CHAPTER 4
The Egg and the First Two Larval Stadia
THE ROLE OF THE EGG IN THE LIFE CYCLE
THE DRAGONFLY EGG provides a bridge between the adult and the larval stages. Because eggs of most species are small and difficult to find in the field, this stage is usually the last to be investigated. Yet it has an important role to play in the life cycle, mainly by determining the season at which larvae begin their development. For example, eggs of some species, usually those laid in spring or early summer, develop promptly and directly after being laid, completing embryonic development in about a month and then hatching. Others, by contrast, show delayed development such that hatching is postponed for several months. This is likely to happen in eggs laid in late summer or autumn which are then liable to overwinter in that stage. Such a pattern of development is achieved by the egg undergoing diapause. Diapause is not the same as dormancy or quiescence, states that can be induced by environmental conditions (e.g. temperature and food) being unfavourable for development at the time. Diapause is a state of suspended development that may occur in any stage of the life cycle (egg, larva or adult) and that typically constitutes an anticipatory response to unfavourable environmental conditions. Species like Lestes sponsa or Aeshna cyanea that habitually lay diapause eggs will typically lay eggs of this kind long before the onset of winter. Such eggs then develop very slowly during the autumn and winter, hatching the following spring, after the water temperature has reached a level at which larvae can survive and forage. We know something about how this pattern of development is regulated in L. sponsa, from experiments conducted at controlled temperature.1 After being laid, eggs need a period of about two weeks (at normal summer temperatures) to reach a stage at which the embryo is almost fully formed and has assumed its final orientation inside the eggshell. The embryo now enters diapause, a physiological state which is completed most rapidly at a temperature characteristic of late autumn in Britain, actually about 10°C. By the time that this next stage has been completed, the ambient temperature has fallen below that at which hatching can occur. In this way the eggs, after completing diapause, are obliged to postpone hatching until spring, at which time they hatch synchronously, producing larvae that then grow rapidly. The situation revealed in the eggs of L. sponsa shows diapause to differ from normal development by having a thermal optimum close to 10°C, which is much lower than that for normal (i.e. non-diapause) development.1
The existence of a diapause in the egg has a large impact on the pattern of larval development and therefore on the life cycle as a whole. One obvious effect of an egg diapause is that the earliest larval stadia, which are assumed to be the most susceptible to low temperature, escape winter temperatures. Species having diapause eggs begin larval development, more or less synchronously, in spring, whereas those having non-diapause eggs hatch in summer or autumn, typically in a staggered fashion that reflects the temporal dispersion of oviposition. We shall see on p.119 that larvae from late-hatching eggs can be at risk from heavy predation from their older, larger conspecifics.
Among the British species an egg diapause, if it occurs, is usually obligate, meaning that it is an unvarying feature of the life cycle. In a few species, however, diapause is optional, or facultative, occurring in some years and not in others. The distribution of egg diapause among the British species is shown in Table 1. These data should not, however, be regarded as the last word, because in some genera (e.g. Somatochlora) it has been shown, by careful experiment, that the existence of diapause in the egg depends on the date in summer when the egg is laid. Thus Somatochlora alpestris and S. arctica lay diapause and non-diapause eggs, the proportion of diapause eggs increasing as the summer advances,2 showing that the diapause is facultative. It has long been known that the egg diapause in some species of Sympetrum is facultative, an example being S. striolatum,3 in which, likewise, the incidence of diapause depends on the date when the egg is laid, and also S. sanguineum,4 but for these two species experiments to determine the factors involved still have to be performed. In Japan, which is home to many species of Sympetrum, several species are known that exhibit facultative diapause in the egg.5
TABLE 1. The incidence of delayed embryonic development in British Odonata.
Note: Various sources, including those listed by Corbet6
and Sternberg and Buchwald.7
a All three species show direct development, but in Germany the proportion of diapause eggs laid by Somatochlora arctica increases steadily as the summer progresses..2
b In Sympetrum sanguineum and S. striolatum the typical pattern of embryonic development is unclear. In these species both direct and delayed development have been recorded.
EXTERNAL MORPHOLOGY AND OVIPOSITION MODE
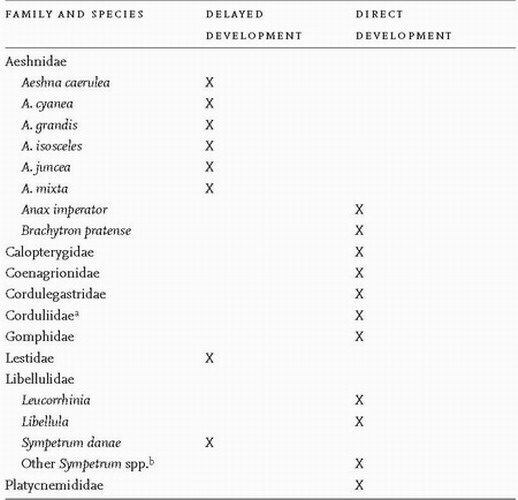
Eggs are usually either spindle-shaped and several times longer than wide, or ellipsoid to subspherical (Fig. 53). In general, eggs of the former type are laid endophytically and the others are laid exophytically (Box 7, p.8o). Eggs laid endophytically tend to be pushed into an incision in the substrate and positioned so that the anterior pole faces the outside, sometimes projecting very slightly outside the substrate. A hazard that such eggs presumably face is that of being sealed inside the stem or leaf by overgrowth of the plant tissue. The eggs of several species that oviposit endophytically are furnished with a cap, funnel or bladelike projection (Fig. 54), apparently formed from the outermost layer of the eggshell, which seems to prevent the plant tissue enclosing the egg and so provides a conduit through which the hatching prolarva can pass when the egg hatches. Such a projection is especially well developed in eggs of Anax, Calopterygidae, Coenagrionidae and Platycnemididae. In Anax the bladelike cone is apparently formed by the outer eggshell sliding away from the inner eggshell as the egg is placed into the substrate.8 A close examination by Steve Cham of hatching in Ischnura pumilio has shown that the cap at the anterior pole does indeed facilitate the passage of the prolarva during hatching.9 Also at
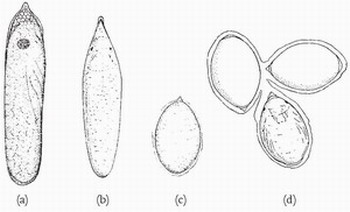
FIG 53. Shapes of dragonfly eggs. (a) Aeshna isosceles (1.5 mm long); (b) Pyrrhosoma nymphula (0.9mm); (c) Sympetrum danae (0.7mm); (d) Libellula depressa (within a gelatinous matrix) (0.9mm). (a) and (b) are laid endophytically and (c) and (d) exophytically. ((a) from Gardner;10 (b) from Gardner and MacNeill;11 (c) from Gardner;12 (d) from Gardner.13)
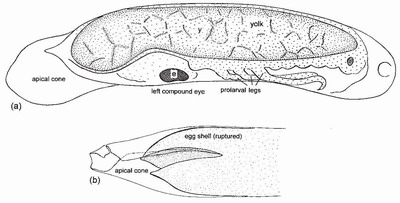
FIG 54. The egg of Anax imperator (length excluding apical cone 1.8 mm). (a) showing the apical cone, the yolk mass, the left compound eye (e) and the prolarval legs; (b) the anterior end after hatching, showing the ruptured egg shell and cone through which the prolarva has passed during eclosion. ((b) after Corbet.14)
the anterior pole of the egg are two or more conical or nipple-shaped projections containing canals (micropyles) through which sperm travels before penetrating the thin envelope enclosing the egg cell. Fertilisation takes place as each egg passes down the uterus during the act of oviposition.
Eggs of some exophytic species are invested by a gelatinous matrix which sometimes has adhesive properties. In riverine gomphids (e.g. Gomphus simillimus), this covering appears to enable the eggs to adhere to the substrate promptly after being laid and so avoid being swept downstream.15 Curiously, no such investment has been found on the egg of Gomphus vulgatissimus in which it might have been expected. The egg of the other exophytic riverine anisopteran in Britain, Cordulegaster boltonii, lacks a gelatinous covering, but this is not surprising because the ovipositing female thrusts each egg into sediment near the bank, where the egg is protected from dislodgement by the current.
EMBRYONIC DEVELOPMENT
The appearance of the egg changes markedly during embryonic development. Immediately after it has been laid, the egg of most species is milky white or pale yellow, and in unfertilised eggs it remains this colour until decomposing. Usually, however, a very high proportion (often 100 per cent) of laid eggs are fertile. After having been demonstrated in the laboratory in a Japanese gomphid,16 parthenogenesis in the Odonata has been confirmed to occur naturally, and to be widespread among populations of Ischnura hastata on the Azores,17 a place where its existence had long been suspected because of the Islands’ isolation, and because collections there consisted only of females.18 Adolfo Cordero and colleagues surmise that parthenogenetic I. hastata may have been infected by a novel endosymbiontic bacterium able to cause this mode of reproduction.19 Parthenogenesis has not been detected in any other species of odonate, or in North American populations of I. hastata, although Wolbachia, bacteria able to induce parthenogenesis in their arthropod hosts, have been found in several species of tropical Odonata,20 but, interestingly, not in I. hastata.19 It is not known whether Wolbachia is associated with the role reversal of the sexes that is a conspicuous feature of species of the Polynesian pseudagrionine genus Nesobasis.21
The fertile egg soon darkens, and after a few days the developing embryo becomes visible inside, the first, most conspicuous signs of development being the pigmented compound eyes. Thereafter, under appropriate lighting and magnification, the legs, labium and abdomen become visible and then the embryo undergoes blastokinesis (or katatrepsis), abruptly reversing its orientation within the egg shell. After blastokinesis the embryo’s head is at the anterior end of the egg, in the position in which hatching will later take place. Species with diapause eggs differ according to whether blastokinesis occurs after winter (Type 1) (e.g. most species of Aeshna and Sympetrum danae) or before winter (Type 2) (e.g. most species of Lestes).22 The ecological significance of this dichotomy is not understood. In the North American Sympetrum vicinum, the eggs of which exhibit facultative diapause, eggs laid early in autumn, at a water temperature above 14°C, conform to Type 2, whereas those laid later, in colder water, conform to Type 1.23 In S. vicinum, as in Lestes sponsa, diapause development proceeds most rapidly at intermediate temperatures. Species whose eggs exhibit direct development (i.e. which lack diapause) typically hatch four to six weeks after being laid at seasonal ambient temperatures (e.g. Brachytron pratense in three weeks;24 Cordulegaster boltonii in 24-43 days25).
SURVIVORSHIP
The point at which within-generation survival begins to be determined is the number of laid, fertilised eggs that hatch. Egg mortality after hatching is caused by predators and parasites. Very little is known about predators of dragonfly eggs
PARASITOIDS OF DRAGONFLY EGGS
A parasitoid is a parasite that invariably kills its host and so, in this respect, resembles a predator. Odonate eggs that are laid endophytically are especially vulnerable to parasitoids which can attain high levels of incidence. Egg parasitoids consume the host from within in the stage in which it is attacked (i.e. very soon after oviposition), and so prevent its subsequent morphological development. Egg parasitoids of Odonata belong to two superfamilies of Hymenoptera: Chalcidoidea and Scelionidea. Most belong to the Chalcidoidea, in which three families and about ten genera are represented.29 This topic has received relatively little attention by researchers and so it is likely that many more host-parasite associations remain to be discovered. Some egg parasitoids, such as the eulophid wasp Tetrastichus polynemae, can act as a hyperparasitoid, developing at the expense of a parasitoid already in a host egg. Adult females of the parasitoid walk or swim under water to locate the host egg in which they oviposit by thrusting the ovipositor deep into the victim.30 Some egg parasitoids (e.g. Mymaridae) are among the smallest of insects (Fig. 55), the adults being only 0.2 millimetres long, which is smaller than some Protozoa. Even the smallest Zygoptera are available to them as hosts. Some Trichogrammatidae can mate inside the host eggshell, doing so on the day of emergence30 and thus completing the whole life cycle in seven to ten days.31 Recorded rates of odonate mortality due to Chalcidoidea range between 12 and 95 per cent.32 In a species of Polynema, an egg parasitoid of Calopteryx virgo, usually only one parasitoid larva develops per host egg, and if more than one parasitoid larva occupies a host egg, only one parasitoid individual completes development there.33 Some such parasitoids, mainly Chalcidoidea, employ elaborate strategies to locate the eggs of the host: mature female wasps sometimes travel around with the host, clinging to its body, and so being well placed to find eggs promptly after they have been laid.34 Wasps of the latter type are mainly Scelionidae.35 Only females have this phoretic relationship with their dragonfly hosts which likewise are predominantly (perhaps exclusively) females. Such an association would enable the scelionid to attack the host egg immediately after it had been laid, and so before the host embryo began to develop and before any protective covering accompanying the egg had had time to harden.35 This, it seems, would be a much more effective way of exploiting the host than having to search for the laid eggs (as some chalcidoids do) and thus sustaining a delay. Very little is known about this fascinating relationship, perhaps partly because, when the female host is captured, the parasitoids probably drop off and so escape detection.
FIG 55. An adult female of the trichogrammatid parasitoid wasp Hydrophylita aquivalans, from right side, inside the egg of a coenagrionid, Ischnura verticalis. The wasp has used its mandibles to make a hole in the eggshell through which it will pass when emerging, before swimming to the water surface using its wings as oars. (From Davis.36)
to which presumably eggs laid exophytically are more vulnerable, although the eggs of a West African species of Malgassophlebia, laid exophytically within a gelatinous investment, are subject to predation by drosophilid larvae;26 and water mites consume laid eggs of zygopterans and libellulids.27 As far as is known, mortality during embryonic development is caused largely by parasitism, mainly by minute wasps which act as parasitoids of eggs laid endophytically (Box 8). Parasitoids of dragonfly eggs can exert heavy mortality: 22.4 per cent of eggs laid by the continental lestid Sympecma paedisca were killed by the parasitoid wasp Anagrus.28
HATCHING
Several hours before the eggshell ruptures, the embryo can be seen making peristaltic movements of the oesophagus, synchronised with rhythmic contractions of the dilator muscles of the pharynx. Thereafter, hatching differs in the two suborders. In Zygoptera the embryo swallows amniotic fluid, supposedly causing water to enter the egg through the micropyles. Increase of pressure ruptures the inner eggshell along a line of weakness and distends the vitelline membrane, pushing the anterior pole away from the egg (Fig. 56). Continual swallowing, accompanied by abdominal distension, causes the embryo to move forward so that its head occupies the chamber formed by the bulging vitelline membrane. At this time the anterior tip of the egg often projects visibly from the oviposition substrate. The prolarva then slides out of the egg, passing through an anteriorly placed cone if one is present (Fig. 56), its exit apparently being eased by posteriorly directed tiny teeth on the head and thorax as well as spines on caudal appendages. The embryo’s continual, active intake of water, through mouth and probably also anus, and the arching of its head and thorax, enable the prolarval cuticle to split on the head and thorax, exposing the stadium-2 larva, which quickly makes its exit, often leaving the exuvia of the prolarva still attached to the now-empty eggshell. The process of hatching, as described here, takes about 20 minutes in Calopteryx virgo,37 but some Coenagrionidae apparently complete the process more rapidly.17 Visible swallowing movements in Coenagrion puella cease immediately after the moult to stadium 2.38
In Anisoptera the eggshell is typically ruptured by an egg burster, a sclerotised crest on the head of the prolarva. The egg burster, which is on the frons of endophytic species and on the top of the head of exophytic species,
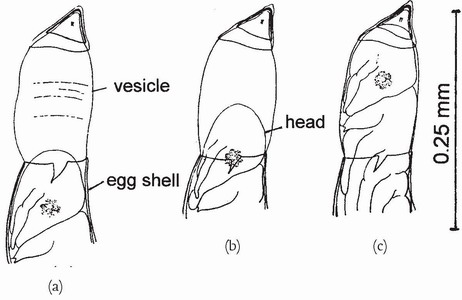
FIG 56. Successive stages (a-c) during egg hatching in Enallagma cyathigerum. At the anterior end of the egg, water enters through the micropyle and distends the vitelline membrane which ruptures the eggshell and forms a vesicle into which the hatching embryo pushes its head. Later the embryo, by swallowing water, will rupture the vesicle and leave the egg. (After Degrange.37)
produces a slit that may extend around the egg. The prominent ‘frontal horn’ on the stadium-2 larva of Anax imperator may represent the relic of the egg burster.39
About 30 seconds to a few minutes after the moult to stadium 2, the main dorsal tracheal trunks fill rapidly with gas, starting in the midgut region and spreading backward and forward. The gas, initially carbon dioxide,40 is soon replaced by nitrogen and oxygen and can then perform a respiratory function.
The precise stimuli that trigger hatching are unknown for British species. For eggs that are submerged, ambient temperature exceeding a threshold may be the stimulus. For eggs positioned out of water, and by analogy with certain tropical species, wetting may be the trigger, as it is for Ischnura pumilio..9 For British species it is not known if hatching follows a diel periodicity.
THE PROLARVA AND STADIUM 2
There are persuasive grounds for regarding the prolarva (Box 9) as a true stadium, as we do here.41 Some observers have been reluctant to regard it as a stadium partly because of its extreme brevity: it often lasts less than a minute and seldom as long as five minutes, although it can greatly postpone moulting if obliged to seek water from a distance. When having to travel to water, the prolarva of the New Zealand lestid, Austrolestes colensonis, can delay the first moult for almost nine hours.42 On reaching water, the prolarva of the Japanese aeshnid Aeschnophlebia longistigma floats for three to five minutes and then moults, but it can survive as a prolarva for 14 hours if it fails to reach water within that time.43 Some other odonates, including the primitive Japanese Epiophlebia superstes, can jump over a dry substrate as prolarvae, sometimes covering in one leap a distance about 100 times their own length.44 Prolarvae travel more effectively on a firm surface: those of the Japanese aeshnid Planaeshna milnei can travel nearly 20 centimetres at each jump over land but much less than 1 millimetre over water.45 The European lestid Lestes viridis oviposits in the woody branches of the willow Salix aurita growing beside a pool. When the eggs hatch, most prolarvae drop directly onto the water of the pool, but some land on the ground, in which case they jump around until they alight on water.46 For endophytic species that habitually oviposit away from water, one may suppose that mortality during the prolarval stage is typically high. For exophytic species that lay eggs in clusters, such as Libellula depressa, prolarvae from late-hatching eggs may suffer predation from their more precocious siblings who have already entered stadium 2 and so are able to attack them.13
THE PROLARVA
The first larval stadium, usually very brief, is the prolarva..41 In appearance it somewhat resembles an Egyptian mummy, the limbs and mouthparts being barely delimited from the rest of the body (Fig. 57). It can wriggle and jump but not walk, swim or feed. Its duration, which can vary from a few seconds to several hours, seems to depend on when it reaches free water, at which time it moults promptly to embark on the second larval stadium – the first mobile, feeding stage. Sometimes the prolarval exuvia may remain attached to the eggshell, as in many species that oviposit exophytically. If the prolarva has to travel to reach free water, moulting can be postponed and the prolarval exuvia may be left floating on the water surface. Few people have observed the behaviour of prolarvae travelling to water from oviposition sites remote from water. The reduced form of the odonate prolarva has a parallel in the first stadium of certain grasshoppers and cicadas and may serve to facilitate smooth emergence from the egg by a larva whose long legs might otherwise get entangled with the edges of the opening in the eggshell.
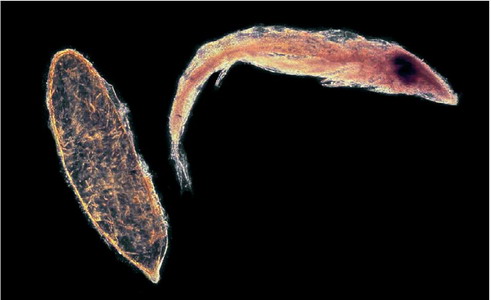
FIG 57. The egg (left) and prolarva (right) of Anax imperator from the collection of Eric Gardner. The length of the egg, including the apical cone, is about 2.2 mm (The Natural History Museum).
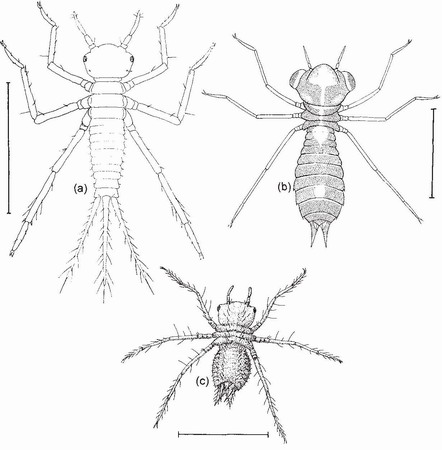
FIG 58. Stadium-2 larvae of (a) Coenagrion mercuriale; (b) Anax imperator; (c) Sympetrum striolatum. Scale lines 1.0 mm. ((a) after Corbet;47 (b) from Corbet,14 (c) from Corbet.48)
The first larval moult discloses stadium 2, this being the first stage that most odonatologists recognise as a dragonfly larva (Fig. 58).
OPPORTUNITIES FOR INVESTIGATION
It is not known how prolarvae from eggs hatching away from water locate the water, but it is likely that they possess some means of orientating towards it. The ability of a prolarva to locate water if it hatches away from it must have very high survival value. Although prolarvae seldom exceed 1 millimetre in length, and are therefore inconspicuous, a description of their behaviour during the journey from hatching site to water could be highly informative and could perhaps indicate how this orientation is being accomplished. In Britain an obvious candidate for such a study would be Aeshna cyanea, which is notorious for laying eggs in substrates which are sometimes a metre or more from water. By analogy with Austrolestes colensonis,42 it is assumed that the prolarva makes its way to water by skipping around on land first. Accounts of the behaviour and orientation of prolarvae during this episode would fill long-standing gaps in our knowledge of this brief but fascinating developmental stage. This might be achieved by marking the positions of extra-aquatic eggs, determining the diel periodicity of hatching, and then waiting to observe some of the later eggs hatching. Alternatively, it would not be difficult to position laid eggs in a laboratory situation so that the passage of prolarvae towards water could be recorded. One should allow for the possibility that prolarvae, like adults, possess a sense that allows them to orient towards a horizontal water surface by perceiving the reflection of polarised light.
Endophytic oviposition away from water might reduce parasitoid attack on eggs. This merits investigation.
Little is known of the temporal pattern of oviposition in the field, but such information would throw light on the population dynamics of larvae of species that lay non-diapause eggs. The provision of surrogate oviposition substrates, to be examined and replaced daily, would make possible acquisition of such data, as well as providing a supply of eggs, the exact age of which was known, for experiments.
Other fruitful topics for investigation include the relationship between temperature and rate of development in diapause and non-diapause eggs. This would throw light on the nature of facultative diapause and its role in seasonal regulation. The requisite apparatus need not be elaborate: containers in which both temperature and daylength could be regulated, and a supply of eggs, the species and age of which were known. Parallel studies in the field would also contribute to our understanding of life cycles. The confinement and regular inspection of eggs (of known age and origin) in nature would yield information about egg survival and rate of embryonic development under natural conditions. It might also yield information about the frequency of parasitoid infection and the identity of the parasitoids involved.
Another informative project would be to capture ovipositing females of Anisoptera with a net of very fine mesh. As described above (pp.104-5), sometimes, by a lucky chance, adult scelionoid wasps (minute egg parasitoids) are caught on ovipositing Anisoptera. Catching adult Anisoptera with a fine-mesh net might reveal other phoretic organisms of interest, as might observing them carefully through a short-focus telescope. Using both techniques, Klaus Sternberg found five female adults of the minute milichiid fly, Desmometopa sp., running around on the head of an adult Cordulegaster boltonii (p.194),49 apparently sharing the dragonfly’s last meal, something that the egg parasitoids may also do when their energy supplies become depleted. Because of the way that odonatologists usually catch adult dragonflies, with robust manipulation of a wide-mesh net, such minute ‘hitchhikers’ are liable to escape notice. Further study of this relationship could not fail to be rewarding.