CHAPTER 3
Habitat Selection and Oviposition
THE HABITAT
THOSE WHO STUDY Odonata soon become aware that each species, as larva and adult, frequents a particular kind of habitat (Box 4). An adult male of Brachytron pratense characteristically patrols the margins of reeds flanking static or slowly flowing water, and the larva clings tightly to floating or submerged stems of reeds or plants of similar diameter. Dragonflies do not exhibit parental care in the strict sense. The closest they come to doing so is when the female, sometimes guided by her male partner, lays her eggs in a site favourable for development of their progeny.
Some species of Odonata, probably only a few, exhibit philopatry, returning when reproductively mature precisely to the water body whence they emerged. The Palaearctic Lestes barbarus does this,1 but many Odonata apparently disperse widely across the countryside seeking water bodies that meet their needs. The arrival of many species of Anisoptera and Zygoptera at ecologically isolated ponds2 and the rapid colonisation by Odonata of newly constructed water bodies are consistent with this conclusion, although most individuals of some other species (e.g. Coenagrion mercuriale) appear to stay close to the emergence site throughout adult life.3 Having arrived at a water body, adults seem to choose whether to localise there or to move on. Many of the adults seen by Jochen Lempert2 arriving at an ecologically isolated pond departed after briefly approaching it closely.
Thus habitat selection is a regular feature of the odonate behavioural repertoire. Indeed, it is difficult to imagine a behavioural trait that plays a more important role in contributing to the inclusive fitness of a species. As we shall see
A PLACE TO LIVE
The habitat of a species (literally its dwelling place) varies with space and time, over a spectrum of levels of scale, complexity and heterogeneity. Plants play a major role as components of a dragonfly’s biotic environment, especially for species that use plants as egg-laying substrates (Box 7, p.8o). So it can be clarifying to apply different terms to different levels of habitat complexity according to the plantscape – the architecture and texture of plants, at the level of the community and at the level of the foliage structure of individual plants. No consensus exists regarding appropriate terms to describe levels within this hierarchy, but we use the following terms in this book: at one end of the scale is the biotope, an area having a characteristic association of plants and other organisms (the biota), for example, a marsh, a waterlogged peat bog, a willow carr, a stream. A habitat is a place within a biotope where a given species lives. A habitat may be subdivided from a functional point of view into places where different activities are centred; so one can speak of an oviposition habitat, foraging habitat and so on. The term microhabitat is usefully applied to an animal’s chosen surroundings at the most intimate level – for example, the deposits of gravel, sand or silt in which a larva burrows, or the matrix of submerged foliage amongst which a larva sprawls. So, whereas a larva’s habitat may be a lake margin, its microhabitat may be the surface of fine silt at a depth of 1-2 metres in the littoral zone. Quite another concept is the niche, which combines behavioural and ecological parameters with the physical habitat. A species’ niche is its functional role and position in an ecosystem. It might also be termed metaphorically its profession or occupation. For example, the niche of a larva of Lestes sponsa could be described as that of an active, surface-living, plant-dwelling, predominantly diurnal, intermediate predator with a high thermal coefficient for growth and an aversion to water bodies occupied by fish.
in Chapter 5, larvae of different species are closely adapted in many respects to different kinds of habitat and microhabitat; it follows that their prospects of survival will depend to a large extent on the eggs from which they originated being placed in a habitat that suits them.
HABITAT SELECTION
Where habitat selection is concerned, it is useful to distinguish between two properties of a habitat: ultimate factors and proximate cues (Box 5). All British species of Odonata develop in water, be it flowing or static. So we have no need to consider here cues that might be used by those tropical species that develop in tree cavities or in moist leaf litter on the forest floor.5 The received hypothesis, due largely to Hansruedi Wildermuth,6 sees habitat selection as a stepwise process involving successive binary decisions, of increasing precision. At each stage, the searching adult will be making a choice based on a proximate cue. A challenge for the investigator is to discover, for each species, the nature of these proximate cues. An exciting advance towards this end has been the discovery7 that some insects find their aquatic habitats on the basis of reflection-polarisation patterns of skylight visible on water surfaces.8 Polarised ultraviolet light is the critical stimulus, reception of which is mediated by microvilli in the compound eyes.9 Odonata are among the aquatic insects able to detect such stimuli.10 There is little doubt that the first proximate cue to be perceived by a searching dragonfly is the presence of a water surface, detected through a response to the polarisation of light reflected from a horizontal surface.11 By analogy with findings from other aquatic insects, this response would allow the dragonfly to discriminate between different kinds of water body. This is because the sensitivity maxima of the reflection-polarisation receptors of some aquatic insects occupy different parts of the visual spectrum that correlate with patterns of reflected light characteristic of different kinds of aquatic habitat.12 Furthermore, the reflection-polarisation pattern visible at the water surface varies, among other things, according to the depth of the water body, the material composition of the bottom and the dissolved organic materials, and so can convey useful information about the suitability of a site for a searching dragonfly.13 There is a physical basis for the process by which a species is attracted to (or avoids in the case of a teneral adult) an aquatic habitat. Anyone who has flown in a light aircraft low over wetlands facing the sun when it is near the horizon will know how conspicuous the smallest patch of surface water can be (even to the human eye which lacks polarisation receptors). So a dragonfly, flying towards the sun, especially at dusk,13 would have a superb sensory mechanism for locating surface water. A response to the wavelength of reflected light can also permit Odonata to recognise plants used as oviposition substrates, on the basis of light reflected from floating leaves, as in Erythromma najas,14 or from the shape of leaf margins, as in Pyrrhosoma nymphula.15 The experiments
ULTIMATE FACTORS AND PROXIMATE CUES
When the attributes of a habitat that play a role in habitat selection are being analysed it is helpful to distinguish between so-called ultimate factors and proximate cues. Ultimate factors are those properties of the environment that exert selection pressure that maintains a specific kind of behaviour. An example would be predation over evolutionary time that affects larval survival. Predation correlates, among other things, with the presence of submerged aquatic plants that provide refuges from predators. The plants could provide proximate cues – features of the environment detectable by adults and to which they respond when choosing a habitat. Proximate cues are presumably reliable indicators of ultimate factors and represent signposts guiding the choice of a habitat in which a species’ inclusive fitness will be enhanced. An example of a proximate cue for habitat selection by a species of Calopteryx would be current speed, used by the female (and perhaps also the male) when choosing a site for copulation and therefore also for subsequent oviposition. Current speed correlates with, among other things, the susceptibility of eggs to parasitismby minute wasps.4 In this case, it has been suggested that current speed, the proximate cue, is being used as an indicator of an ultimate factor, parasitism risk, which affects the inclusive fitness of the ovipositing female and her male partner, who are unable to detect the level of potential parasitism directly.
leading to these conclusions have greatly increased our understanding of the crucial, early stages in habitat selection. It is no exaggeration to say that a response to polarised light is the most important mechanism that guides Odonata in their selection of habitats and oviposition sites.16
The determining role played by horizontal reflection polarisation in habitat selection explains how dragonflies sometimes alight, or oviposit, on unsuitable substrates, such as the bodywork of cars,17 reflections from which are strongly polarised,18 and oil lakes by which dragonflies (such as Aeshna mixta, Anax imperator and Sympetrum vulgatum) can be deceived, attracted and trapped.19 The observation of Cordulegaster boltonii striking the surface of an asphalt road with its abdomen20 presumably has a similar explanation. It is not uncommon for riverine dragonflies to be deceived by roads: Hansruedi Wildermuth watched a male of the riverine aeshnid, Boyeria irene, searching intensely for females along the border of a road that had structures resembling those at streams.21
The occasional occurrence of some normally riverine Odonata in standing water22 may result from agitation of the water surface that might make it resemble flowing water. Some Odonata are notoriously catholic in their habitat choice: Aeshna cyanea larvae have been found in small coastal pools just below the splash zone.23
Here, sounding a cautionary note, we should mention a remarkable observation of an adult Cordulegaster boltonii (an obligate stream-dweller) following precisely, for more than 100 metres, the course of a stream that had been totally enclosed within a culvert hidden beneath pavement.24 Clearly much remains to be elucidated about proximate cues that dragonflies use for habitat selection.
Having arrived at a water body, the dragonfly must exercise choice based on features that we know empirically to be correlated with its ecological tolerance, such as the size and shape of the water body, the disposition of emergent plants around the margin, the degree of shading, the riparian ground cover (which may comprise meadows or woodlands), and floating plants or algal mats. Mowing of riparian meadows lowered the density and changed the species composition, density and dispersal rate of zygopterans occupying a lowland brook near Freiburg.25 This result of habitat modification emphasises the great importance of terrestrial habitats for dragonfly assemblages and therefore, one supposes, for habitat selection.
Some species exhibit a wide range of ecological tolerance, that is to say they are eurytopic. By definition, they exercise little further choice once they have located a body of water. Among British Odonata, eurytopic species include Enallagma cyathigerum and Ischnura elegans. To learn more about the later steps (if any) in habitat selection, we must look at so-called stenotopic species, namely those with a narrow ecological tolerance. It is from such species that the notion derives that some species occur exclusively in habitats possessing certain attributes. Although the concept is appealing to the tidy minded, and although (in field experiments) some species do indeed exhibit marked and highly predictable selectivity when choosing oviposition substrates, few if any species comply strictly with this concept. In field experiments Platycnemis pennipes can exhibit marked and highly predictable selectivity when choosing an oviposition substrate (e.g. flower heads of the water lily Nuphar lutea)26 but this may reflect little more than the alternatives offered in the choice experiment, because this species can sometimes be abundant at sites where this water lily is absent. For many years it was supposed that the Palaearctic Aeshna viridis reproduced only in habitats containing the Water Soldier, Stratiotes aloides, a floating plant with spiny leaves in which females oviposit and larvae dwell. A survey by Oliver Leyshon and Norman Moore revealed that Aeshna isosceles is also closely associated with this plant.27 As with A. viridis, the association, though strikingly close, is not invariable. In a survey at Castle Marshes, Barnby, Suffolk, during 1991-2, F-0 exuviae of A. isosceles were found in nine dykes and sections with S. aloides and in none without. Exuviae were confined to dykes having a high density of S. aloides, and all exuviae were found within 1 metre of an S. aloides plant, many being attached to it. Likewise, most adult male and female A. isosceles were observed on dykes containing abundant S. aloides, and oviposition was confined to dykes having a dense population of this plant. Interestingly, A. isosceles is virtually confined to water bodies with S. aloides in Britain but not on the continent of Europe. The reason for the close association between dragonfly and plant in Britain is unknown. However, abundance of S. aloides indicates unpolluted water and therefore a rich aquatic invertebrate fauna.28 Thus it may be that, at the edges of its range (as in Britain), or in areas of low background productivity, the presence of S. aloides is a useful proximate cue indicating a rich source of prey for larvae. In Britain, A. isosceles is now restricted to a few grazing marshes which are relatively isolated from polluted water.29 In the Netherlands and Germany, where Aeshna viridis is associated with S. aloides, if such habitats are left unmanaged, in due course they become unsuitable for continued occupancy by S. aloides because this plant represents a successional stage. As it happens, farmers harvest the S. aloides for pig food and by doing so arrest ecological succession and thus secure the continued occupancy of water bodies by the plant and therefore also by A. viridis!30 Exceptions to the association between A. viridis and S. aloides exist, notably in the Netherlands31 and in the eastern Palaearctic where the species is associated with willow bushes.32 So the concept of linkage to a certain plant species still remains theoretical if considered in the absolute, at least as far as Odonata are concerned, although several species, in temperate and tropical regions, show a very high correlation between habitat occupancy and the presence of a given species of plant.33
One result of stenotopy is that a species having very circumscribed habitat requirements will, by definition, encounter less competition for space and other resources. By the same token a stenotopic species will probably be more vulnerable to habitat modification. An extreme example is the small, delicate coenagrionid Nehalennia speciosa, now a severely threatened, continuously declining, vanishing relic in mainland Europe. It occupies an unusually narrow ecological niche, analysed in detail by Rafal Barnard and Hansruedi Wildermuth,34 and exhibits poor dispersal.
We conclude that a neat, mechanistic explanation of the correlation between the occurrence of certain species and habitat type remains elusive, supporting the realist’s dictum that ‘the only thing that fits a pigeon hole is a pigeon’. Be this as it may, in Britain the presence of S. aloides may well be a proximate cue for A. isosceles for a stem habitat (Box 4, p.70).
The fact remains that species of Odonata can be usefully associated with certain habitat types if these are categorised at the appropriate level of detail. For example, as described in Chapter 2, each of the following habitat types in Britain has its own characteristic assemblage of Odonata: lowland rivers, streams and upland rivers, bogs, moorland and lowland wet heath, levels, fens and grazing marshes, and ponds and gravel pits.35
An intensive study of discrete populations of Coenagrion mercuriale in Britain has shown this species to be associated with several habitat features, the most important of which are a channel substrate consisting primarily of silt, wide underwater ledges, and in-channel emergent dicotyledons and bankside monocotyledons; and its presence is negatively associated with bankside trees.36 Although this generalisation applies to habitat selection by C. mercuriale in Britain, it is not necessarily valid throughout the species’ range. In continental Europe this species can be found in habitats of at least two kinds: calcareous spring mires in the foothills of the Alps and meadow streams and ditches.37 So C. mercuriale may occupy what appear to be very different types of habitat in different parts of its range, and the same is true of Ceriagrion tenellum, the distribution of which is limited by winter cold in the north-eastern part of its range.38 This heterogeneity of habitat selection may be one evolutionary route to population isolation and subsequent speciation. The process may begin with the occurrence within a species’ range of habitats that are unevenly suitable for survival, perhaps across stages of the life cycle. Oxygastra curtisii, for example, is found only in lakes in Switzerland, but in southwest Europe almost exclusively in running water.39
When species are found to be occupying what appear (to the observer) to be very different habitats, it can be salutary to bear in mind that there may be some attribute common to both types of habitat that has escaped notice. For example, Ischnura pumilio in Britain was formerly thought to be acidophilic because it seemed to be restricted to acid seepages. Then populations were found in seepages in chalk pits, after which it was concluded that its habitat requirement was shallow, warm water.40
Another example of a species that appears to have more narrowly constrained
METAPOPULATIONS
Aquatic habitats occupied by dragonflies vary considerably in the opportunities they offer for reproduction and population maintenance. This realisation underlies the concept of the metapopulation, as a group of populations within which population dynamics operates at two levels: within patches and between patches.54 One subpopulation may go extinct, later to be re-established by colonisation from another subpopulation. A study of Aeshna subarctica by Klaus Sternberg55 illustrates the way in which habitats of varying suitability contribute to the reproductive potential of a species. Components of metapopulations in southwestern Germany exist in three kinds of habitat, designated according to the opportunities they offer for reproduction.
1) Stem habitats (Fig. 44) function as distribution centres, their large (‘source’)53 populations being self-supporting for many years.
2) Secondary habitats represent population reserves, their small (‘sink’)56 populations being self-supporting only for some years and depending on immigration to survive; for Lestes viridis, a species that breeds in temporary as well as permanent water bodies, temporary ponds contain sink populations in dry years and are quickly recolonised after the local population has been extinguished.57
3) Latency habitats (also harbouring sink populations) are the least productive (of emerging adults), being inhabited mainly by larvae. Adults (usually only females) are found sporadically. Such habitats contribute to the stability of a metapopulation by providing stepping stones for the colonisation of other habitat types.
Survival of a metapopulation of Aeshna subarctica depends on intensive interaction and exchange among several subpopulations occupying different types of habitat. The concept of metapopulations has important implications for nature conservation, especially in regard to habitat management and to the interpretation of distribution records.
FIG 44. The Fish Pond, Wokefield Common, Berkshire, viewed from the northern end in late May 1953. At that time it was a stem habitat for Anax imperator and Pyrrhosoma nymphula. In 1952 more than 4,300 individuals of A. imperator and several hundred P. nymphula emerged from it. The expanse of water in the foreground covers a sward of Shoreweed, Littorella uniflora; in the middle distance are floating mats of Marsh St. John’s Wort, Hypericum elodes; and beyond them is a large patch of Broad-leaved Pondweed, Potamogeton natans. Most emergence of A. imperator took place on the oak trees and willow bushes at the southern end near the dam. (From Corbet.58) (Photo S. Beaufoy.)
habitat associations in Britain than in the rest of its range is Platycnemis pennipes. In Britain the species is restricted to slow-flowing rivers, whereas in northern France it is more widespread. Large populations can be found on ordinary farm ponds in Brittany. A similar ‘edge-of-range effect’ is found in other insect groups (Chapter 2).
The occurrence of Calopteryx splendens appears to be positively correlated with the height of riverside vegetation, and to be negatively correlated with the height of bankside trees and the bank.41
When flying above water, both sexes of Calopteryx dimidiata (a Nearctic species closely related to the British species of Calopteryx) can detect oviposition substrates that are completely submerged.42 This raises the possibility that searching adults might be able to detect the presence in a water body of submerged predators, such as fish or frogs. Because fish are major predators of odonate larvae, especially Zygoptera, an ability by the ovipositing female to detect fish would seem to be of high selective value. In North America, though apparently not in Europe, there are species assemblages of Odonata (especially in the genus Enallagma) that are confined either to lakes with fish or to lakes without fish – the so-called ‘winterkill’ lakes43 – and, correlated with the fact that their main predators are fish in the former and anisopteran larvae in the latter, the innate antipredation behaviour of their larvae differs accordingly.43 Yet, curiously, it seems that searching adults of species inhabiting fishless lakes cannot discriminate between lakes with and without fish,44 despite the obvious selective advantage of doing so. At the present state of our knowledge, differences in species distribution among lakes are provisionally attributed not to active selection of different ‘lake types’, but to the low propensity of adults to disperse from their natal lakes,45 coupled, presumably, with poor survival in lakes with fish.
When one reflects on the wide range of microhabitats occupied by larvae, whose morphology and behaviour differ according to whether they are burrowers, claspers, hiders or sprawlers (p.121), it is remarkable that adults seem to make an appropriate choice so frequently. Both sexes do so by choosing a rendezvous, and females, sometimes with male assistance, do so by choosing an oviposition site, which may or may not share properties with that rendezvous.
Certain species offer us strong clues about habitat choice. Examples are to be found among so-called ‘species pairs’, both members of which occupy rather similar habitats, but seldom occur together, such as Calopteryx virgo and C. splendens, and, in Germany, Cordulegaster boltonii and C. bidentata. Early studies by Rudolf Zahner46 suggested that C. splendens differs from C. virgo in avoiding streams less than 60 centimetres wide, or free water with an area of less than 0.5 metre. Both species, typically but not invariably, avoid lakes (perhaps acting on an area assessment) and, by analogy with congeneric species (such as C. haemorrhoidalis),4 females of both probably use current speed as a cue during the precopulatory courtship display.
Proximate cues that serve for habitat selection can be inferred by noting the common or invariable attributes of habitats occupied by the species in question. On this basis, Enallagma cyathigerum is inferred to need standing water with vertical structures along the shoreline (perhaps used for oviposition and emergence).47 Hansruedi Wildermuth believes this species to be restricted to water bodies with a rather large water surface, with patches of vegetation occupying no more than an area of about 10 x 10 metres.48 In some places, Erythromma najas apparently needs floating Yellow Water Lily, Nuphar lutea, or White Water Lily, Nymphaea alba, shallow water (no more than 4 metres deep), marginal reeds and woodland close by.49 However, other studies have revealed that floating mats of algae or Potamogeton natans, Broad-leaved Pondweed, can also be suitable. Erythromma viridulum needs fine-leaved, submerged vegetation, parts of which must reach the water surface.50 For Coenagrion mercuriale it appears that the main proximate cues are water velocity and the nature of the aquatic and bank vegetation.51 Habitat selection by Gomphus vulgatissimus may depend on water velocity which is itself a correlate of sediment particle size.52 Females of Onychogomphus uncatus and Ophiogomphus cecilia oviposit in running water in slowly flowing stretches, where relatively fine sediment will accumulate.53
CHOICE OF OVIPOSITION SUBSTRATE
Species that oviposit endophytically (Box 7) can easily be seen to discriminate among the substrates into which they place their eggs, using the numerous sensory receptors on the ovipositor.59 A degree of discrimination probably exists also in species that oviposit exophytically, although this may be less obvious. For example, an ovipositing female of Libellula depressa touches the water surface with the tip of her abdomen many times before starting to oviposit, presumably testing the substrate, which may be floating plants, especially algae. The laid eggs then form discrete clumps resting on the vegetation.60
In nature, egg-laying females of endophytic species pay attention to a wide range of substrates (usually plants). On the other hand, they show a high level of discrimination during choice experiments. For example, although ovipositing females of Platycnemis pennipes have been seen to associate in nature with at least 30 species of plant,61 in choice experiments they exhibited a strong preference for ovipositing in flower heads of the Yellow Water Lily, Nuphar lutea, discriminating with respect to colour, age, size, and verticality of the bloom.26 Several factors may contribute to this apparent paradox. If a preferred substrate is hard to find, a female may recognise a hierarchy of progressively less-favoured substrates on which she may land and probe; further, as Andreas Martens and others have stressed, a female seen to make oviposition movements on a substrate is not necessarily laying eggs there. It is not always possible to distinguish between simulated and actual oviposition by simple observation. Among Platycnemis pennipes ovipositing in flower heads of Nuphar lutea, 89 per cent of apparent oviposition activity resulted in actual oviposition. On Spiked Water-milfoil, Myriophyllum spicatum, this value was 83 per cent. For the rest of the time spent on these plants, females probed without laying.61 On the whole, very brief visits to a substrate did not result in oviposition there. But for stays of more than 40 seconds the duration of oviposition behaviour correlated closely
OVIPOSITION
Depending on the species and the substrate, dragonflies lay eggs (oviposit) in various ways, most of which are encountered in British species. The three main ways in which eggs are laid are:
1) endophytically, the eggs being inserted into plants or other substrates, (Figs 45 & 46), such as mud, using the valvulae of the ovipositor;
2) exophytically, the eggs being washed off the tip of the abdomen (Fig. 47) onto the water surface; and
3) epiphytically, the eggs being stuck to vegetation at or above the water surface.
British Odonata that oviposit endophytically comprise all Zygoptera and all Aeshnidae. Functionally, Cordulegaster boltonii also belongs in this category because it thrusts its eggs into sand or mud at the edge of a stream, using a hyperdeveloped, sclerotised ovipositor. Species that oviposit exophytically comprise all Libellulidae and Corduliidae, and Gomphus vulgatissimus. Sometimes the two species of Somatochlora oviposit epiphytically by placing eggs on the surface of moss near the water’s edge. Some libellulids in other countries possess bilateral, projecting foliations on the eighth abdominal tergite which the female uses, when ovipositing exophytically and in flight, to scoop up a drop of water and flick it, together with some eggs, toward or onto the bank. In some such species, this behaviour places the eggs above the prevailing water level, thus delaying hatching until the water level rises and submerges them. No British species has yet been recorded as ovipositing in this way, though it is possible that species of Orthetrum may occasionally do so.
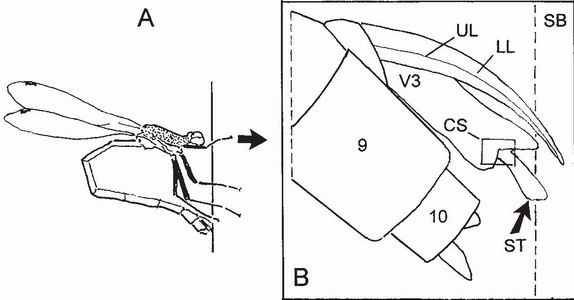
FIG 45. Diagram of component parts of the endophytic ovipositor of Lestes sponsa when in contact with an oviposition substrate, such as a plant stem (accompanying male not shown). A) posture of female (body length about 38 mm); B) placement of ovipositor (much enlarged); CS, location of campaniform sensilla; LL, lower leaves of ovipositor; SB, substrate; ST, stylus; UL, upper leaves of ovipositor; V3, third valvulae; 9, 10, abdominal segments. (Redrawn after Matushkina and Gorb.59)

FIG 46. Aeshna grandis ovipositing endophytically into a decaying log (Robert Thompson).
FIG 47. Tip of abdomen, seen from below, of an exophytic anisopteran, Sympetrum striolatum. Abdominal segments 8 and 9 are numbered. (After Askew.61)
with the total number of eggs laid.62 Simple observations of oviposition movements do not necessarily constitute a record of reproduction at a site or of substrate choice.
Our understanding of substrate choice is confounded by actions that appear to be wayward or maladaptive. There are bizarre examples, mostly of female aeshnids, laying eggs (or at least performing oviposition movements) in manifestly unsuitable substrates that happen to be close to a pond. A female Aeshna cyanea laid at least one egg in a brown, woollen, knitted pullover worn by Roderick Dunn, then Secretary of the BDS, as he sat beside a pond;63 and another female A. cyanea was seen making oviposition movements upon a rubber boot worn by Sally Corbet, another member of the BDS, as she stood near a pond.64 Hansruedi Wildermuth, the distinguished Swiss odonatologist, standing barefoot by his garden pond, received attention from a female A. cyanea who set about ovipositing in his ankle. She tickled him but did not penetrate, showing that she was probing, not ovipositing.65 A fourth female of A. cyanea was seen by Mike Parr, then President of the Worldwide Dragonfly Association, making oviposition movements upon the rump of his dog (a beagle called Oscar), as the latter lay on mown grass by a pond’s edge.66 Interestingly, Oscar exhibited noteworthy indifference throughout. However, it would be premature to conclude that A. cyanea, when using anomalous substrates, chooses only those associated with odonatologists. This species often lays in decaying wood near pond margins. Perhaps the award for the most bizarre, inappropriate substrate should go to the female A. cyanea seen by Bernard Schmidt probing the skin of a male Yellow-bellied Toad, Bombina variegata. As soon as the probing commenced the toad submerged, washing the dragonfly into the water whereupon she was grasped by a conspecific male who copulated with her.67 Another observation deserving mention involved an aeshnid making oviposition movements on the back of a carp protruding above the water surface at Trentham Park, near Stoke-on-Trent.68 The dragonfly, which was photographed but not closely examined, could have been A. cyanea. We can expect to encounter further observations of this kind. It is to be hoped that, in some cases, it may be possible to discover whether or not eggs have actually been laid in such apparently unsuitable substrates.
The anomalous oviposition substrates we have described may share certain textural properties with decaying wood, although it is not obvious what these properties might be. What could be the selective value, if any, of such substrate choices? We need to understand the ultimate factors that correlate with the

FIG 48. A tandem pair of Lestes sponsa before ovipositing endophytically into the stem of a Horsetail, Equisetum (Robert Thompson).
choice of oviposition substrate. A knowledge of how prolarvae reach the water after the eggs hatch, about which we know almost nothing at present, should illuminate this question.
ARRANGEMENT OF LAID EGGS
It has long been known that the laid eggs of Zygoptera are arranged in patterns characteristic of a given species.69 Matushkina and Gorb have investigated the sensory systems that regulate the positioning of eggs in an egg set – the eggs laid during a bout.59 The ovipositor is richly endowed with sense organs, including campaniform sensilla (usually associated with strain detection) which occur on the upper and lower leaves of the ovipositor and at the base of the style (Fig. 45, p.81). Lestes sponsa lays up to four eggs in each incision, adjusting the angle of each to the direction of the egg line so that this angle diminishes with each successive egg laid. The angle of the whole egg line, which is usually related to the orientation of fibres in the substrate, is regulated by the styli. Many eggs can be laid by many females in a single plant stem, and it has been suggested that regular positioning may help to keep eggs of successive sets apart and so reduce damage to eggs laid previously.59 Egg sets can be arranged in one of three patterns: irregular; regular simple; and regular complex, where several eggs occupy one incision. Such arrangements probably reflect physical properties of the oviposition substrate.70 Egg sets of Zygoptera have been found in dicotyledonous leaves from the Cretaceous period (about 90 million years before present).71
GUARDED OVIPOSITION
During guarded oviposition the female’s last copulation partner remains close to her while she oviposits. If he retains his grip on her head (as in Anisoptera) or prothorax (as in Zygoptera), the behaviour is termed ‘contact guarding’, resulting in ‘tandem oviposition’ (Fig. 50). If the male relinquishes his grip but hovers near the female, the behaviour is termed ‘non-contact guarding’. Among British species, tandem oviposition occurs in all Zygoptera except the two species of Calopteryx, Enallagma cyathigerum, Ischnura pumilio and I. elegans; it occurs facultatively in certain Libellulidae and Anax parthenope. All species that oviposit in tandem sometimes also do so alone. The species of Calopteryx engage in non-contact guarding, and in Enallagma cyathigerum the oviposition flight begins in tandem and then the female eludes the male by descending beneath the water surface to oviposit while he remains above, hovered or settled, often close to the stem she used for her descent.
Unattached males can be aggressive towards tandem pairs, trying to dislodge the male partner, sometimes even submerging to achieve a takeover.72 Lestes sponsa males exhibit unusually possessive guarding during oviposition. This may reflect several circumstances that bear on sperm competition (pp.253): high female receptivity after copulation; high male capacity to resist takeover; high male density and operational sex ratio; and a short interval between copulation and oviposition.73 Several species of Anisoptera, mainly Libellulidae, exhibit guarded oviposition, the choice of mode being facultative:74 when potential interference by conspecific males is high, the tandem mode is liable to be adopted, but when such interference is low, non-contact guarding is more common.75 One obvious consequence of tandem oviposition is that the accompanying male, because of his physical position, is preventing any rival from usurping him as parent of the eggs the female is laying. If the accompanying male can prevent his female partner from copulating again before she has laid most or all of her current clutch of eggs, he can sometimes virtually ensure that it will be his sperm that fertilises her eggs. His status as father of her current progeny can, however, be jeopardised if she postpones oviposition after copulating with him because, by about 24 hours after copulation, the sperm in her storage organs becomes mixed so that the sperm she receives last ceases to enjoy priority for fertilising any eggs that are then laid.76 So another benefit to the male that may accrue from contact or non-contact guarding may be that after copulation he can induce his partner to oviposit promptly. Such a function of guarding has not yet been demonstrated unequivocally for Zygoptera, but the male of some Anisoptera (such as Orthetrum coerulescens) has been seen to nudge his partner immediately after copulation in a way that induces her to begin ovipositing.77 Ingenious field experiments, conducted by Andreas Martens and Gunnar Rehfeldt of Braunschweig University, have revealed other selective benefits of guarded oviposition. In several species of small Zygoptera, namely Platycnemis pennipes, Coenagrion puella, C. pulchellum, Pyrrhosoma nymphula and Ceriagrion tenellum, the male adopts the ‘Agrion’ or sentinel position when the female is ovipositing (Fig. 50). This posture has several consequences that may enhance the fitness of both partners:
- the male greatly extends his field of vision, thus being able to detect predators (such as frogs) sooner;
- the tandem pair can alight on (and therefore use) smaller areas of substrate than would otherwise be the case because the male requires no horizontal space for himself;
- by analogy with the obelisk posture, the vertical posture of the male may reduce his thermal gain under bright sunlight, perhaps meeting a physiological need for species that oviposit during the heat of the day; and
- the vertical posture may offer greater protection from takeover by rival males than the horizontal posture adopted by certain Anisoptera.78 When libellulids contact guard during exophytic oviposition, the male himself contributes to the oviposition manoeuvres by making movements that lower and raise the female. An example is Sympetrum striolatum, in which the active role of the male can be demonstrated by observing the flight of a male in tandem with a dead female.79
The other mode of post-copulatory guarding is non-contact guarding, in which the male stays close to, but not linked to, his most recent copulation partner while she oviposits, although he may not remain with her during the whole bout of oviposition. The flexible nature of this behaviour, often seen in species of Sympetrum, presumably reflects the trade-off for the male between protecting his genetic investment in the progeny of the guarded female and being free to respond to mating opportunities with new females.80 A female whose guarding partner leaves her may oviposit more slowly thereafter.81
The Japanese coenagrionid Pseudagrion pilidorsum sometimes continues to contact guard his mate while she submerges, but balances contact and non-contact guarding according to the need to defend his territory (above the water surface) from other males.82 The male of the European Enallagma cyathigerum hardly ever accompanies his mate when she submerges; he normally releases the female as soon as she submerges unless there is strong harassment from other males, in which case he may retain the tandem grip until he becomes completely submerged.82
It is rare among Zygoptera for the female always to oviposit alone, although this is the case in most species of Ischnura, including the two that occur in Britain. The reproductive behaviour of I. elegans is anomalous in another respect: females exhibit aggressive behaviour toward males that encroach on their oviposition sites.83 A remarkable type of guarding, quite unlike anything so far described in this chapter, is shown by Sympetrum depressiusculum, a Palaearctic species that exists in high-density populations in southern France. There males form tandems with females away from water at nocturnal roosting sites in the early morning (where presumably females are relatively easy to find and grasp) and retain their grip until about three hours later when copulation and oviposition (in tandem) ensue.84 This type of association has been termed ‘precopulatory guarding’ and has been interpreted as a means whereby, when competition for receptive females is intense, a male that finds a female can sequester her until a time of day when the ambient temperature has risen enough to permit copulation and oviposition. This type of guarding has not yet been recorded in any British species of dragonfly, although in one British species, Lestes sponsa, males have been seen to form tandems away from water and then to escort females to the oviposition site.73 The behavioural difference between populations may perhaps reflect the number of perching (or roosting) sites close to the water’s edge.
Non-contact guarding occurs regularly among the British species of Calopteryx, and has been reported occasionally among aeshnids. There is an example purportedly involving Anax imperator in continental Europe,85 but this record has subsequently received critical scrutiny.86 The North American migrant, A. junius, regularly oviposits in tandem and occasionally makes landfall in western Europe.87 Anax junius is not easy to distinguish from A. imperator except in the hand. The possibility cannot be dismissed that the anactines seen ovipositing in tandem by Balança and Visscher were not A. imperator but A. junius, which at that time had not yet been recorded from Europe. (It might also have been A. parthenope!) This possibility must remain hypothetical because no voucher specimens were retained. Among aeshnids worldwide, guarding of either kind is rare and facultative.88
GROUP OVIPOSITION
Females sometimes oviposit in conspicuous conspecific groups. A participating female may do this with or without a male in tandem. Such groups can comprise many individuals which, although very close to one another, show little or no interaction. Sometimes Lestes sponsa does this at a density of more than ten tandem pairs per stem.89 British species that routinely exhibit group oviposition include Coenagrion puella, C. pulchellum, Platycnemis pennipes (Fig. 50). Erythromma najas, E. viridulum and Pyrrhosoma nymphula.15,90 It has also been witnessed, perhaps as an exception, in Aeshna grandis.91 In the species of Zygoptera the accompanying male is often in the sentinel position. Theoretically there are two quite different ways in which such aggregations of females could come about.
First, many females, acting independently, could each be attracted to the same highly suitable oviposition site. Second, each new arrival could come because she had been attracted to the presence of conspecific females (or tandem

FIG 50. Platycnemis pennipes ovipositing endophytically, exhibiting tandem and group oviposition (Steve Cham).
pairs) already there. By ingenious use of models and by skilful experiment, Andreas Martens has shown that aggregations of ovipositing Pyrrhosoma nymphula,15 Platycnemis pennipes,92 Coenagrion mercuriale93 and Coenagrion puella94 arise because tandem pairs are positively attracted to pairs already at the site. The phenomenon and its behavioural explanation are securely founded, and this mechanism of aggregation may well apply to other species too. For example, tandems of Coenagrion mercuriale land preferentially on a substrate where a single motionless male is in the sentinel position.93 It is interesting to speculate about ways in which such behaviour might enhance inclusive fitness. Gunnar Rehfeldt has listed two, both of which serve an antipredation function:
1) species that aggregate occupy water bodies frequented by frogs so that group oviposition may enhance survival because the presence of tandems may indicate a low risk of predation by frogs;95 and
2) if several pairs are together in a group, a successful frog is likely to catch only one pair at a time, thereby alarming the rest who can then escape.96
UNDERWATER OVIPOSITION
Predation risk is not confined to species that oviposit above the water surface. Species employing the third mode – underwater oviposition – risk predation by fish, newts and aquatic insects, including beetles (such as Cybister, Fig. 51), waterbugs (such as Notonecta) and even conspecific anisopteran larvae.98 Underwater oviposition is the primary mode in Enallagma cyathigerum in which the oviposition flight begins in tandem and ends with the female submerging by climbing down a plant stem (p.85). Her male partner may accompany her under water, but usually remains above the surface. When she reappears, he or another male grasps her.99 A female E. cyathigerum may descend as far as 1 metre beneath the surface.100 During submergence she probably obtains oxygen from the physical-gill action of the air bubble surrounding her (presumably unwettable) body and wings. Her fore wings, shielded by the closed hind wings, remain dry so that she can take off, perhaps with assistance from a male, after regaining the surface. Sometimes a female briefly interrupts oviposition to visit the surface, presumably to replenish the air in the bubble that invests her body. Several species, including Enallagma cyathigerum, that oviposit under water make rocking movements while submerged or when experiencing oxygen deprivation, flexing and extending the legs at the coxal joints on alternate sides. Peter Miller interpreted such movements as preventing the accumulation of an unmixed
FIG 51. Predation during oviposition. A larva of the Diving Beetle, Cybister lateralimarginalis, attached to the abdomen of a female Aeshna grandis which it had grasped while the latter was ovipositing. (Photograph by J. Ott.97)
boundary layer of oxygen-depleted water around the body.99 Most oviposition by E. cyathigerum witnessed by Miller was into green stems of submerged macrophytes where high oxygen levels could be expected as a product of photosynthesis.
After completing a bout of oviposition, a female Enallagma cyathigerum rises to the surface simply by floating upwards, using for lift the buoyancy of the air bubble around her. Once at the surface, she makes herself conspicuous to searching males by flexing and extending her abdomen repeatedly; she usually lies on her side, whereupon a male almost always grasps her, turning her dorsal side uppermost and either carrying her away in tandem or towing her to a support as a prelude to a brief flight in tandem before trying to copulate with her. Females not assisted in this way probably drown because they seem unable to rise from the surface unaided.99 As far as the male is concerned, his ‘rescue’ behaviour will be rewarded if the female still contains unlaid eggs, accepts copulation and resumes oviposition soon afterwards, although Peter Miller found that almost half the females surfacing spontaneously after oviposition contained no eggs ready to lay and that only about 25 per cent contained at least 50 residual eggs and so would have offered a paternity opportunity for a rescuing male.99
Underwater oviposition has both costs and benefits. A cost is lowered survival through predation or drowning. Possible benefits include thermoregulation by evaporative cooling, freedom from interference by searching males, freedom to choose an oviposition site, a heightened assurance that the laid eggs will not be subject to desiccation after a fall in water level, and a reduced likelihood that the eggs will be parasitised by Hymenoptera (p.104).
Until recently it was assumed that only Zygoptera oviposited under water. Now it is known that at least two species of Australian Aeshnidae can do so,101 but it is a rare event in Anisoptera. In Japan, Aeshna juncea ovipositing in mud has been seen to submerge completely except for the head and wings.102 Aeshna grandis will back down into the water to the level of the hind wings.
PREDATION
We may expect predation to be greater on dragonflies ovipositing while settled than on those doing so in flight. The main predators of species ovipositing endophytically are frogs, fishes, birds (such as wagtails) and certain aquatic insects, including anisopteran larvae.98 The tandem position does not necessarily protect the female from predators that approach from beneath the water surface because the female’s abdomen often probes the underside of a floating leaf. As we have already seen, guarded oviposition, especially in the sentinel position, serves as anti-predation behaviour as far as predators visible from above the surface are concerned. Underwater oviposition involves an obvious trade-off between freedom from male interference on the one hand, and increased exposure to attack by aquatic predators, especially fish and waterbugs (such as Notonecta), on the other. The anti-predation value of tandem oviposition is illustrated by ovipositing females of Coenagrion puella when harassed by Notonecta. Most solitary females that were grasped were killed, and those solitary females who escaped left the site, whereas females ovipositing in tandem remained.103
Females preoccupied with oviposition may be especially susceptible to predation. Hornets, Vespa crabro, have been seen to prey on aggregated tandems of Sympetrum sanguineum.104
DIEL PATTERN
Once a female has been inseminated and is ready to oviposit, she risks being interrupted by interference from searching males. One strategy, as we have seen, is to oviposit in tandem. Another is to oviposit under water. With the exception of Anax parthenope, neither option seems to be available to female aeshnids which (in British populations at least) are not known to oviposit in tandem. Moreover, aeshnids, with some notable exceptions, differ fundamentally from most Zygoptera and Libellulidae in that several days elapse between copulation and oviposition.105 A female bent on oviposition runs the risk of being detected and grasped by a searching male when she arrives at an oviposition site. It is probably to avoid or mitigate such interference that, in some habitats, females of Aeshna cyanea prefer to oviposit early or late in the day when males are not present. At one such habitat in southern Germany, receptive females arrived at the rendezvous, which was also an oviposition site, throughout the day, whereas most of the non-receptive females (namely those coming solely to oviposit) arrived in the evening, after most males had departed.106 Likewise, at a habitat in the southeastern Alps, females of Aeshna juncea oviposited in small numbers throughout the day but showed a clear peak of abundance during evening twilight when no male was present.107 Non-receptive females of Cordulia aenea have been observed to oviposit late in the day or during overcast weather when fewer males are present at water.108 In Germany, females of Aeshna mixta and A. cyanea oviposit in the early morning, thereby escaping the attentions of conspecific males, but no such strategy is exhibited by Coenagrion puella or Ischnura elegans.109 This raises the possibility that endothermic species can use this strategy whereas Zygoptera cannot. The diel periodicity of ovipositing Anax imperator coincides with that of males, but females possess effective means of rejecting males:110 they flex the abdomen downwards, almost at right angles, as they fly away close to the water surface. Some coenagrionids behave likewise. David Thompson has pointed out that this posture constitutes an ‘honest signal’ to males that the female is a poor prospect for mating because it can only be adopted when her abdomen is devoid of eggs.111 Evidently pursuing males quickly get the message! Non-receptive females of some corduliids exhibit unequivocal escape behaviour. Females of Somatochlora arctica and S. metallica were seen arriving inconspicuously at small water bodies, flying very low and ovipositing at hidden places amongst plants. As soon as one was detected by a male she fled from the water towards adjacent forest, followed by the male. Sometimes, when a male came very close, a female would plunge into riparian sedge and feign death, whereupon her pursuer would stop and hover at the site, apparently unable to locate her.112
RATE
The rate of oviposition (the number of eggs laid per unit time) correlates with the oviposition mode. Endophytic species, as might be expected, lay eggs relatively slowly, at about 1-22 eggs/minute.113 The rate may vary according to the type of substrate. Five species of Coenagrionidae have been observed to lay at between 2.0 and 7.1 eggs/minute.114 Exophytic species, by contrast, may lay at rates greatly exceeding 1,000 eggs/minute. It has been demonstrated that the rate in exophytic species is positively correlated with environmental temperature,115 and one may assume that the same is true for endophytic species. Another factor that can influence oviposition rate in exophytic species is persistence of the tandem link: in Sympetrum sanguineum the rate declines after the transition from contact to non-contact guarding.81 The suggestion by Wesenberg-Lund that exophytic oviposition (clearly a derived evolutionary condition) may have been selected for partly in response to predation risk116 becomes even more plausible when one reflects on the extent to which exophytic oviposition reduces the duration of oviposition and the concomitant exposure to hazard. Early advantages of endophytic oviposition may have included the positioning of eggs in a microclimate free from extremes of temperature and humidity and in an environment relatively free from the risk of predation. A well-developed ovipositor was a feature of the first recognisable progenitors of dragonflies, the Protodonata, known from lower Upper Carboniferous deposits laid down more than 300 million years ago.117
FECUNDITY
The total number of fertile eggs laid by a female during her lifetime equates to her fecundity, also known to evolutionary biologists as her lifetime reproductive success (LRS), a term which suitably emphasises the quantitative nature of her inclusive fitness, measured by her numerical and genetic contribution to the next generation.
During a female’s life she lays eggs discontinuously, in successive pulses or episodes, typically separated by intervals of 1-5 days. In a study of Sympetrum danae, for example, the interval between successive episodes averaged 4.7 days and ranged from 1 to at least 20 days.118 Interepisodal intervals estimated for other British species were 1-3 days for Aeshna cyanea,119 1 day for Calopteryx virgo120 and 1-17 days for Coenagrion puella.121 Some of the higher recorded values for this interval may be overestimates: the female concerned may have been visiting (and ovipositing in) other habitats unknown to the observer between successive sightings and so may have avoided notice and registration. Weather permitting, we would expect a general estimate of 1-5 days for the interclutch interval to be realistic.
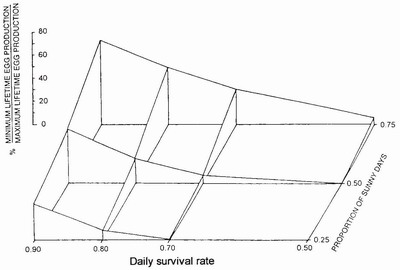
If one knows the average number of eggs laid during each episode, known as a clutch, and also the number of episodes (which depends on a female’s expectation of life), one can estimate the lifetime egg production – a direct measure of fecundity. The calculation requires extensive data of high quality and so is unlikely to become available for more than a very few species. The work of Michael Banks and David Thompson has provided such data for Coenagrion puella in Britain.121 Their results, which they have analysed in depth, reveal that the mean number of clutches per female was 3.85 (maximum 15) and that potential fecundity for long-lived females exceeded 4,000 eggs during two consecutive years at a medium-sized pond in The Wirral, England. Clutch size, which varied with ambient temperature, body size and interclutch interval, ranged from slightly more than 100 to 400. Depending on the weather during the flying season, and especially the degree to which sunny days were clumped, fecundity varied between years by a factor of 10. Chance played a dominant role in determining fecundity because a female who began her reproductive life during an unbroken succession of sunny days produced many more eggs than a female whose reproductive life coincided with cloudy weather. Unsurprisingly, the same dependence of fecundity on weather exists in Calopteryx virgo.119 We conclude from these two careful studies that generalisations regarding LRS in
FIG 52. The effect of daily survival rate and the proportion of sunny days on lifetime egg production by Coenagrion puella, derived from a simulation model (sample size 30 for each simulation). (After Thompson.122)
a changeable temperate climate will always be elusive, depending as they do on local conditions, especially the weather.
A simulation model based on his studies of Coenagrion puella enabled David Thompson122 to construct the framework of dependence shown in Fig. 52. Two dependent variables have a major effect on interyear variation in lifetime egg production: reproductive life span and number of clutches.121 The pre-eminent importance of life span is evident also from a study of LRS of Lestes sponsa in Belgium.123 The use of clutch size in estimating LRS can be subject to error when eggs fail to hatch, either because they have not been fertilised or because they have been parasitised. For example, 22.4 per cent of eggs laid by the continental lestid, Sympecma paedisca, were parasitised by the wasp Anagrus,124 whereas water mites consume laid eggs of zygopterans and libellulids.125
OPPORTUNITIES FOR INVESTIGATION
This chapter has highlighted topics likely to be rewarding for future investigation, and the cited sources describe approaches and methods that are appropriate. For investigators with suitable opportunities, a project likely to be of great value in increasing understanding of odonate biology in general is the estimation of LRS. The studies of Calopteryx virgo by Claire Lambert120 and of Coenagrion puella by Michael Banks and David Thompson121 provide worthy models to follow. As with most field research on Odonata, a prerequisite for success is a favourable study site. This should be a stem habitat (Box 6, p.76) inasmuch as it should support a continuous succession of large populations, and it should be readily accessible and possess a vegetation structure sufficiently robust to resist being downgraded by frequent visits. Such habitats exist, though their numbers are decreasing, and they have sometimes become famous among odonatologists as foci for studies that have acquired benchmark status because of the rich information they have yielded.
For investigators who lack the opportunities to study LRS or other topics that require daily visits to a site throughout the flying season, there are many other useful projects to be pursued. For example, it would be rewarding to conduct tests in the field on choice of substrates by ovipositing females, especially by those that lay eggs endophytically. Again, one needs access to a habitat where one can count on finding many ovipositing females. We learnt on p.82 how Aeshna cyanea will sometimes lay in bizarre, unnatural substrates near a pond. Presumably primary attributes of an acceptable oviposition substrate for most endophytic species are substrate texture, position with respect to the water’s edge, perhaps colour, and orientation with respect to the sun’s azimuth. All these variables are susceptible to experimental manipulation. There are many different kinds of plastic materials, including polystyrene products, of varying rigidity, that have a sponge-like texture and could be exposed in various positions as surrogate oviposition substrates. If presented in the form of moveable tiles, or plaques, they could readily be monitored for the presence of eggs. The feasibility of this approach has already been demonstrated in laboratory studies, using polystyrene to harvest eggs of the North American coenagrionid Argia moesta.126
Surrogate substrates could also be used to explore further the distinction between apparent and actual oviposition and thus to document further the relationship between a female’s length of stay and the number of eggs that she lays. Studies using surrogate substrates could also have value as a means of obtaining eggs (for which the exact time of laying was known) for laboratory studies of the role of temperature in determining the rate of embryogenesis and diapause development – areas of developmental biology that have been neglected in British Odonata, about a seventh of which have eggs in which diapause is obligate (p.99). Eggs of known provenance could also provide material for taxonomic studies of egg morphology and of early larval stadia, two topics that urgently need documentation. And, with appropriate arrangements, one might tackle the elusive question of how prolarvae of species that oviposit near, but away from, water make their way there before moulting to the second stadium.
The matter of whether ovipositing females can detect the presence of fish in a water body could be approached by constructing similar, adjacent ponds, some with and some without fish in them, and monitoring the result.