Studies of humans who have been unfortunate enough to acquire brain damage have provided a rich source of information for cognitive neuroscientists. The basic premise behind the approach is that, by studying the abnormal, it is possible to gain insights into normal function. This is a form of “reverse engineering,” in which one attempts to infer the function of a component (or region) by observing what the rest of the cognitive system can and can’t do when that component (or region) is removed. In this way, lesions “carve cognition at its seams” (McCarthy & Warrington, 1990).
Patient-based neuropsychology has tended to take two broad forms. In one tradition, which I shall call classical neuropsychology, attempts have been made to infer the function of a given brain region by taking patients with lesions to that region and examining their pattern of impaired and spared abilities. This type of research has benefited greatly from the development of imaging methods that enable more accurate lesion localization and quantification. It also provides an important source of constraint on functional imaging data. In the second tradition, which I shall call cognitive neuropsychology, the pattern of spared and impaired abilities in and of themselves has been used to infer the building blocks of cognition—irrespective of where they are located in the brain. This approach has been particularly informative for guiding the development of detailed informationprocessing models and provides the cognitive framework that underpins much imaging research. The schism between these traditions has run deep. For example, many journals either tacitly or explicitly favor one approach over the other. Moreover, each tradition has tended to rely on its own methodology, with classical neuropsychology favoring group studies and cognitive neuropsychology favoring single-case studies. The development of cognitive neuroscience has led to something of a reconciliation of these traditions, and this textbook discusses both. The key point that one needs to bear in mind is this: the method one chooses should be appropriate to the question one is asking. It will be argued in this chapter that group studies are more appropriate for establishing lesion-deficit associations, whereas single-case studies are particularly helpful for establishing how cognitive processes might be subdivided.
Key Terms
Group studies
In neuropsychology, the performance of different patients is combined to yield a group average.
Single-case studies
In cognitive neuropsychology, the data from different patients are not combined.
Transcranial magnetic stimulation (TMS)
Non-invasive stimulation of the brain caused by a rapidly changing electrical current in a coil held over the scalp.
Transcranial direct current stimulation (tDCS)
Non-invasive stimulation of the brain caused by passing a weak electrical current through it.
Naturally occurring brain lesions are “accidents of nature” that occur because of stroke, tumor, head injury, or other types of brain damage. A complementary approach, that in many ways resembles the logic of the lesion method, involves magnetic stimulation of the intact brain to produce what has been described as “virtual lesions” (e.g. Pascual-Leone et al., 1999). This method is called transcranial magnetic stimulation (TMS). The method makes contact with the literature from the classical neuropsychology tradition with its emphasis on lesion location. However, it can also be used to test information-processing theories of cognition because it can provide information on the timing of cognitive processes. The method has a number of advantages over traditional lesion methods. A newer method is based on the principle of electrical stimulation and is termed transcranial direct current stimulation (tDCS) (Nitsche et al., 2008). Like TMS it can be used to temporarily disrupt cognitive function (a virtual lesion approach). However, it can also be used to boost cognitive function which has important implications for rehabilitation as well as for exploring the brain basis of cognition.
Ways of Acquiring Brain Damage
Brain damage can be acquired in a number of ways, as summarized below:
Neurosurgery
Operations are occasionally performed in cases of severe epilepsy in which the focus of the epileptic seizure is surgically removed. One of the most famous cases in neuropsychology, HM, had dense amnesia after part of his medial temporal lobe was surgically removed (see Chapter 9). Another surgical procedure formerly used to reduce epileptic seizures spreading across the brain was to sever the fibers of the corpus callosum. This operation was referred to as the split-brain procedure. Patients who have undergone this intervention have only mild impairments in daily living, but the impairments can be observed in laboratory conditions in which stimuli are presented briefly to each hemisphere (for a review, see Gazzaniga, 2000). Surgical intervention was also previously common in psychiatric patients (see the discussion on the prefrontal lobotomy in Chapter 14). In general, surgical procedures are only carried out in the absence of suitable pharmacological treatments.
Strokes (or cerebrovascular accident; CVA)
Disruptions to the blood supply of the brain (called strokes or cerebrovascular accidents, CVA) can result in global or local death of neurons. If an artery ruptures, this leads to a hemorrhage and an increase in intracranial pressure (typically relieved by surgery). People born with aneurysms are more susceptible to rupture. These are localized regions of over-elastic artery that may balloon and rupture. Blood vessels may also become blocked if, for example, a fatty clot gets pushed from a large vessel into a smaller one (an embolism) or a stationary clot becomes large enough to block the vessel (thrombosis). Other vascular disorders include angiomas (tangled and tortuous blood vessels liable to rupture) and arteriosclerosis (hardening of the vessel walls).
Traumatic head injuries
Whereas vascular disorders tend to affect older people, traumatic head injuries are the most common form of brain damage in people of less than 40 years of age. They are particularly common in young men as a result of road traffic accidents. Traumatic head injuries are classified in two ways, “open” or “closed,” depending on whether the skull is fractured. Open head injuries often have more localized injuries; whereas closed head injuries have more widespread effects (as the brain ricochets in the skull) and often produce loss of consciousness.
Tumors
The brain is the second most common site for tumors (after the uterus), and brain tumors are often spread from other parts of the body (these are called metastatic tumors). Tumors are caused when new cells are produced in a poorly regulated manner. Brain tumors are formed from supporting cells such as the meninges and glia (termed “meningioma” and “gliomas,” respectively). Tumors adversely affect the functioning of the brain because the extra cellular material puts pressure on the neurons, disrupting functioning and possibly leading to cell death.
Viral infections
A number of viruses target specific cells in the brain. These include herpes simplex encephalitis (HSE), human immunodeficiency virus (HIV), and Creutzfeldt-Jakob disease (CJD).
Neurodegenerative disorders
Most western societies have a large ageing population that will, if anything, continue to get larger and older. In 1900, 4 percent of people were over the age of 65; in 2030, 20 percent of the population is estimated to be over 65. An increase in life expectancy is bringing about an increase in degenerative illnesses that affect the brain. By far the most common is dementia of the Alzheimer type (or DAT). This is associated with atrophy in a number of regions of the brain, with memory loss (amnesia) typically being the earliest noted symptom. Other neurodegenerative diseases include Parkinson’s disease and Huntington’s disease (see Chapter 8), Pick’s disease (often the medical diagnosis in cases of semantic dementia), and multi-infarct dementia (caused by many small strokes that can be hard to distinguish from DAT).
Key Terms
Split-brain
A surgical procedure in which fibers of the corpus callosum are severed.
Strokes
Disruption in the blood supply to the brain; also called cerebrovascular accidents (CVA).
Aneurysm
Over-elastic region of artery that is prone to rupture.
In 1990, two very unusual patients came to the attention of Roberto Cubelli (Cubelli, 1991). One patient, CF, was unable to write any vowel letters and left gaps in their place (“Bologna” → B L GN). Another patient, CW, made spelling errors selectively on vowels (e.g. “dietro” → diatro); equivalent errors were not found in his spoken language. By contrast, Kay and Hanley (1994) report a different patient who made spelling errors selectively on consonants (e.g. “record” → recorg). The basic logic behind the cognitive neuropsychological approach is that a difficulty in one domain relative to an absence of difficulty in another domain can be used to infer the independence of these domains. In the case of the patients just discussed, the implication was that the brain has separate neural resources for the processing of written vowels relative to consonants. These neural resources need not lie in different locations of the brain (at least on a millimeter or centimeter scale), but might reflect two different populations of interspersed neurons. Note, also, that it is not clear that one can conclude that the only function of these neurons is the coding of consonants and/or vowels. The difference could be relative and, indeed, without testing a whole range of other stimuli (e.g. digits), it is unwise to conclude exclusivity of function. Nonetheless, it is reasonable to conclude that there are some neural resources predominantly implicated in written vowel processing relative to consonants and vice versa.
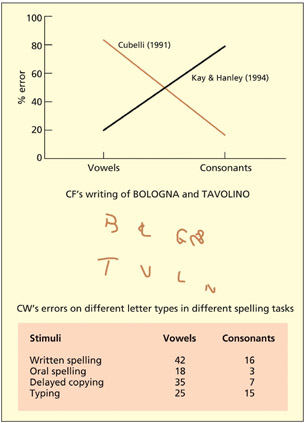
Some patients produce spelling errors selectively on either consonants or vowels. This may imply separate neural resources for coding consonants and vowels.
If a patient is impaired on a particular task (task A) but relatively spared on another task (task B), this is referred to as a single dissociation. If the patient performs entirely normally on task B compared with a control group, this has been termed a classical single dissociation, whereas if the patient is impaired on both tasks but is significantly more impaired on one task, this is referred to as a strong single dissociation (Shallice, 1988). In either of these instances, one inference is that task A and task B utilize different cognitive processes with different neural resources. However, other inferences could also be made.
It could be the case that both task A and task B use exactly the same cognitive/neural resources as each other, but task B requires more of this resource than task A (i.e. task B is harder). If brain damage depletes this resource, then task B may be relatively or selectively impaired. This has been referred to as a task-resource artifact (Shallice, 1988). Another explanation of a single dissociation is in terms of a task-demand artifact (Shallice, 1988). A task-demand artifact is when a single dissociation occurs because a patient performs one of the tasks suboptimally. For example, the patient may have misunderstood the instructions or have adopted an unusual strategy for performing the task. Task-demand artifacts can be minimized by assessing the patient’s general intellectual functioning, giving clearer instructions or training, using ecologically valid tests, and repeating the same (or similar tests) on several occasions.
Key Terms
Single dissociation
A situation in which a patient is impaired on a particular task (task A) but relatively spared on another task (task B).
Task-resource artifact
If two tasks share the same neural/cognitive resource but one task uses it more, then damage to this resource will affect one task more than the other.
Task-demand artifact
One task is performed worse than another because the task is performed sub-optimally (but not because some aspect of the task is compromised).
Double dissociation
Two single dissociations that have a complementary profile of abilities.
Dysgraphia
Difficulties in spelling and writing.
Syndrome
A cluster of different symptoms that are believed to be related in some meaningful way.
In general, almost all neuropsychological studies are aimed at proving that two or more tasks have different cognitive/neural resources and disproving the task-resource and task-demand explanations even if this is not explicitly stated in these terms. In the case of Cubelli’s patients, a task-demand artifact can easily be ruled out because the same task (i.e. writing) was performed in both conditions. One of the most powerful ways of discounting a task-resource artifact is to document a double dissociation, which merely refers to two single dissociations that have a complementary profile of abilities. To remain with the current example, Kay and Hanley’s patient could write vowels better than Cubelli’s patient, whereas Cubelli’s patient could write consonants better than Kay and Hanley’s.
So far, the discussion has emphasized the importance of dissociations between deficits, but what about associations of deficits? For example, if for every patient that resembled Cubelli’s there were 10, 20, or 100 times as many patients who had comparable dysgraphia for both consonants and vowels, then would this diminish the findings of the dissociation? Some researchers would suggest not. There are some theoretically uninteresting reasons why two symptoms may associate together, the main reason being that they are close together in the brain and so tend to be similarly affected by strokes (or whatever) in that region. For example, patients with difficulties in recognizing faces often have difficulties in perceiving colors, but this probably reflects neuroanatomical proximity rather than suggesting a “super-module” that is specialized for both. It is the (double) dissociations between the two that count from a theoretical point of view.
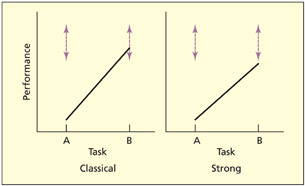
In a classical dissociation, performance on one task lies within the control range (shown by dotted lines). In a strong dissociation, both tasks fall outside the control range, but one task is significantly more impaired than the other.
Needless to say, this particular viewpoint has attracted controversy. It has been argued that it is important to know how common a particular dissociation is in order to rule out that it hasn’t been observed by chance (Robertson et al., 1993). For example, if brain damage affects some written letters more than others in a random fashion, then it would still be possible to find patients who appear to have selective difficulties in writing vowels, but it would be a chance occurrence rather than meaningful dissociation. Other researchers have focused more on associations between symptoms (so-called syndromes) rather than dissociations. The use of the double dissociation itself has been subject to criticism (see Dunn & Kirsner, 2003). Some have argued that the use of double dissociation implies an endorsement of the notion of modularity (e.g. as specified by Fodor, 1983; see Chapter 1). However, it need not. Shallice (1988) discusses why this argument is wrong by setting up the following thought trap: if modules exist, then double dissociations are a reliable way of uncovering them; double dissociations do exist, therefore modules exist. The way out of this trap, however, is to ask the question: can non-modular systems produce double dis soci ations? It has been demonstrated that other types of cognitive architecture, such as interactive con nectionist models, can produce double dissociations (Plaut, 1995). The reason why they do so is interesting. It reflects the fact that these systems also contain units that are functionally specialized for certain types of process/ information, even though the system is interactive, and even though these units may respond (to a greater or lesser degree) to a range of stimuli.

A task-resource artifact can arise because one task uses more of a cognitive/neural resource than the other (i.e. one task is harder). One could construe brain damage as depleting the amount of resource available. In this instance, at moderate brain damage the patient can still perform the easy task normally. A single dissociation need not reflect different cognitive/neural substrates for the tasks.
Some have argued that the reliance on double dissociations is flawed because it requires the study of “pure” cases (Dunn & Kirsner, 2003). However, it need not (Shallice, 1979). First of all, one must be careful to state what is meant by a pure case. For example, imagine that the dysgraphic patients mentioned above also had amnesia. Would the fact that they were not “pure dysgraphic” exclude them from study? This might depend on the theoretical stance one adopts. If one’s theoretical model assumes that writing and memory are independent (as most do), then studying writing in isolation is entirely feasible.
It is worth stating that finding a double dissociation between two patients on two tasks is only part of the neuropsychologist’s toolkit. To interpret their spared and impaired performance, one requires evidence from a range of other relevant tasks. For example, to fully interpret the dysgraphic patients’ impairments it would be interesting to know if they could copy vowels and consonants, or recognize them visually. The types of error that patients produce can also be an important source of information, irrespective of their performance level (i.e. how good or bad they are). For example, the independence of consonants and vowels was initially inferred from the types of errors made in dysgraphia (Caramazza & Miceli, 1990) and not from the double dissociation logic. The double dissociation is useful, but it is not a panacea.
Caramazza’s assumptions for theorizing in cognitive neuropsychology
Although the use of single cases of brain-damaged individuals to study normal cognitive/brain function began in the mid-nineteenth century, attempts to formalize the logic of this approach were lacking for many years. Caramazza provided one of the first serious attempts to do so in the 1980s (Caramazza, 1986, 1992; Caramazza & Badecker, 1989; Caramazza & McCloskey, 1988; McCloskey & Caramazza, 1988).He suggested that three underlying, and unstated, assumptions underpinned almost all neuropsychological studies to date:
- The fractionation assumption. The first assumption is that damage to the brain can produce selective cognitive lesions. Note that the assumption is stated with reference to a lesion within a particular cognitive model and not to a lesion to a particular region of the brain (although the two may, of course, be correlated). Caramazza’s arguments were concerned with using observations of brain-damaged individuals to inform theories of cognition (cognitive neuropsychology), not to localize cognitive processes in the brain (classical neuropsychology).
- The transparency assumption. The transparency assumption states that lesions affect one or more components within the preexisting cognitive system, but they do not result in a completely new cognitive system being created. This assumption is needed because one wishes to study the abnormal in order to understand the normal, and not just to study the abnormal as an end in itself.
- The universality assumption. The universality assumption is that all cognitive systems are basically identical.
Key Term
Transparency assumption
Lesions affect one or more components within the preexisting cognitive system but do not result in a completely new cognitive system being created.
Caramazza acknowledges that these assumptions may, under some situations, not hold true. It is a matter for empirical research to determine the extent to which they are true and, hence, the validity of any inference that can be drawn from the study of brain-damaged individuals. Critics have pointed to a number of potential difficulties with the assumptions. Kosslyn and van Kleek (1990) have suggested that whether selective cognitive impairments will be observed (the fractionation assumption) depends on the neural architecture. For example, selective deficits may be more likely if neurons performing a given operation are clustered together rather than distributed around the brain, and if the neurons are dedicated to one operation rather than shared by many operations. Nevertheless, selective cognitive impairments can be observed and so the fractionation assumption appears to hold true at one level, even if there are some cognitive processes that may be hard to uncover by the lesion method by virtue of an atypical neural architecture.
The transparency assumption is potentially the most problematic. Basically, one needs to assume that brain damage removes one component of cognition, but does not create, from scratch, a rearranged or different cognitive system. Examples of brain plasticity, and rehabilitation and recovery after brain damage, might at first appear to be convincing arguments against transparency. But they need not be. For example, imagine that a patient has severe problems in speaking after a stroke (i.e. aphasia) but that these problems ameliorate over time. This could be taken as prima facie evidence that the brain has somehow reorganized itself after the stroke. However, it could be that the preexisting cognitive model has just been reinstated rather than that a whole new way of performing the task has been created. As such, this would not be a violation of the transparency assumption. Plasticity at a neural level is a pervasive aspect of brain function (see also Chapter 9), and need not imply behavioral change or functional change. It is important to point out that the assumption is more likely to hold true for brain damage acquired during adulthood than childhood (Thomas & Karmiloff-Smith, 2002). It is also worth pointing out that the transparency assumption refers to the cognitive organization of the cognitive system and not necessarily its location. Consider the case of an epileptic child who has his left hemisphere removed and then learns to speak using the right hemisphere (Vargha-Khadem et al., 1997a). Is that a violation of the transparency assumption? It could be, but it need not be. It depends on whether the new right hemisphere system is cognitively equivalent to the one in the left. The transparency assumption refers to the comparability between premorbid and postmorbid cognitive systems, and not on where such systems are located. Although the debate remains open about the validity of this assumption, a good rule of thumb is that the transparency assumption is less likely to be violated in adult relative to child cases, and when studied soon after injury relative to later in time (or if the cognitive profile after injury remains stable over time).
The universality assumption, that all cognitive systems are basically the same, may also be problematic to neuropsychology. But Caramazza has argued that it is equally problematic for other methods within cognitive neuroscience. Basically, one needs to assume that an individual (or individuals) are representative of the population at large in order to make generalizations to normal cognition. Individual differences, such as they are, are attributable to “noise” (e.g. variations in performance related to time) or other factors that may be related to the efficiency of the cognitive system (e.g. expertise) but need not reflect qualitative differences in the way the task is performed. Of course, if there are individual qualitative differences, then this is theoretically interesting. Finding a framework to explore and account for these differences is a challenge for cognitive neuroscience in general, rather than patient-based neuropsychology in particular. Caplan (1988), however, has argued that individual differences are more of a problem for singlecase studies relative to other methods because this method gives exaggerated importance to exceptional findings. But this could be construed as the strength of this method rather than a weakness—assuming that the individual differences can be ascribed to something of theoretical interest rather than just “noise.”
The case for single-case studies
Caramazza and McCloskey (1988) have gone as far as to suggest that the singlecase study is the only acceptable method in cognitive neuropsychology. The titles of the papers debating this position tell a story of their own. The original paper, entitled “The case for single patient studies” (Caramazza & McCloskey, 1988), was interpreted as the case against group studies. A subsequent paper, “The case against the case against group studies” (Zurif et al., 1989), defended group studies on the grounds that “syndromes [i.e. associations of symptoms] are what the world gives us.” This provoked a paper with a particularly amusing title: “Clinical syndromes are not God’s gift to cognitive neuropsychology: A reply to a rebuttal to an answer to a response to the case against syndrome-based research” (Caramazza & Badecker, 1991). To understand this heated debate, it is necessary to take a step back and consider the argument as initially laid out.
Consider first the logic of testing participants in the non-brain-damaged population. One may recruit a sample of participants (S1 to Sn) and make the assumption, valid or not, that they have broadly equivalent cognitive systems (M). One may then conduct an experiment (E), making the further assumption that all participants carry it out in equivalent ways (i.e. no task-demand artifacts), and derive a set of observations (O1 to On). In this instance, it is argued that it is quite feasible to average the observations of the group because it is assumed that the only difference between the participants is “noise” (i.e. variations in performance over time, differences in speed or ability).
Consider next the situation in which one wishes to test a group of brain-damaged patients (P1 to Pn). As before, it is assumed that each has (before their lesion) essentially the same cognitive system (M) and that each is given the same experiment (E) and complies with the experiment in the same way. However, each patient may have a different lesion to the cognitive system (L1 to Ln) and so difference in observed performance may be attributable to differences in lesion rather than between-patient noise and, as such, averaging across patients is not possible. Determining where the lesion is in the cognitive system can only be determined on the basis of empirical observation of each case in turn. It is crucial to bear in mind the distinction between a lesion to a cognitive component (which is relevant to the discussion here) and an anatomical lesion. At present, there is no magic way of working out what the precise cognitive profile of a given patient will be from a structural lesion (except in the most general terms). Thus, establishing the cognitive impair ment re quires cognitive testing of individual patients.
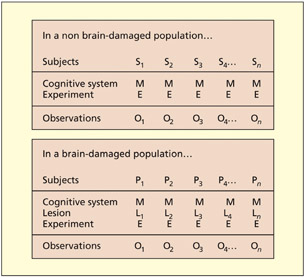
Caramazza has argued that it is possible to average observations (O1 to On) across different non-braindamaged participants (S1 to Sn) because they are assumed to have the same cognitive system (M) that performs the experiment (E) in comparable ways. The same logic may not apply to brain-damaged patients (P1 to Pn) because each patient will have a different cognitive lesion (L), which cannot be known a priori.
What if one were to establish that a group of patients had identical lesions to the same component of the cognitive system, could one then average across the patients? Caramazza has argued that, although legitimate, the study be comes a series of single-case studies, not a group study, and so the unit of interest is still the single case. To establish that they had the same lesion, one would have to carry out the same set of experiments on each individually. As such, one would not learn any more from averaging the set of patients than could be learned from a single case itself. The objection is not against the idea of testing more than one patient per se, but rather averaging the results of many patients assumed (but not proven) to be equivalent.

The use of single cases is not peculiar to neuropsychology. For example, it is the mainstay of archaeology and anthropology. In 1974, Donald Johanson discovered a partial skeleton of a single primate, Lucy, from 3.18 million years ago, which had walked upright and had a small brain. Previous theories had suggested that brain enlargement preceded the ability to walk upright. This single case proved this not to be so. Note that Johanson did not have to provide a group of “Lucys” for his findings to be acceptable to the scientific community.
Some of the common objections against the use of the single-case study are that one cannot create a theory based on observations from only a single case, or that it is not possible to generalize from a single case. The counterarguments are that nobody is trying to construct whole new theories of cognition based on a single case. Theories, in neuropsychology and elsewhere, must account for a wide range of observations from different sources, both normal and brain-damaged. For example, cognitive models of reading are able to account for different observations found in skilled readers and also account for the different types of acquired dyslexia (drawn from several different single cases). They must also account for the pattern of performance (e.g. the types of error made) as well as the level of performance (i.e. following the logic of dissociations). Although nobody wishes to construct a theory based on a single case, observations from single cases constitute valid data with which to test, amend, and develop theory. As for the argument that it is not possible to generalize from a single case, the counterquestion would be “generalize to what?.” It is entirely plausible to generalize from a single case to a model of normal cognition. It is, however, much harder to generalize from one single case to another single case. Two patients with a stroke may have very different cognitive profiles (i.e. one cannot generalize from one case to another), but it should nevertheless be possible for each particular case to generalize to some aspect of normal cognition.
Evaluation
The argument presented above has emphasized the point that single-case studies are a valid methodology and they may have a particularly important role to play in determining what the components of cognitive systems are. The discussion has also argued that the term “lesion” can be construed both in terms of disruption to a component in a cognitive model, as well as a region of organic brain damage. Does this mean that group studies have no role to play at all? It will be argued that group studies do have an important role to play, and that they may be particularly suited to addressing different types of question from the single-case approach.
The introduction to this chapter discussed the historical schism that exists between cognitive neuropsychology, which is aimed at developing purely cognitive accounts of cognition, and classical neuropsychology, which is aimed at developing brain-based accounts of cognition. Both approaches fit well within a cognitive neuroscience framework. The cognitive neuropsychology tradition enriches the conceptual framework and provides a testable hypothesis about what the likely neural components of cognition are (although not necessarily where they are). The classical neuropsychology tradition provides important contrastive data with functional imaging. There are several reasons why regions may appear active or inactive in functional imaging tasks, and a region of activity need not imply that a region is critically involved in that particular task. Studies of patients with lesions in that area do enable such conclusions to be drawn. The lesions of patients, however, are typically large and rarely restricted to the region of interest. Thus, to be able to localize which region is critical for a given task, several patients may need to be considered.
Ways of grouping patients
How does one decide the principle by which patients should be grouped in order to associate lesion sites with deficits? There are at least three approaches in the literature:
- Grouping by syndrome. Patients are assigned to a particular group on the basis of possessing a cluster of different symptoms. This approach is particularly common in psychiatric studies (e.g. of schizophrenia), but there are equivalent approaches in neuropsychology (e.g. the aphasia subtypes identified by Goodglass and Kaplan, 1972).
- Grouping by cognitive symptom. Patients are assigned to a particular group on the basis of possessing one particular symptom (e.g. auditory hallucinations; difficulty in reading nonwords). They may also possess other symptoms, but, assuming that the other symptoms differ from case to case, the method should be sensitive to the symptom under investigation.
- Grouping by anatomical lesion. Patients are selected on the basis of having a lesion to a particular anatomical region. This region may have been identified as interesting by previous functional imaging studies. This method need not require that patients have damage exclusively to the region of interest. The patients may have additional damage elsewhere, but, assuming that the other lesions differ from case to case, the method should be sensitive to the region in question (Damasio & Damasio, 1989).
There is no right or wrong way of deciding how to group patients, and to some extent it will depend on the precise question being addressed. The method of grouping cases by syndrome is likely to offer a more coarse level of analysis, whereas grouping according to individual symptoms may provide a more fine-grained level of analysis. In general, the syndrome-based approach may be more appropriate for understanding the neural correlates of a given disease pathology rather than developing theories concerning the neural basis of cognition.
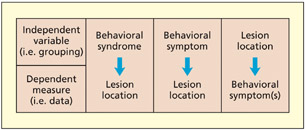
There are at least three different ways of grouping patients to carry out a lesion-deficit analysis.
The method of grouping patients by symptom (2 in the list above) and then finding out what regions of damage they have in common is relatively new. This is made feasible by new techniques that compare the location of lesions from MRI scans of different patients on a voxel-by-voxel basis thus producing a fine-grained statistical map of the likely lesion “hot spot” (Rorden & Karnath, 2004). For example, it has been used to separate out the different contributions of frontal regions in tests of executive function (Shammi & Stuss, 1999; Stuss et al., 2002). One advantage of working forward from a symptom to a lesion location is that it could potentially reveal more than one region as being critically involved. For example, let’s assume that a deficit can arise from damage to either region X or region Y. If one were to initially group patients according to whether they have damage to region X and test for a deficit (3 in the list above), then one could falsely conclude that region X is the key region that gives rise to this deficit and the method would not detect the importance of region Y. The main situation in which one would group patients by lesion site and then test for the presence of a particular symptom (3 in the list above) is if one has a specific testable prediction about what the region is critical for (e.g. the region has been implicated by functional imaging studies).
Caveats and complications
Key Terms
Edema
A swelling of the brain following injury.
Diaschisis
A discrete brain lesion can disrupt the functioning of distant brain regions that are structurally intact.
There are at least two caveats and complications that warrant further discussion. The first concerns the ability of current structural imaging techniques to identify lesions. The second concerns the inferences that can be drawn from lesion-deficit associations that can, if not articulated properly, lapse into neophrenology.
Damasio and Damasio (1989) discuss how certain types of neuropathology are more suited to lesion-deficit analysis than others, at least with current techniques. The most suitable lesions are those in which dead tissue is eventually replaced by cerebrospinal fluid. This is frequently the case in stroke (at least in the chronic rather than acute phase), in damage resulting from the herpes simplex encephalitis (HSE) virus and following neurosurgery. Identifying the site of a lesion caused by a tumor is particularly problematic when the tumor is in situ, but is less problematic once it has been excised. Certain tumors (e.g. gliomas) may infiltrate surrounding tissue and so have no clear boundary, and physical strain around the tumor may cause swelling (termed edema). This distorts the true size and shape of the brain tissue and may render neurons inoperative even if they are not destroyed. Similar arguments apply to the presence of leaked blood during hemorrhage, and the intracranial swelling associated with closed head injury. In general, reliable lesion images are best obtained 3 months after onset and when the neuropsychology testing is carried out at a similar time to the structural imaging (Damasio & Damasio, 1989).
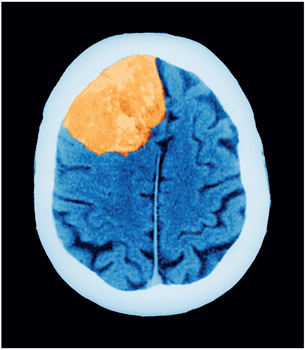
A tumor (here shown on a CT scan) can make it hard to estimate lesion size, and the distortion in the shape of the brain makes it hard to map onto a standard atlas.
On finding that a function (F) is disrupted following a lesion to region X, it is tempting to conclude that function F is located in region X or, worse still, that the purpose of region X is to implement F. These conclusions, and the second one in particular, are tantamount to endorsing a neophrenological view of brain structure–function relationship. Before jumping to such a conclusion, one would need to consider a number of other questions. Is this the only function of region X? Do other regions contribute to the performance of function F, or is this the only region that does so? On finding that a function (F) is disrupted following a lesion to region X, a more cautious conclusion is that region X is critical for performing some aspect of function F. This assertion does not assume that region X has a single function, or that function F has a discrete location. It is also important to note that even a very discrete brain lesion can disrupt the functioning of distant brain regions that are structurally intact; this is termed diaschisis. For example, structural lesions to the left frontal lobe can result in markedly #x003C;pg>reduced activity in other distant regions (e.g. left inferior posterior temporal lobe) during a letter judgment task (Price et al., 2001). This can occur even though this distant region is not lesioned and may function normally in other contexts. The implications are that damage to one region can disrupt the functioning of another, intact, region when these two regions work together to implement a particular cognitive function.
Evaluation
Group studies of patients can be important for establishing whether a given region is critical for performing a given task or tasks. Two broad methods are favored, depending on the hypothesis being addressed. The first method involves establishing (on a case-by-case basis) whether a patient is impaired on a given task and then determining the lesion location(s). The second method involves selecting the group on the basis of a lesion to a predefined area and then establishing what functional deficits the group has. This second method is important for testing predictions derived from functional imaging research.
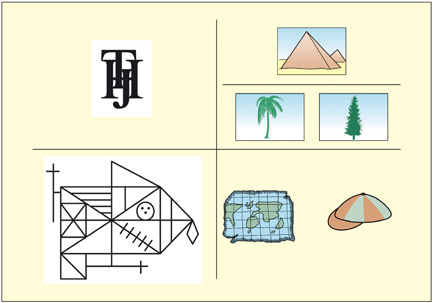
The purpose of a neuropsychological assessment is to ascertain a patient’s level of functioning relative to that expected based on his or her premorbid functioning (Cipolotti & Warrington, 1995a). Some common neuropsychological tests are shown; clockwise from top left: patients with visual recognition problems find it hard to identify overlaid letters relative to non-overlaid ones (from BORB; Riddoch & Humphreys, 1995); patients with semantic memory impairments may find it hard to match the palm tree to the pyramid (Howard & Patterson, 1992); patients with aphasia may find it hard to decide whether things rhyme (from PALPA; Kay et al., 1992); patients with memory problems may be able to copy but not remember this figure
Key Term
Behavioral neuroscience
Cognitive neuroscience in nonhuman animals.
The two main methods that use non-human animals that are considered in this textbook are single-cell recordings (discussed in Chapter 3) and lesion methods. Both of these methods have been greatly assisted by structural MRI scanning enabling individual differences in each animal’s brain anatomy to be taken into consideration when placing electrodes and lesions, and also for determining the extent of lesions in vivo. When non-human animals are used in this way, it is typically referred to as behavioral neuroscience rather than cognitive neuroscience. The implication of this difference in terminology is that humans think but animals behave, or, rather, we know that humans think but we can’t be so sure about other animals.
Although lesion methods in humans rely on naturally occurring lesions, it is possible—surgically—to carry out far more selective lesions on other animals. Unlike human lesions, each animal can serve as its own control by comparing performance before and after the lesion. It is also common to have control groups of animals that have undergone surgery but received no lesion, or a control group with a lesion in an unrelated area. There are various methods for producing experimental lesions in animals (Murray & Baxter, 2006):
- Aspiration. The earliest methods of lesioning involved aspirating brain regions using a suction device and applying a strong current at the end of an electrode tip to seal the wound. These methods could potentially damage both gray matter and the underlying white matter that carries information to distant regions.
- Transection. This involves cutting of discrete white matter bundles such as the corpus callosum (separating the hemispheres) or the fornix (carrying information from the hippocampus).
- Neurochemical lesions. Certain toxins are taken up by selective neurotransmitter systems (e.g. for dopamine or serotonin) and, once inside the cell, they create chemical reactions that kill it. A more recent approach involves toxins that bind to receptors on the surface of cells, allowing for even more specific targeting of particular neurons.
- Reversible “lesions.” Pharmacological manipulations can sometimes produce reversible functional lesions. For example, scopolamine produces a temporary amnesia during the time in which the drug is active. Cooling of parts of the brain also temporarily suppresses neural activity.

A family of macaque monkeys.
Studies of non-human animals have also enabled a more detailed anatomical understanding of the brain and, in particular, the anatomical connectivity between regions. In non-human animals, injecting the enzyme horseradish peroxidase into axons carries a visible tracer back to the cell bodies that send them. The tracer can be visualized at post-mortem. This enables one to ascertain which regions project to a given region (Heimer & Robards, 1981).
While the vast majority of neuroscience research is conducted on rodents, some research is still conducted on non-human primates. In many countries, including in the EU, neuropsychological studies of great apes (e.g. chimpanzees) are not permitted. More distant human relatives used in research include three species of macaque monkeys (rhesus monkey, cynomolgus monkey, and Japanese macaque) and one species of New World primate, the common marmoset. There are a number of difficulties associated with the use of animal models in neuropsychology, not least the concern for the welfare of the animals. Scientists working with these species must provide a justification as to why the research requires primates rather than other animals or other methods, and they must justify the number of animals used. It is also important to have careful breeding programs to avoid having to catch animals in the wild and to protect the animals from viruses. It is important to give them adequate space and social contact. Another disadvantage of animal models is that there are some human traits that do not have obvious counterparts in other species. Language is the most obvious such trait; consciousness is a more controversial one (see Edelman & Seth, 2009).
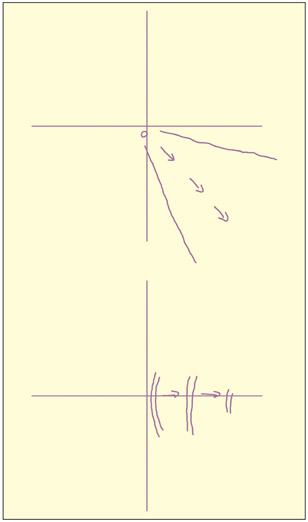
An example of two phosphenes produced by stimulating area V5/MT. Left hemisphere V5/MT stimulation produces right visual field phosphenes moving away from the center. The first was described as “movement of a single point in a static field” and the second as “drifting right, not continuous.”
Attempts to stimulate the brain electrically and magnetically have a long history. Electric currents are strongly reduced by the scalp and skull and are therefore more suitable as an invasive technique on people undergoing surgery. In contrast, magnetic fields do not show this attenuation by the skull. However, the limiting factor in developing this method has been the technical challenge of producing large magnetic fields, associated with rapidly changing currents, using a reasonably small stimulator (for a historical overview, see Walsh and Cowey, 1998). Early attempts at magnetic stimulation were successful at eliciting phosphenes (Magnussen & Stevens, 1914), but this was probably due to stimulation of the retina rather than the brain (Barlow et al., 1947). It was not until 1985 that adequate technology was developed to magnetically stimulate focal regions of the brain (Barker et al., 1985). Since then, the number of publications using this method ology has increased rapidly. Typically, the effects of transcranial magnetic stimu lation (TMS) are small, such that they alter reaction time profiles rather than elicit an overt behavior. But there are instances of the latter. For example, if the coil is placed over the region of the right motor cortex representing the hand, then the subject may experience a sensation or involuntary movement in the left hand (given that the right motor cortex sends movement signals to the left part of the body). If the coil is placed over the right visual cortex, then the subject may report visual sensations or “phosphenes” on the left side (given that the right visual cortex represents the left side of space). Even more specific examples have been documented. Stewart et al., (1999) stimulated a part of the visual cortex dedicated to motion perception (area V5/MT) and reported that these particular phosphenes tended to move. Stimulation in other parts of the visual cortex produces static phosphenes.
How does TMS work?
TMS works by virtue of the principle of electromagnetic induction that was first discovered by Michael Faraday. A change in electric current in a wire (the stimulating coil) generates a magnetic field. The greater the rate of change in electric current, the greater the magnetic field. The magnetic field can then induce a secondary electric current to flow in another wire placed nearby. In the case of TMS, the secondary electric current is induced, not in a metal wire, but in the neurons below the stimulation site. The induced electric current in the neurons is caused by making them “fire” (i.e. generate action potentials) in the same way as they would when responding to stimuli in the environment. The use of the term “magnetic” is something of a misnomer as the magnetic field acts as a bridge between an electric current in the stimulating coil and the current induced in the brain. Pascual-Leone et al. (1999) suggest that “electrodeless, noninvasive electric stimulation” may be more accurate, although it is a less catchy term.
A number of different designs of stimulating coil exist, and the shape of the coil determines how focused the induced current is. One of the most common designs is the figure-of-eight coil. Although the coil itself is quite big, the focal point of stimulation lies at the intersection of the two loops and is about 1 cm2 in area. If you have access to TMS equipment, try holding the coil a few centimeters above your arm. When the pulse is released, you should feel a slight harmless twinge on a small area of skin that is representative of the area of direct stimulation of the brain.
The “virtual lesion”
TMS causes neurons underneath the stimulation site to be activated. If these neurons are involved in performing a critical cognitive function, then stimulating them artificially will disrupt that function. Although the TMS pulse itself is very brief (less than 1 millisecond), the effects on the cortex may last for several tens of ms. As such, the effects of a single TMS pulse are quickly reversed. Although this process is described as a “virtual lesion” or a “reversible lesion,” a more accurate description would be in terms of interference. The neurons are being activated both from an internal source (the task demands themselves) and an external source (the TMS) with the latter disrupting the former. Of course, if the region is not involved in the task, then interference would not occur in this way.
What is the “Visual” Cortex of a Blind Person Used for?
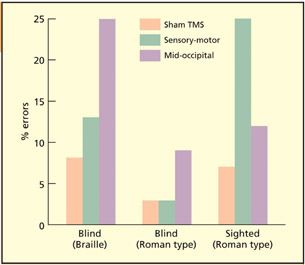
TMS over mid-occipital “visual” cortex impairs tactile identification in the blind, but not in blindfolded sighted people, whereas TMS over sensorimotor (tactile) cortex impairs tactile discrimination in sighted individuals.
Could whole regions of the brain normally dedicated to one type of processing (e.g. vision) take on a completely different functional characteristic (e.g. touch)? A number of studies have investigated the functioning of the visual cortex (in the occipital lobes) in people who were blind from a very early age.
Sadato et al. (1996) conducted a brain imaging study demonstrating that early blind Braille readers showed activity in their primary visual cortex (V1) during Braille reading. This was not found for late blind or sighted individuals with their eyes closed. However, functional imaging methods can reveal increases in activity that may not be functionally critical. It could be, for instance, that the blind readers are trying to use the visual cortex during Braille reading but that this activity is not actually contributing to task performance. To address this, lesion methods are appropriate. Given that early blind people with late brain damage restricted to occipital regions are rare (but see Hamilton et al., 2000), TMS avails itself as the most appropriate method.
Cohen et al. (1997) studied tactile identification of Braille letters in early blind individuals, and tactile identification of embossed letters in roman type in both early blind and (blindfolded) sighted individuals. When they placed their finger on the letter, a train of TMS pulses was delivered. The TMS was delivered to a number of sites, including the mid-occipital (“visual” cortex), the sensory-motor (tactile/motor cortex) and “air” as the control condition. For the blind participants, TMS over mid-occipital regions impaired tactile letter discrimination. This suggests that the “visual” cortex is used for touch in the early blind. Sighted people show disruption when TMS is applied over sensory-motor cortex. It is perhaps surprising that blind people do not additionally show an effect here. It could be that, because they are more skilled, they require a higher intensity of TMS for disruption to be induced. There is evidence for plasticity in somatosensory, as well as mid-occipital, regions in the blind as the region of the brain representing their reading fingers is enlarged by as much as two or three times (Pascual-Leone & Torres, 1993). Similar TMS studies have revealed cortical enlargements are found for skilled racquet players (Pearce et al., 2000), and cortical reductions found for limb amputees (Cohen et al., 1991). These suggest that level of use is critical for plasticity.
Is it likely that any brain region can substitute for the function of another? In this instance, the function of the brain region is largely the same (i.e. it makes fine-grained spatial discriminations) even though in one instance it responds to vision and in another to touch. However, more recent research suggests that the occipital cortex in blind individuals can support tasks of a very different nature (e.g. verb generation; Amedi et al., 2004).

The coil is held against the participant’s head, and a localized magnetic field is generated during performance of the task.
TMS has a number of advantages over traditional lesion methods (Pascual-Leone et al., 1999). The first advantage is that real brain damage may result in a reorganization of the cognitive system (a violation of the transparency assumption) whereas the effects of TMS are brief and reversible. This also means that within-subject designs (i.e. with and without lesion) are possible in TMS that are very rarely found with organic lesions (neurosurgical interventions are an interest ing exception, but in this instance the brains are not strictly premorbidly “normal” given that surgery is warranted). In TMS, the location of the stimulated site can be removed or moved at will. In organic lesions, the brain injury may be larger than the area under investigation and may affect several cognitive processes.
| Advantages of TMS over organic lesions |
Advantages of organic lesions over TMS |
|
|
| • No reorganization/compensation |
• Subcortical lesions can be studied |
|
|
| • Can be used to determine timing of cognition |
• Lesions can be accurately localized with MRI (effects of TMS are less well understood spatially) |
|
|
| • Lesion is focal |
|
|
|
| • Lesion can be moved within the same participant |
• Changes in behavior/cognition are more apparent |
|
|
| • Can study functional integration |
|
Will TMS completely replace traditional neuropsychological methods? Probably not. For one thing, TMS is restricted in the sites that can be stimulated, i.e. those beneath the skull; stimulations elsewhere cannot be studied with TMS. Moreover, the spatial extent of the changes induced by TMS is not fully understood and it is possible that more distant brain structures receive stimulation if they are connected to the stimulation site (Paus, 1999). In contrast, organic lesion localization using MRI is more tried and tested. Another advantage of traditional neuropsychology is that the “accidents of nature” turn up some unexpected and bizarre patterns. For example, some patients can name body parts, but not point to named parts of their body (Semenza & Goodglass, 1985); and some patients can draw a bicycle, but not recognize a drawing of a bicycle (Behrmann et al., 1994). Perhaps these sorts of pattern could also be observed with TMS, but nobody would think to look for them without the patient-based observations. Indeed, the effects of TMS “lesions” are often only observable through slowed reaction times and not through error rates or the externally observable behavior that characterizes most neurological deficits.
Using TMS to study functional integration
The uses of TMS described so far come within the framework of functional specialization: i.e. trying to understand the functional contributions of particular regions to certain aspects of cognition. A complementary approach is functional integration; i.e. trying to understand how one region influences another or how one cognitive function influences another. One way in which this is achieved is by undergoing a session of focal TMS and then studying how this affects the communication between brain regions using fMRI (Bestmann & Feredoes, 2013). (Note: for safety reasons TMS cannot be done in the scanner itself). Another approach is to use TMS to examine competition between brain regions. If there are different processes competing in the brain, then eliminating one process from the competition (using TMS) might have a beneficial effect on the other.
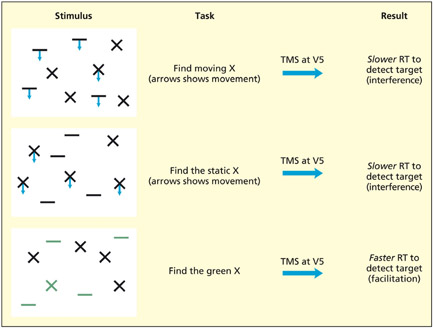
The participants must search for the presence or absence of a specified target (e.g. moving X) in an array of other items. TMS was applied over area V5/MT (involved in visual motion perception) at various points during search. If motion was relevant to the search task, then performance was impaired, but if motion was irrelevant to the search task, then performance was facilitated.
The brain divides up the visual world into different attributes such as color, shape and motion and these different attributes are essentially represented in different regions of the brain (see Chapter 6 for discussion). One theoretical question is: “Do these regions compete with each other, and does attending to one attribute (e.g. motion) have positive or negative consequences for irrelevant attributes (e.g. color)?” To answer this question, Walsh et al. (1998b) presented participants with arrays of different shapes made up of different colors that were either moving or static. The task of the participants was to determine whether a prespecified target (e.g. a moving cross, a static cross, a green cross) was present or absent in the array as quickly as possible. TMS was delivered at area V5/MT (specialized for visual motion perception) at a number of different time intervals, but, for simplicity, the overall pattern across time only will be discussed here. In the first two examples, motion is needed to discriminate between targets and distractors because relying on shape alone will not help (some Xs move and some Xs are static). Unsurprisingly, a virtual lesion to V5/MT disrupts this visual search, as has been found for organic lesions to this area (McLeod et al., 1989). The unexpected finding comes when there is no motion at all and the participants must find a target based on color and form (a green X). In this instance, a virtual lesion to V5/MT facilitates search efficiency. This suggests that different visual areas may compete with each other and eliminating an irrelevant visual area can improve the operation of relevant ones.
Practical aspects of using TMS
When designing experiments using TMS (or when evaluating other people’s choice of design), there are three main considerations: when to deliver the pulses, where to deliver the pulses, and selection of appropriate control conditions (for a good overview, see Robertson et al., 2003). Finally, given that the brain is being stimulated, one must be fully aware of safety and ethical considerations when performing TMS experiments.
Timing issues—repetitive or single pulse?

TMS can be used to establish when in a task a given region is critical. In this experiment, participants had to search for a visual target in an array that was either present or absent. TMS applied over the parietal lobes disrupted performance, but only in specific time windows, with present trials occurring earlier (100 ms; purple line) than absent trials (160 ms; green line). A temporal dissociation such as this could not be observed in patients with irreversible organic brain damage.
The issue of when to deliver the pulse is crucial to the success, or otherwise, of a TMS experiment. On rare occasions, the time taken for a stimulus to be registered in a given brain region is known by previous research using other techniques. For example, single-cell recordings suggest that it takes 100 ms for a visual stimulus to be registered in the primary visual cortex (area V1), and TMS studies in which a single pulse is delivered close to this critical window can render the subject effectively “blind” to the stimulus (Corthout et al., 1999). On most occasions, information such as this will not be known. In this situation, there are a number of options. First, one could make the time of pulse delivery a variable in its own right. For example, if a stimulus is presented for 500 ms, the TMS pulse (or pulses) could be delivered in different time windows (0–50 ms, 50–100 ms, . . . 450–500 ms). This experimental design could thus provide important information about the timing of cognition, as well as providing information about the necessity of that region. An alternative solution is to use a train of pulses during the task (i.e. repetitive or rTMS). In this situation, the experiment becomes potentially more powerful in its ability to detect the necessity of a region, but it would not be possible to draw conclusions about timing because it would be unclear which pulse (or pulses) was critical. Whether or not single-pulse or rTMS is used is not only related to whether timing is an independent variable, but also to the nature of the task itself. Some tasks may require several pulses for TMS to exert interference. The reasons why this might be are not fully understood, but it is a general rule of thumb that TMS studies of perceptual processes have often used single-pulse designs, whereas studies of “higher” cognition (e.g. memory, language) have often used rTMS (Walsh & Rushworth, 1999).
How to hit the spot?
To conduct a TMS experiment, one needs to make some assumptions about which regions of the brain would be interesting to stimulate. In some instances, functional resolution is all that is needed. Just as with the arguments concerning classical versus cognitive neuropsychology, one may wish to establish that a given task/behavior can be selectively disrupted (in which case, the location of the stimulation site is not relevant to the type of conclusion drawn).
Positions on the head can be defined relative to landmarks, such as those used in the EEG system of electrode placement. Skull landmarks include the inion (a bony protrusion at the back of the skull), the anion (the bony ridge between the eyebrows), and the vertex (midway between the anion and inion, and midway between the ears). For example, one published way of approximately locating area V5/MT (dedicated to visual motion perception) is by marking a spot 5 cm in front of the inion, and 3 cm up from it (Walsh et al., 1998a). The spot can be physically marked by placing an X on the skin, or by marking the position on a taut swimming cap. If a precise location is not known before the study, then one could stimulate, say, six different spots lying in a 2 · 3 cm grid, drawn on a swimming cap relative to a fixed skull landmark. Different adjacent positions could then serve as control conditions in the analysis.
Structural and functional MRI can also be used to locate candidate regions of stimulation taking into account individual differences in brain anatomy and skull shape (this is called frameless stereotaxy). A structural or functional MRI scan can be obtained prior to TMS and then online digital registration (using specialist software) enables the position on the skull to be identified. Alternatively, the TMS could be performed prior to a structural MRI scan in which the stimulation sites used have been marked in such a way as to render them visible on the scan. Cod liver oil tablets, attached to the head, have been used previously (Hadland et al., 2001).
What is the appropriate control condition?
Two possible control conditions for TMS experiments have already been considered. First, one can compare performance when the same region is stimulated in critical and non-critical time windows. Second, one can compare stimulation in critical and non-critical regions. Some consideration needs to be given to the selection of the non-critical region. Using regions adjacent to the critical region can provide extra clues about the spatial size of the region of interest. In studies in which there is good reason to believe that the cognitive function is lateralized, one could use the same site on the opposite hemisphere as a control. A further advantage in using the control conditions mentioned above is that peripheral effects of TMS can be minimized. These include the loud sound of the pulse and twitches caused by inadvertent stimulation of the facial nerves and muscles. The latter can be more pronounced at some sites and so using adjacent regions or opposite hemisphere regions would minimize this. “Sham TMS,” in which the coil is held in the air rather than against the head, is not an ideal control condition, and having no TMS at all as a control condition is also not desirable. Another control condition that can be used in TMS experiments is a task control. Thus, the same region can be stimulated at the same times, but with some aspect of the task changed (e.g. the stimuli, the instructions).
Evaluation
TMS is an interesting addition to the cognitive neuroscientist’s toolkit. It is able to ascertain the importance of a given region by stimulating that region during task performance. As such, it is related to other lesion methods that are used for establishing the importance of a given region, but it has certain advantages over the organic lesion method. The main advantage lies in the fact that the interference is short-lived and reversible. It can also be used to explore how regions interact with each other (functional connectivity) and shed light on the timing of cognitive processes.
Safety and Ethical Issues in Tms Research
Researchers need to bear in mind a number of safety issues when conducting TMS experiments. It is essential to be aware of the local regulations that apply in your own institution, but the following points are likely to be important:
- The most recent safety and ethics guidelines come from a consensus of leading researchers in the field that offers guidance on issues such as the number and intensity of pulses (Rossi et al., 2009).
- Whereas single-pulse TMS is generally considered to be safe, repetitive-pulse TMS carries a very small risk of inducing a seizure (Wassermann et al., 1996). Given this risk, participants with epilepsy or a familial history of epilepsy are normally excluded. Participants with pacemakers and medical implants should also be excluded. Credit cards, computer discs and computers should be kept at least 1 m away from the coil.
- The intensity of the pulses that can be delivered is normally specified with respect to the “motor threshold”; that is, the intensity of the pulse, delivered over the motor cortex, that produces a just noticeable motor response (for a discussion of problems with this, see Robertson et al., 2003).
- During the experiment, some participants might experience minor discomfort due to the sound of the pulses and facial twitches. Although each TMS pulse is loud (~100 dB), the duration of each pulse is brief (1 ms). Nonetheless, it is mandatory to protect the ears with earplugs or headphones. When the coil is in certain positions, the facial nerves (as well as the brain) may be stimulated, resulting in involuntary twitches (e.g. blinking, jaw clamping). Participants should be warned of this and told they can exercise their right to withdraw from the study if it causes too much discomfort.
- It is generally believed that a single session of TMS has no long-term consequences. However, repeated participation in experiments could conceivably have longer-term effects—either positive or deleterious. A number of studies report an improvement in mood in depressed individuals following repeated frontal lobe stimulation (George et al., 1995). But this study involved repeated stimulation on a daily basis. Except in cases of therapeutic intervention, it is good practice not to test the same participants many times over a short interval.
Key Terms
Cathodal tDCS
Decreases cortical excitability and decreases performance.
Anodal tDCS
Increases cortical excitability and increases performance.
The use of electrical currents to stimulate the brain has a long and checkered history, with the most notorious noninvasive method being electro-convulsive therapy (ECT) used to “treat” psychiatric illnesses. Unlike ECT, the method of transcranial direct current stimulation (tDCS) uses a very weak electric current. Direct current involves the flow of electric charge from a positive site (an anode) to a negative site (a cathode). In tDCS, a stimulating pad (either anodal or cathodal) is placed over the region of interest and the control pad is placed in a site of no interest (sometimes on the front of the forehead, or sometimes on a distant site such as the shoulders). After a period of stimulation (e.g. 10 min) a cognitive task is performed and this can be compared with sham stimulation, or anodal and cathodal stimulation can be directly contrasted. Cathodal tDCS stimulation tends to disrupt performance (i.e. it is conceptually equivalent to a virtual lesion approach) whereas anodal tDCS stimulation tends to enhance performance (Nitsche et al., 2008). For example, anodal stimulation over visual cortex leads to an enhanced early visual ERP component (N100) and enhances the ability to detect weak visual stimuli, whereas cathodal stimulation has the opposite effects (Accornero et al., 2007; Antal et al., 2001).
Stagg and Nitsche (2011) provide a summary of the likely neurophysiological mechanisms. It is important to consider the immediate effects of direct current stimulation and the aftereffects separately. Animal models of direct current stimulation followed by single-cell recordings have shown that anodal stimulation increases the spontaneous firing rate of neurons whereas cathodal stimulation reduces the firing rate. The immediate effects of stimulation are believed to occur on the resting membrane potential rather than modulation at the synapse. However, the aftereffects of stimulation are likely to occur due to changes in synaptic plasticity influencing learning and perhaps affecting different neurotransmitter systems. Anodal stimulation affects the GABA system (this neurotransmitter has inhibitory effects) whereas cathodal stimulation affects the glutamate system (this neurotransmitter has excitatory effects).
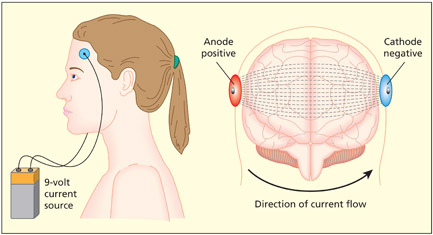
The method of tDCS uses a very weak electric current applied using stimulating pads attached to the scalp. Direct current involves the flow of electric charge from a positive site (an anode) to a negative site (a cathode).
The current safety guidelines recommend upper limits on the size of the current and the surface area of the stimulating electrodes (Nitsche et al., 2003). If the current is concentrated on a small electrode, then it can cause skin irritation. However, unlike TMS, participants often cannot tell whether the machine is switched on or used as sham (there is no sound or twitching). As such there is very little discomfort.
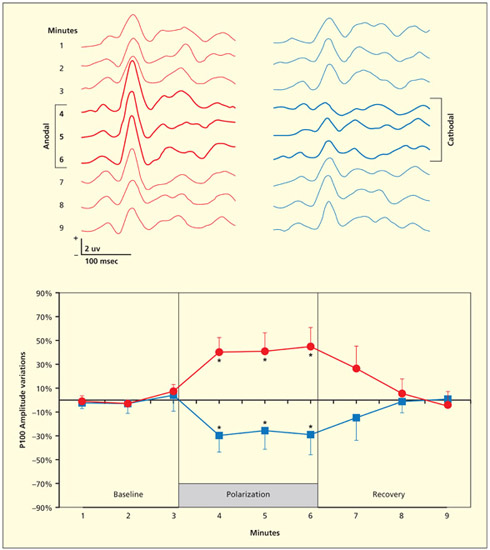
tDCS applied over the visual cortex (for 3 min) disrupts the amplitude of an ERP component (P100) elicited in response to viewing a black and white checkerboard. Anodal stimulation increases the amplitude, but cathodal stimulation reduces it.
Repeated sessions of anodal tDCS are becoming increasingly used for cognitive enhancement (of normal brains) and neurorehabilitation (of damaged brains). For instance, repeated sessions of tDCS over the primary motor cortex leads to increased cortical excitability and greater hand functionality in patients with motor impairments following stroke (Hummel et al., 2005). In this study, the treatment was compared with sham and the procedure was double blind (i.e. neither participant nor experimenter knew which condition they were in). Other studies using anodal tDCS have reported improvements in language following stroke (Monti et al., 2008) and improved working memory in patients with Parkinson’s disease (Boggio et al., 2006).
Summary and Key Points of the Chapter
- A double dissociation between two patients occurs when patient 1 is significantly better than patient 2 on task A, and patient 2 is significantly better than patient 1 on task B. The standard interpretation of this is that task A and task B utilize some different neural resources.
- The use of single cases has led to important insights into the way in which cognitive components are organized and may be fractionated.
- Group studies of patients are important for making links between lesion location and behavioral deficits, and provide an important source of converging evidence for functional imaging data.
- Transcranial magnetic stimulation (TMS) works by stimulating a region of cortex placed beneath a current-carrying coil. This stimulation temporarily interferes with ongoing cognitive activity in that region and, therefore, provides information about the necessity of that region for performing the task. This has been termed a “virtual lesion.”
- Transcranial direct current stimulation (tDCS) has a poorer temporal and spatial resolution to TMS, but has the advantage of being able to facilitate cognitive function (anodal tDCS).
Example Essay Questions
- What assumptions must one accept to be able to draw inferences about normal cognition from adults with brain damage? Are these assumptions plausible?
- Critically evaluate the role of group studies in neuropsychological research.
- What are the advantages and disadvantages of using single cases to draw inferences about normal cognitive functioning?
- How have TMS and tDCS studies contributed to our knowledge of brain plasticity?
- Compare and contrast lesion methods arising from organic brain damage with TMS and tDCS.
Visit the companion website at www.psypress/cw/ward for:
- References to key papers and readings
- Video lectures and interviews on key topics with leading psychologists Elizabeth Warrington and author Jamie Ward, as well as demonstrations of and lectures on brain stimulation
- Multiple choice questions and interactive flashcards to test your knowledge
- Downloadable glossary
Recommended Further Reading
- A special edition of Cognitive Neuropsychology (1988), Vol. 5, No. 5, is dedicated to methodological issues related to single-case and group studies in neuropsychology.
- A special section of the journal Cortex (2003), 39(1), debates the use of dissociations in neuropsychology.
- Pascual-Leone, A., Davey, N. J., Rothwell, J., Wassermann, E. M., & Puri, B. K. (2002). Handbook of transcranial magnetic stimulation. London: Arnold. A detailed account of the methods and uses of TMS, with a strong clinical slant.
- Walsh, V. & Pascual-Leone, A. (2003). Transcranial magnetic stimulation: A neurochronometrics of mind. Cambridge, MA: MIT Press. A detailed account of the methods and uses of TMS, with a cognitive neuroscience slant.
















