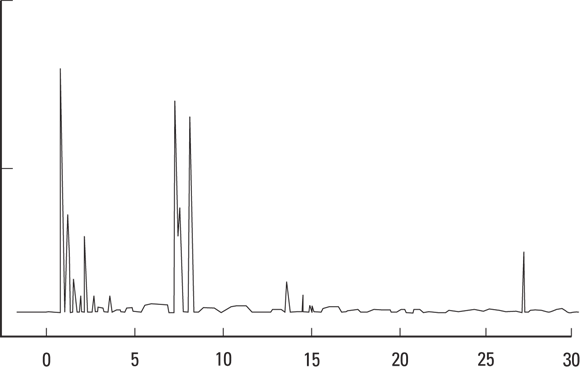
Illustration by Nan Owen
FIGURE 8-1: A gas chromatograph. Each peak represents a different component of the unknown substance being tested.
Often, technicians need only the results from the GC to determine the composition of the unknown sample. But if they need further information, they can combine the GC results with either mass spectroscopy or infrared spectrophotometry results (see the sections that follow).
Mass spectroscopy
No two substances have the same chemical fingerprint, and each compound’s fingerprint can be determined with mass spectroscopy (MS).
The GC and MS can be directly connected. This combination is called a gas chromatograph-mass spectrometer (GC-MS). The GC feeds each gaseous compound it separates directly into the MS, and the MS determines the compound’s mass spectrum. Virtually any substance can be identified using this combination.
Infrared spectrophotometry
Each compound has a different infrared absorption spectrum and can be identified by its spectrum using infrared spectrophotometry (IR). This test determines the amount of infrared light absorbed by the compound in question and results in a chemical fingerprint of the compound in question.
IR also can be combined with GC, and the GC-IR combination rapidly gives results that are as accurate as the ones provided by the GC-MS combination.
Drawing conclusions from testing
Although this investigative method can’t be completely relied upon, the person examining the sample may be able to say that it possibly (or perhaps even probably) came from a particular source.
Investigating Homicidal Fires
Arson may not be the only crime at fire scenes, which unfortunately often contain a body or two. Just because a body is found at a fire scene, however, does not mean the victim died because of the fire. The cause and manner of death is a matter for the medical examiner (ME) and homicide investigators, who are called in whenever a body turns up at a scene. They work to identify the victim and figure out how he died, using clues like the location of the body and evidence of smoke inhalation.
The ME must answer one all-important question: Was the victim alive at the time the fire started and burned? If the victim was alive, the fire may have been an accident, though murder is still possible if the killer/arsonist thought the victim was dead when in fact he wasn’t. If the fire was indeed arson and the ME determines the victim was dead prior to the fire beginning, then murder is obviously more likely.
Determining the cause and manner of death depends upon careful evaluation of the fire scene and the corpse during autopsy. The ME looks at several aspects of the body to help make this determination, including the position it was in, the carbon monoxide (CO) levels of its blood and tissue, and the presence or absence of soot in the lungs and airways.
Location, location, location
The position of the body is extremely important. Where the victim died, the state of the body, and the items around the body provide investigators vital information about that person’s death (and about the fire itself).
Say, for example, that the corpse is found on a bed, and the ME determines that the cause of death is smoke inhalation. Investigators conclude that the fire’s origin was a cigarette in contact with bedding. Under these circumstances, the death may be accidental. But did the victim smoke? If no one is able to clear up this question for investigators, the ME can measure the nicotine level in the victim’s urine. A high level indicates that the victim was indeed a smoker. If not, the cigarette may have been an instrument for arson.
Poisoned by air
Asphyxia (or suffocation) from inhaling smoke and carbon monoxide (CO) causes most fire-related deaths. The ME tests the victim’s blood and tissues for CO. A normal level is less than 5 percent, but it can be slightly higher in smokers. In victims of CO asphyxiation, the level ranges from about 45 to 90 percent. Finding a high CO level suggests that the victim died from smoke and CO inhalation, but a low level implies that the victim died before or at the time the fire started.
Interpretation of the CO level also depends upon evaluation of natural disease in the deceased. An older person with severe coronary artery disease will die from a lower level of CO than a young person with a healthy body.
Besides a high blood CO level, finding soot in the mouth, throat, lungs, and airways suggests that the victim was alive as the fire burned and inhaled these materials in a struggle for oxygen. Conversely, if the CO level is low and no soot is present in the airways, the ME considers other causes and manners of death, and homicide becomes a strong possibility.
What if a burned body is found bound, gagged, and shot? The ME performs blood CO levels to determine the cause of death. Did the gunshot or the fire do in the victim? Finding high blood CO levels and soot within the airway and lungs leads the ME to conclude that the victim lived through the gunshot and died as a result of CO inhalation caused by the fire. If the victim has a low blood CO level, the gunshot is the more likely cause of death. This finding can be critical to prosecutors if there were two perpetrators in the crime. If one pulled the trigger and the other started the fire, one is the murderer and the other an accomplice.
Evaluating Explosive Situations
Fires and explosions are similar reactions in that both result from a combination of fuel and oxygen. The difference is simply the rate of that reaction. Fires consume their fuel (wood, paper, trees) more slowly than explosions, which consume their fuel (gasoline, dynamite) almost instantaneously, in part because the material is confined. If ignited in an unconfined space, the material simply burns, but if you tightly pack the material into a container, it explodes when you ignite it.
Explosions create numerous problems for investigators. The explosive device and any surrounding structures are heavily damaged, if not completely destroyed. Unless a secondary fire occurs, investigators usually can determine the point of origin with ease; however, finding fragments of the device or any igniters or timers may be difficult.
Defining explosives
Explosives are categorized as either high or low by the speed of their resulting pressure wave. Low explosives typically move at rates of 1,000 meters per second or less, and high explosives may reach speeds as high as 8,500 meters per second.
The most readily available and commonly used low explosives are black powder and smokeless gunpowder. A combination of sugar and potassium chlorate makes another easy explosive. Bombers need not be very sophisticated.
- Initiating explosives are very sensitive to these effects. Because of the potential for unexpected detonation, home-manufactured bombs rarely use initiating explosives. These explosives more often appear in primers and blasting caps, where they initiate other, less-sensitive noninitiating explosive materials. Mercury fulminate and lead azide are commonly used in this way.
- Noninitiating explosives are less sensitive and more commonly used in commercial and military applications. These explosives include dynamite, TNT (trinitrotoluene), RDX (pentaerythritrol tetranitrate), and PETN (cyclotrimethylenetrinitramine). Although you can still find dynamite, it and other nitroglycerine-based explosives have largely been replaced by ammonium nitrate–based explosives. ANFO, an easily made explosive material, is a mixture of ammonium nitrate and fuel oil.
Investigating a bombing scene
Searching the scene of an explosion requires the same attention to detail as does the search of a fire scene. Finding fragments from the explosive device, igniter, and timer can be crucial to determining the type of explosive used and, ultimately, the person responsible for the bombing. In addition to locating these fragments, investigators direct their searches toward collecting debris to test for unexploded residue, which almost always is present.
After finding out what particular explosive was used, investigators focus on finding the seller and buyer of that explosive.
 Locating where a fire began is the cornerstone of fire and arson investigation. Evaluating materials found where the fire started can help investigators determine whether it was accidental or incendiary.
Locating where a fire began is the cornerstone of fire and arson investigation. Evaluating materials found where the fire started can help investigators determine whether it was accidental or incendiary. Investigators estimate the intensity of the fire at any particular location by assessing the fire’s effect on structural materials. Steel beams buckle whenever the fire is extremely intense, and glass melts at around 1,500 degrees Fahrenheit. Cracking and flaking (spalling) on walls and floors indicate areas of high heat. Similarly, wooden beams, floors, and walls may char, leaving a pattern that looks like alligator skin. When that happens, smaller scales tend to be near the hottest point of the fire. For years, investigators believed that spalling and alligatoring meant that an accelerant had been used, but these myths have been debunked. These findings, for reasons I state earlier, simply mean that the fire was hot enough in that area to produce these changes; they can occur without the presence of an accelerant and can be far removed from the actual point of origin.
Investigators estimate the intensity of the fire at any particular location by assessing the fire’s effect on structural materials. Steel beams buckle whenever the fire is extremely intense, and glass melts at around 1,500 degrees Fahrenheit. Cracking and flaking (spalling) on walls and floors indicate areas of high heat. Similarly, wooden beams, floors, and walls may char, leaving a pattern that looks like alligator skin. When that happens, smaller scales tend to be near the hottest point of the fire. For years, investigators believed that spalling and alligatoring meant that an accelerant had been used, but these myths have been debunked. These findings, for reasons I state earlier, simply mean that the fire was hot enough in that area to produce these changes; they can occur without the presence of an accelerant and can be far removed from the actual point of origin. Spontaneous combustion (an internal chemical reaction that starts a fire) is rare but can occur when combustible materials are contained in a tight space —when oil-soaked rags are kept in a small, closed pantry, for instance. Slow oxidation of the oils releases heat. If the rags are in an enclosed space, the heat has nowhere to go and may reach the ignition temperature of the oil or the rags.
Spontaneous combustion (an internal chemical reaction that starts a fire) is rare but can occur when combustible materials are contained in a tight space —when oil-soaked rags are kept in a small, closed pantry, for instance. Slow oxidation of the oils releases heat. If the rags are in an enclosed space, the heat has nowhere to go and may reach the ignition temperature of the oil or the rags. The heads of matches contain diatoms, which are single-celled organisms found in the kind of earth used in match production. The shells of these tiny creatures contain silica, a tough compound that survives fires. Interestingly, match manufacturers use different materials, so different diatom species show up in their matches. Because each species of diatom has a unique shell structure, identifying these shell remnants often identifies the brand of matches used by the arsonist.
The heads of matches contain diatoms, which are single-celled organisms found in the kind of earth used in match production. The shells of these tiny creatures contain silica, a tough compound that survives fires. Interestingly, match manufacturers use different materials, so different diatom species show up in their matches. Because each species of diatom has a unique shell structure, identifying these shell remnants often identifies the brand of matches used by the arsonist. Ammonium nitrate is an oxygen-rich oxidant that can be found in fertilizers. Bombs produced from this substance were involved in the Oklahoma City and 1993 World Trade Center bombings.
Ammonium nitrate is an oxygen-rich oxidant that can be found in fertilizers. Bombs produced from this substance were involved in the Oklahoma City and 1993 World Trade Center bombings.