CHAPTER 5
The Larva: Survival Under Water
THE ROLE OF THE LARVA IN THE LIFE CYCLE
THE LARVA IS THE only stage in the life cycle in which significant growth in size occurs. Being confined to water, the larva has evolved in directions quite different from those of the adult. Larvae show much greater diversity in body shape and behaviour than adults, a reflection of their occupancy of different microhabitats, which in turn appears to reflect adaptations to reduce predation, mainly by fish, but also by larvae of other dragonflies. In Britain the larval stage occupies an environment that is relatively well insulated against extremes of temperature. From this refuge, the time of emergence to the less protected adult is regulated by the larva’s responses to seasonal variables such as daylength and temperature (p.172). The ways in which such regulatory responses enable each species to position the adult stage at a constant and characteristic time of year (p.169) are marvellous to behold, and to unravel them provides a continuing challenge for the investigator.
RESPIRATION
Comparative morphology tells us that the form of the respiratory system in dragonfly larvae – a network of gas-filled tubes, or tracheae – shows that they have descended from terrestrial ancestors that must have breathed through paired apertures, or spiracles, on the thorax and abdomen.1 Throughout most of the larval life the spiracles are closed, and the tracheae fill with gas, spontaneously, early in stadium 2. The routes by which oxygen passes from the water to the tracheal system differ markedly in the two suborders.
In Zygoptera most respiratory exchange occurs via the three caudal appendages, often called caudal lamellae when they are leaflike, at the tip of the abdomen. The caudal lamellae have a large surface area, and are richly supplied with fine branches of the tracheal system. The lamellae also function as fins and for enhancing swimming speed during escape behaviour. Being large and conspicuous, they need to be camouflaged. Each lamella has a breaking joint at the base, allowing it to become detached readily if grasped by a predator, enabling the larva to escape, rather in the manner of a lizard jettisoning its tail.2 The lamellae are not essential for respiration: a larva can survive without them, at least until dissolved oxygen in its surroundings becomes seriously depleted. Some respiratory exchange occurs also through the surface of the body, especially via parts that are richly tracheated, like the wing sheaths. The shape and relative size of caudal appendages change greatly during larval development:3 in the earliest stadia they are relatively large and often spearlike, bearing long setae which are assumed to be sensory (Fig. 59). Small larvae readily use them for aggressive or defensive display, whipping them forwards to jab at a potential adversary, of the same or another species. Such behaviour is evident as early as stadium 2 in larvae of Calopteryx splendens.4 During the last four or five stadia of
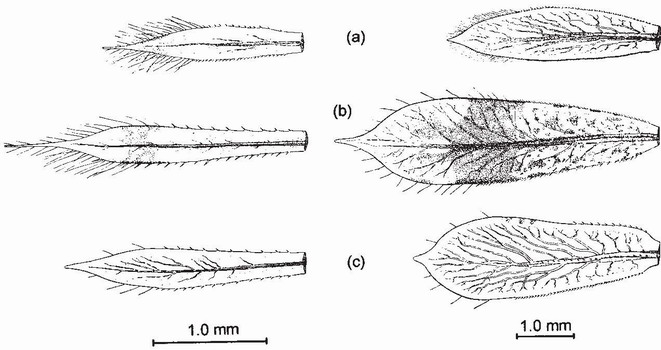
FIG 59. Middle caudal appendage of three species of Coenagrionidae, viewed from the right side, showing the change of shape during development. (a) Coenagrion mercuriale; (b) Pyrrhosoma nymphula; (c) Ceriagrion tenellum. Left, in approximately stadium 7 (body length 3.5mm); right, in the final stadium. (From Corbet..3)
many species of Zygoptera, the appendages diminish in size relative to abdomen length and become progressively more leaflike (Fig. 59), presumably coming to play a larger role in respiration and emergency locomotion.
In Anisoptera, gaseous exchange occurs across the richly tracheated inside wall of the rectum which is typically furnished with elaborately patterned gills arranged in bunches or tufts (collectively known as the branchial basket). The rectal epithelium is irrigated by pulsating movements of the abdomen which draw in and expel water from outside. The requisite changes in pressure are effected by a transverse, muscular diaphragm inside the abdomen. The diaphragm performs other vital functions besides respiration: by increasing the strength and frequency of pulsations the larva can expel water from the anus so vigorously that it can swim by jet propulsion to elude predators, to chase prey and, occasionally, to hover, head uppermost, just beneath the water surface to forage (Box 10). Sometimes the diaphragm helps forcibly to eject faecal pellets, which, for reasons unknown, can be propelled for 30 centimetres or more away from the larva’s body.5 Another vital function that relies on the diaphragm is the sudden extension of the labium during prey capture (Box 10).
BOX 10
OPERATION OF THE LARVAL LABIUM DURING PREY CAPTURE
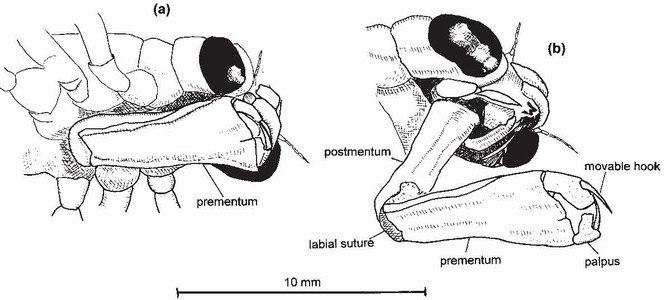
The larval labium as developed in Odonata is a highly specialised organ found only in this order. It derives from the fusion of the second maxillae to form a robust, hinged extensible organ consisting of two parts, the postmentum and prementum9 (Fig. 60).
The prementum bears two palpi, each armed with a moveable hook; and the palpi open like pincers to grasp the prey. The explosive protraction (extension) of the labium results from its being an energy-storage mechanism, not unlike that causing the leap of a flea, locust or click beetle. Its successful operation requires the precise co-ordination and rapid operation of three processes: contraction of the abdominal diaphragm (in Anisoptera) or the abdomen (in Zygoptera), which increases internal pressure of the haemolymph; simultaneous closure of the anal valve (to prevent exhalation); and release of the mechanism that locks the labium in the resting position.10 The primary flexor muscles of the labium, together with a locking device, keep it in the resting position while energy is stored before protraction. Release of the locking mechanism causes explosive protraction of the labium, enabling the moveable hooks on the labial palpi to open and then grasp the prey (Fig. 60) whereupon the captured prey is drawn back to the mouth by muscular action which restores the labium to the resting position, folded and locked beneath the head..10 The time taken to achieve full protraction has been recorded as about 15 msec in some Anisoptera11 and 40 msec in some Zygoptera.12 The co-ordination achieved by Anisoptera when operating this mechanism is remarkable: sometimes larvae of Aeshnidae and Libellulidae can be seen using jet propulsion to hover (in a jerky manner) just below the water surface while simultaneously using the labium to catch small prey, such as mosquito larvae or microcrustacea, that have assembled beneath the surface. At other times the ascent can appear relatively smooth: Sandra Sell was amazed to see a late-stadium larva of Anax imperator rise vertically to the water surface, with no visible means of support, to use the labium to capture a fly that had settled above it.13
FIG 60. Head of an aeshnid larva, viewed obliquely from beneath right side, (a) with labium in resting position and (b) a few milliseconds after the onset of the labial strike. In (b) the locking mechanism holding the prementum in the resting position under the head has just been released, allowing blood pressure built up by the muscular abdominal diaphragm to launch the labium towards the target. The labial palps will be opened just before the target is engaged. (Modified from Weber.14)
FORAGING
Dragonfly larvae are catholic feeders, taking predominantly living prey which they detect, visually or by touch, and usually by its movement. Their default mode is to ambush prey as it swims or crawls within range of the labium which is then extended, extremely rapidly, to grasp the prey and draw it back to the mouth, where it is crushed by the mandibles (Box 10). Selection pressure on the rapidity of labial extension has probably been intense: in an aeshnid this may happen so fast that a small tadpole does not initiate a startle response (thus alerting conspecifics) until after being struck by the labium.6 As ambush foragers, like spiders and praying mantids, dragonfly larvae are using energy in a very efficient way,7 at least as long as suitable prey organisms continue to come close to a larva’s perch. Their prey not infrequently includes dragonfly larvae, of their own as well as other species. This intra-odonate predation is most likely to occur when larval density is high, when prey is in short supply, and when the largest and smallest larvae differ in size by two or more stadia.8 Thus, when emergence is poorly synchronised and when oviposition (and therefore egg hatching) are temporally dispersed, intra-odonate predation (either intraspecific or interspecific) can be expected to be most pronounced. One effect of this will be to reduce the size range of the surviving larval population, as the smallest larvae suffer disproportionately from predation.
Cannibalism (i.e. intraspecific predation) may be a decisive factor for survival.15 Brachytron pratense larvae of all sizes show active sibling cannibalism when prey is scarce.16 Temporal priority (reflected in size differences) confers dominance on larger larvae and may cause smaller larvae to show anti-predation behaviour.17 If larvae of different size (or age) cohorts were to occupy different microhabitats, this might serve to mitigate intraspecific predation; and, as mentioned later (pp.119-121), the banding pattern of larvae of Anax imperator and some other aeshnids may make them less visible to older conspecifics in semivoltine populations.
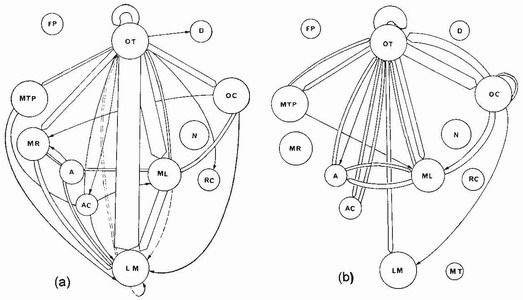
Although it is realistic to regard dragonfly larvae as primarily ambush foragers, they sometimes show remarkable versatility in their foraging behaviour. To obtain a balanced perspective of their foraging capabilities, we can consider some of their opportunistic behaviour. For example, although many experiments have shown that the ways in which prey move plays a major role in their detection and capture, some species, such as Anax imperator,18 are able to detect, recognise and capture prey that is virtually immobile or moves extremely slowly, such as a snail, and then stalk and capture it. Thereafter the sequence of actions larvae use to manipulate and ingest the prey depends on the prey type (Fig. 61): for example, a caddisfly larva inside its case will be handled differently from a snail in a shell. Even more surprising perhaps is the finding that certain zygopteran larvae (e.g. the New Zealand coenagrionid Xanthocnemis zealandica) may return to feed off the carcass of another zygopteran larva, rather in the manner of a fox or jackal coming back to scavenge for second helpings.19 Aeshnid larvae sometimes vary the ambush feeding mode by actively stalking prey. This behaviour, which aeshnids are most likely to adopt when hungry,19 can be well observed when tadpoles are available as prey. The aeshnid larva is alerted to the tadpole’s presence each time the latter wriggles and then, when the wriggling stops, seems to remember where the tadpole was and stalks it accordingly. Christine Blois18 and Richard Rowe20 have shown how the predatory sequence can be recorded and analysed in a systematic way and have thereby revealed the scope that exists for fruitful studies of behaviour in this field (Fig. 61).
At the family level, species, especially among Anisoptera, segregate as larvae into broad categories within which eye structure, larval shape, behaviour, microhabitat and growth rate are correlated. This diversity has led to larvae being classified into types, based mainly on eye specialisation and behaviour.21 Another classification can be based on behaviour, morphology and microhabitat occupancy (Table 2). These two classifications, which do not conflict, provide a template against which we can assemble information about larval behaviour and ecology.
Taking into account only Anisoptera, we may consider examples of the four main categories. These should not be regarded as discrete, exclusive categories, but rather as foci, among which larvae move and towards which they gravitate depending on prevailing environmental conditions, as determined by the array of microhabitats that are available, prey availability, predator pressure, etc. Notwithstanding this generality, certain species of Cordulia and Somatochlora exhibit behaviour that combines features of more than one type,23 and this may be true of corduliids in general. Somatochlora flavomaculata, depending on its microhabitat, which can be submerged vegetation as well as semiliquid peat mud, can show leg movements characteristic of sprawlers or shallow burrowers.24 In general, the behavioural types offer opportunities for niche differentiation leading to compromises in which microhabitat use, voltinism and sensitivity to predation by fish are highly correlated.25 Foraging mode depends partly on prey availability and activity: for example, in experimental situations, Coenagrion hastulatum and Aeshna juncea used the ambush mode when prey density was high, but the active mode when it was low or prey were sedentary.25 Among anisopteran larvae four focal categories have so far been recognised (Table 2).
FIG 61. Flow diagrams showing precapture behavioural sequences for an F-0 larva of Anax imperator with (a) a larval caddisfly, Phryganea sp., and (b) an aquatic snail, Limnaea sp., as prey. Solid lines denote successful, and broken lines denote unsuccessful, labial strikes. The width of bars and linking lines is proportional to frequency, only values exceeding 1 per cent being shown. Capture of a caddisfly larva is characterised by a relatively simple, brief phase between orientation of the head (OT) and labial protraction (LM); capture of a snail includes an initial, extended, preparatory phase that entails orientation of the head (OT) and body (OC) interrupted by slow walking (ML) and spells of immobility (A). Postcapture sequences for the two prey types (not shown) also differ markedly. Other abbreviations: AC, body (of predator) moves forward horizontally but legs retain their position on the substrate; D, larva turns away from prey and abandons precapture sequence; FP, prey escapes; MR, larva walks rapidly over substrate; MT, larva uses fore legs to groom antennae, mouthparts and legs; MTP, legs trample substrate; N, larva swims; and RC, walks backward. (After Blois.18)
Claspers, typically aeshnids (Figs 62 & 63), usually cling tightly to a stem or stick. The body is typically elongate, and the compound eyes are large and prominent. Brachytron pratense (Fig. 63) is a good example of this type and, when in the resting position, the dark larva is well camouflaged. Species of Aeshna adopt much the same position, being greenish if they are amongst water plants, but they are much more flexible in their choice of substrates. Aeshna cyanea (Fig. 64), for example, can occasionally be found in rock pools26 or even cement tanks27 that are almost devoid of perches. Claspers that occupy perches near the water surface (e.g. Anax imperator) typically have large compound eyes and respond promptly and actively to movement, readily stalking or pursuing
TABLE 2. Categories of larval Anisoptera in relation to behaviour, morphology and micro habitat occupancy (from Corbet22).
| 1. Clasper: | compound eyes large, symmetrical; active; abdomen elongate; a visual forager; clings to vegetation near water surface: for example, Aeshna cyanea. |
| 2. Sprawler: | compound eyes small, less symmetrical; active; abdomen squat; a visual and tactile forager; occupies vegetation near water surface: for example, Sympetrum striolatum. |
| 3. Hider: | 3.1 compound eyes small, asymmetrical; sedentary; abdomen squat, setose; a tactile forager; occupies fine detritus: for example, Libellula depressa sometimes. 3.2 compound eyes small, asymmetrical; sedentary; abdomen squat, setose; a tactile forager; lives amongst coarse leaf litter: for example, Cordulia aenea. |
| 4. Burrower: | 4.1 shallow burrower: compound eyes small, asymmetrical; sedentary; abdomen elongate, setose; a tactile forager; lives amongst fine stones or sand; legs perform digging movements: for example, Cordulegaster boltonii; Libellula depressa sometimes. 4.2 deep burrower: no British example. |
Note: These designations are focal points rather than discrete categories. Under different circumstances or in different stages of development, larvae of a given species may move from one category to another.
potential prey if it fails to come near enough to grasp with the labium (Box 10, p.114). Odonatologists who keep A. imperator in a glass-fronted aquarium will become accustomed to a larva turning its head to track the movements of anyone passing nearby. Such active species tend also to grow more rapidly than others and to forage more aggressively. A counterpart among the Zygoptera (which do not on the whole conform to the types in Table 2) are larvae of lestids which are more responsive to movement and grow more rapidly than coenagrionids of similar size, under identical experimental conditions.28 Like larvae of A. imperator, those of lestids occupy perches near the water surface, where the water is usually warmest and the light intensity highest. It is noteworthy that in Britain A. imperator can be semivoltine, requiring two years to complete larval development.29 This means that larvae in the earliest stadia have to exist alongside larvae a year older which, as aeshnids, would not be averse to making a meal of them.30 Close to the water surface, where such larvae live, the incident
FIG 62. Larva of Aeshna caerulea, a clasper and sprawler, in stadium F-0. The wing sheaths cover the first four abdominal segments (Robert Thompson).

FIG 63. Larva of Brachytron pratense, a clasper, in stadium F-0 (Robert Thompson).
FIG 64. Larva of Aeshna cyanea, a clasper and sprawler, in stadium F-0. It is shown in stalking mode. Excellent stereoscopic vision serves aeshnid larvae well when they are capturing prey (Robert Thompson).
light forms a mottled pattern of light and shade caused by the ripples on the surface. In their first year, larvae of A. imperator are strikingly banded with black and white, but they lose this pattern when they graduate to the larger size cohort.31 It is tempting to regard this age-dependent pattern as camouflage that helps to protect them from predation by the senior size cohort. It may be no coincidence that early stadia of several Aeshna species, most of which are semi-or partivoltine, are mottled with black and white. A similar progression occurs in some other aeshnids.32
A second category, sprawlers, are squat in shape and typically have a spoon-shaped prementum. They sprawl, usually with the legs extended laterally, amongst macrophytes, close to the water surface. Examples are species of Sympetrum (Fig. 65), known for their capacity for rapid growth.
Hiders, typified by Corduliidae, are somewhat similar in shape to sprawlers, but tend to be less active and to hide amongst leaf litter and detritus near the bottom. The setae on the body of some hiders gather particles from the sediment and help to camouflage the larva; other hiders are smooth and their bodies do not accumulate detritus. The larvae develop much more slowly than do sprawlers. Sympetrum striolatum, a sprawler, can complete larval development in little more than three months,33 whereas Cordulia aenea, predominantly a hider,34 and a
smooth one, may often require three years or more for the same purpose.35 Larvae of Libellula quadrimaculata and Orthetrum coerulescens, the bodies of which are invested with setae, sometimes behave as hiders and sometimes as burrowers.
Species in the last category, burrowers, are squat, dark, and often thickly invested with hair-like setae, and use their legs to cover the body with sediment. As in sprawlers and hiders, the prementum is spoon-shaped, except in Gomphus vulgatissimus, in which it is more or less flat. The shape of the legs, and the movements they make, are well suited for digging, especially in G. vulgatissimus.36 The species of Libellula and Orthetrum found in Britain are mainly shallow burrowers and, especially in L. depressa and O. coerulescens, achieve additional camouflage when fine silt adheres to the setae on the dorsal surface. Libellula fulva, however, is also a sprawler, living amongst plant debris at the bottom of ponds like Cordulia aenea. Its possession of dorsal spines seems to indicate that it is a sprawler more than a burrower.37 Cordulegaster boltonii (Fig. 67) also
FIG 66. F-0 larva of Cordulia aenea, at different times a hider, sprawler and shallow burrower (Steve Cham).

FIG 67. F-0 larva of Cordulegaster boltonii, a shallow burrower which is widespread in Europe. The tips of the pointed compound eyes and the anal pyramid project slightly above the sediment when the larva is buried, a posture typical of a cordulegastrid larva (Hansruedi Wildermuth).
belongs in this category,37 inhabiting silt that accumulates near the margins of streams. This species is not regarded as an obligate burrower; it occupies a variety of cryptic microhabitats, changing its preference according to the stage of development.37 The larva is large and ponderous, and it can lend zest to a field trip to see a fully grown one clamber out of a mud sample. This example reminds us that not all odonate larvae can be expected to fit neatly into the focal categories that have been identified in Table 2. For example, some Somatochlora larvae combine behavioural and morphological features of sprawlers, shallow burrowers and hiders.37 Also, the activity mode varies with hunger and density or availability of prey.19 Burrowers tend to have conical compound eyes that project dorsally, extending above the surface of the sediment, and when at rest they point the tip of the abdomen upwards, presumably to avoid inhaling silt with the respiratory current. In Britain all burrowers are classified as shallow burrowers because they rest just below the surface of the bottom sediment, but in other countries, notably tropical Africa, there are genera (e.g. Neurogomphus) whose larvae burrow deeply, having a narrow, cylindrical abdomen terminating in a long, slender respiratory siphon that may occupy up to one third of the total body length.38 Such larvae are classified as deep burrowers. Larvae of burrowers select their substrates according to particle size, the smallest larvae of C. boltonii occupying much finer sediment than do larger larvae.39 Larval development of C. boltonii may take three or more years,35 so this arrangement may reduce the likelihood of predation by larger larvae on their smaller conspecifics. In one of the few studies of the diet of Cordulegaster larvae,40 no conspecific larva featured among the gut contents.
Diets of larvae are very diverse and include almost any animal that is palatable and not too large to handle. The diet during the last few stadia of Aeshna juncea, A. caerulea, Somatochlora arctica and Leucorrhinia dubia in a northern bog included Cladocera and larvae of Chaoborus, Chironomidae and Trichoptera.41 Larvae in a population of Pyrrhosoma nymphula (Fig. 68) studied by John Lawton near Durham began by eating small Crustacea such as Daphnia and Chydorus, the lower limit of prey length being about 0.8 millimetres, and then, as they grew larger, fed on progressively larger prey, including small Crustacea (copepods and ostracods), chironomid larvae and oligochaete worms.42 Occasional items included other Zygoptera, water mites and dipteran larvae. Different prey items present different costs and benefits to the predator. For example, handling time can vary from five or six seconds for an F-0 larva of Cordulia aenea or Leucorrhinia dubia consuming Cladocera or Copepoda43 to 19 minutes for an F-0 larva of Aeshna juncea eating the case-bearing trichopteran Limnephilus pantodapus.44 The largest claspers sometimes catch and eat small fish. One of the obstacles to be overcome when
FIG 68. Larva of Pyrrhosoma nymphula, a clasper, in stadium F-0. Its swollen wing sheaths show that it is in an advanced state of metamorphosis. The specimen illustrated is in an alert mode and may have detected a prey item or a conspecific (Robert Thompson).
larvae are being reared in captivity is to find a ready supply of living prey, tailored in size to the size of the larvae. For larger larvae this is seldom difficult because a wide variety of animals can be used, including mosquito larvae and Artemia, the brine shrimp. For the smallest dragonfly larvae, whose prey is little known, cultures of large Protozoa are likely to serve the purpose well.
Many investigators use the voided faecal pellets (each conveniently enclosed in the sloughed peritrophic membrane) as a source of information about the prey of larvae. This method has much to recommend it, not least because larvae do not have to be killed, and because the pellets can be conveniently stored for later inspection, each labelled with the size of its originator. An added advantage is that the investigator quickly becomes adept at recognising prey organisms from inspection of minute and triturated body parts, an admirable though arcane skill that unfortunately has little practical application beyond the realm of freshwater biology and palaeoecology!
After the mandibles have fragmented the prey, the gizzard in the midgut grinds the prey into small parts. A larva taken from the field can be measured, kept until a faecal pellet is voided, and then released.
The stadium-2 larva is usually endowed with yolk derived from the egg, which is visible as a glistening, pale cream bolus occupying the midgut, and which may exempt the larva in that stadium from having to capture prey. After its yolk has been exhausted, in stadium 3 and beyond, the larva must obtain all its energy from its prey.
A continuing challenge faced by all organisms is to achieve a positive energy balance: in the long term their energy intake must equal or exceed their energy expenditure. Attempts to elucidate the way in which animals meet this challenge constitute the field of ecological energetics.45 A truly remarkable feature of animals, especially opportunistic predators like dragonflies, is that they continually make appropriate choices as to how they should direct their activities so as to husband, and use, energy effectively. Energy acquisition through feeding need not always exactly match simultaneous energy expenditure (through growth and movement) because an animal can sometimes compensate for a temporary deficit by drawing on its own stored food reserves.
A quantitative estimate of the energy acquired and the energy expended at the level of the individual or the population is termed an energy budget (Box 11). The first energy budget calculated for an aquatic invertebrate animal was constructed by John Lawton for an odonate, the semivoltine Pyrrhosoma nymphula, in northern England, at the level of a population (Table 3) and a representative individual (Fig. 69).46 The species is consistently semivoltine throughout Britain,35 and Lawton’s study covered two years.
The reasoning underlying the estimation of an energy budget is explained in Box 11.
A noteworthy feature in Table 3 is that population energy flow was similar in two year classes in which larval population dynamics (as expressed in average numbers/m2, mortality rates, and numbers emerging) differed considerably.
The energy efficiency of foraging (i.e. food energy consumed divided by energy expended during its collection) for P. nymphula (1.6-3.6) is very low
ENERGY TRANSFORMATION
Energy is the capacity to do work, which includes growth. Plants typically obtain their energy by capturing solar energy and transforming it (by photosynthesis) into chemical energy which is stored in the form of simple organic compounds. Animals obtain their energy by consuming plants (if they are herbivores) or other animals (if they are carnivores). The chemical energy acquired by an animal can all be accounted for as growth, respiration and excretion.
The amount of energy an animal acquires per unit of food intake depends on its assimilation efficiency (AE), expressed as a percentage of dry weight of food consumed – (dry weight of faeces produced)/(dry weight of food consumed). Most species of dragonfly that have been studied have an AE exceeding 70 per cent.47 Pyrrhosoma nymphula, in stadium 2, can achieve an AE of about 95 per cent and in stadium F-0 this value falls to between 75 and 90 per cent, depending on the type of prey.46 The high AE of dragonflies perhaps compensates for their low feeding rate, a reflection of their default lifestyle as sit-and-wait predators. The energy content of food assimilated is conventionally expressed in joules (J), and can be estimated from its dry weight or by combustion, thus making it possible to compare the energy content of widely different substances. Energy consumed by respiration can be assessed by measuring the amount of carbon dioxide evolved. By such techniques the energy budget during larval life can be determined. The values in Table 3 and Fig. 69 show how energy was allocated by two successive year classes in a larval population of P. nymphula.
compared with that of many other animals, for example a bumblebee (4.4-20.2) or a largemouth black bass (3.8-10.3).48 Lawton interpreted this low value as follows: larvae were feeding conservatively, in that at no time were they feeding at close to their maximum daily rates, but this was still (just) a viable food-gathering strategy because of the very high efficiency with which the food was being assimilated in this species (87.8 per cent) and the low energy cost of being an ambush-type predator.49
The dry weight of a population, which is expressed as its biomass, is a
TABLE 3. Energy flowa in a larval population of Pyrrhosoma nymphula in two successive year classes. (From Lawton46)
| YEAR | ||
| CATEGORY OF ENERGY FLOW | 1966-7 | 1967-8 |
| Consumption | 35.57 | 35.91 |
| Growth | 16.55 | 15.08 |
| Respiration | 13.31 | 15.41 |
| Exuviae | 2.10 | 1.26 |
| Faeces | 3.61 | 4.16 |
Note:a (kJm2/year)
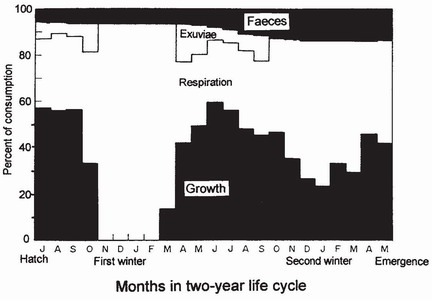
FIG 69. Monthly energy utilisation for growth, respiration and production of exuviae and faeces as a percentage of consumption in a representative larva of Pyrrhosoma nymphula during its two-year development in nature. Based on pooled data from both year classes in two ponds in northern England. (After Lawton.46)
measure of central importance in studies of biological productivity, itself a criterion of richness much used by ecologists when comparing habitats. Analyses of this kind make it possible to determine the proportion of macroinvertebrate biomass contributed by dragonflies in a community of freshwater organisms. For example, a population of Leucorrhinia larvae in acidified fishless lakes accounted for about 50 per cent of the total biomass of macroinvertebrates down to a depth of 6 metres.50 Usually predators make up a smaller biomass than their prey, but sometimes the biomass of odonate larvae exceeds that of their prey, indicating that they are playing a major role in regulating their prey.51 In such a case stability is maintained because the prey has a much higher turnover rate than the predator. This enables the predator to survive on a relatively low prey biomass.
Among invertebrates, odonate larvae are large, obligate predators and so can be expected to have considerable impact on community structure. When Cordulegaster boltonii larvae invaded an acidic, spring-fed stream this species became the new top predator. Food-web complexity changed markedly, presumably as a result of the opportunistic foraging style of C. boltonii, its large size range and the dietary shifts during its development.52 It reduced the abundance of the former top predator, the trichopteran Plectrocnemia, and the stonefly Nemurella. Global warming is thought to have triggered the invasion, which perhaps gives us a foretaste of what will happen if C. boltonii ever becomes established in Ireland.
Odonate larvae have often been (uncritically) promoted as promising agents of biological suppression of insect pests (e.g. mosquitoes). But, for compelling reasons,53 such introductions are unlikely to be effective, except in the very short term, and in closely confined habitats, such as treeholes54 or artificial water-storage containers.55
INTERACTIONS WITH OTHER ORGANISMS
The main groups of organisms likely to be associated with larval Odonata in Britain are reviewed in this section. A more extensive account is given elsewhere.56
Comments on some of the organisms listed in Box 12 may help the observer to recognise them and understand the nature of the association.
THE MAIN GROUPS OF ORGANISMS LIKELY TO BE ASSOCIATED WITH LARVAL ODONATA IN BRITAIN
Epibionts include algae, Protozoa, Rotifera, bivalve molluscs and tube-dwelling chironomid larvae attached to the outside of the body, as well as the early stages of mites that are later ectoparasites of adults after emergence.
Pathogens are presumably present, but virtually nothing is known about them.
Parasites include Protozoa (Sporozoa, Gregarinida) that inhabit the gut and can be carried over to the adult stage, and several major groups of invertebrates (especially Trematoda, Cestoda and Aschelminthes) that use odonate larvae as intermediate hosts in a parasite life cycle that is completed by reproduction in a definitive host that is usually an habitual predator of odonate larvae, such as a fish, amphibian or bird.
Predators. Apart from Odonata (of their own and other species) the main predators of dragonfly larvae are fish and birds and perhaps amphibians, as well as some of the larger aquatic beetles (Coleoptera) and bugs (Hemiptera: Heteroptera).
Epibionts
The juvenile, dispersal stage of the freshwater mussel Dreissenia polymorpha sometimes attaches to the outside of larvae of Gomphidae, Corduliidae and Libellulidae57 and is found on F-0 exuviae after the carrier has emerged. Attachment to such a site presumably results in the premature death of the mussel. The attachment of larval cases of chironomids to larvae of Gomphus vulgatissimus58 and Somatochlora metallica59 may reflect a need by some commensal organisms for a secure substrate and, in flowing water, for a substrate that does not get buried in silt and that consistently faces upstream.60
Parasites
Larvae ingest sporocysts of gregarine Protozoa which develop in the host’s gut to form immature sexual forms, or gamonts, each of which can attain a length of 0.8 millimetres.61 In a severe infection, gamonts come to pack the larva’s gut and seriously impair digestion, causing malnutrition and sometimes death.
When the density of gregarines reaches 300-500 per host, the gut wall can rupture, killing the host.62 Enallagma cyathigerum has been recorded as a host of gregarines.62 Gregarines are found also in the gut of adults, sometimes, perhaps, having been carried over from the larval stage. The gregarine burden in adults is inversely correlated with survival of both sexes of the North American coenagrionid, Enallagma praevarum.63
Adult Calopteryx haemorrhoidalis infested by gregarines exhibit lower mating success, and the intensity of their wing pigmentation is negatively correlated with their parasite load.64 This finding may indicate how a potential partner could assess the reproductive fitness of a potential mate (by monitoring the intensity of wing pigmentation). Gregarines infest Calopteryx splendens early in the prereproductive period and are more numerous in older hosts. Infested adults accumulate less fat during the prereproductive period and consequently are less able to secure and maintain territories later.65
Parasites include several other major groups66 and commonly infest both larvae and adults. Infestation often occurs during the larval stage. For example, metacercariae of the digenetic trematode Haematolaechus (a fluke) invade zygopteran larvae at the base of the caudal appendages or the intersegmental membranes.67 Invasion of Anisoptera commonly takes place through the rectum, presumably being assisted by the inhalation that serves the respiratory system. When cercariae are invading the rectum, dragonfly larvae (e.g. Sympetrum) have been seen first to suspend respiratory movements for several seconds, then to exhale vigorously, and finally to try to catch and eat the cercariae.68
Many genera of digenetic trematodes are found as internal parasites of odonate larvae and, if the larva survives, are carried over at emergence to the host’s adult stage. Occurrence of trematodes in Odonata is sometimes correlated with the presence in the vicinity of genera of molluscs that form the trematode’s first intermediate host. The odonate larva is typically the second intermediate host. The metacercariae form cysts inside the larva, sometimes assuming the appearance of galls located in muscular or fatty tissue. The parasite’s life cycle is completed when a vertebrate predator such as a fish, frog or bird ingests the dragonfly larva. An unpleasant fluke infection of poultry, which can be fatal to the birds, is caused by a species of Prosthogonimus. It is now known that birds can contract this parasite by consuming adult dragonflies but, in the 1920s, long before this fact was established,69 folk wisdom maintained that, to prevent infection, birds should be denied access to the water’s edge when dragonflies were emerging there or when adults were present in large numbers.70 Libellula quadrimaculata is known to be a host, and its intermittent occurrence at very high density and its propensity for mass migration are thought to make it a formidable disseminator of this parasite. In countries where dragonfly larvae form part of the human diet (e.g. in Southeast Asia), humans may contract the flukes by ingesting uncooked dragonfly larvae.71 The effect of trematode infection on the survival of larval or adult dragonflies is unknown and would be difficult to determine, partly because it may sometimes be indirect, as when a parasitised individual becomes more vulnerable to predation by virtue of its weakened condition,72 and partly because the dragonfly may be harbouring other kinds of debilitating parasite. Similarly, some tapeworms, which are ingested as eggs by odonate larvae, form cysts within the odonate larva and then complete the life cycle in a predatory bird such as a heron.
Predators
Predators of odonate larvae, apart from other Odonata, aquatic beetles and Hemiptera, are mainly vertebrates. Many species of bottom-feeding fish consume large numbers of larvae73 and predators of less importance include amphibians (frogs and newts), reptiles (snakes), water birds (such as kingfishers and herons)74 and leeches.75 In the tropics, crocodiles, especially when young, consume large anisopteran larvae, often when these are emerging at the water’s edge at night.76
There is little doubt that fish are the most influential predators of odonate larvae. Odonate biomass is greater in fish-free waters, although there is no correlation between fish presence and odonate species richness.77 The impact of fish varies according to the species of Odonata, presumably reflecting the dragonfly’s antipredation behaviour. For example, in Swedish lakes the abundance of larvae of Aeshna juncea and Leucorrhinia dubia correlates negatively with the presence of fish, but for Coenagrion hastulatum and Libellula quadrimaculata there is no correlation, and for Erythromma najas and Cordulia aenea this correlation is positive.78 We have observed that the introduction of Rudd, Leuciscus erythrophthalmus, into a garden pond was followed by a sustained drop in the numbers of Sympetrum striolatum (a sprawler) but no noticeable change in the numbers of Pyrrhosoma nymphula (a cryptic clasper) which continued to be abundant.
Predation of larvae by other Odonata (of the same or different species) can be substantial, contrary to early assumptions. In some species, for example Ischnura elegans79 and Pyrrhosoma nymphula,46 intraspecific predation is virtually nonexistent, but records from North America show that it can be heavy in Aeshna juncea, in populations where a three-year life cycle obliges larvae of different size cohorts to coexist80 and where populations of coexisting species have contemporaneous components differing widely in size.81 Evidently cannibalism (i.e. intraspecific interodonate predation) can be expected when larvae of different sizes coexist in time and space. Experiments in the laboratory and in field cages show that, under such circumstances, a size difference of two or more stadia renders such predation highly probable.80 Zygopteran larvae seem to be more vulnerable than Anisoptera to intra-odonate predation, especially if fish are absent. In middle North America, where there are numerous species of Enallagma, including E. cyathigerum, their larvae are subject to predation, either by fish (where these are present) or by large Anisoptera (such as Anax) in habitats that lack fish.82 In North America the antipredation behaviour exhibited by larvae of Enallagma differs in lakes with and without fish, depending on the kind of predator they are exposed to. In lakes containing fish, larvae respond to a predator’s approach by becoming immobile and thus escaping the attention of fish, whereas in lakes without fish, where the top predators are Anisoptera, Enallagma larvae have developed much greater swimming speeds and enhanced physiological ability to fuel strenuous activity.83 This equips them well to swim away, a strategy which helps them to escape from an anisopteran larva but which would invite capture in the presence of a fish. This dichotomy of antipredation responses in North American species of Enallagma larvae reflects the selective pressure exerted on them by predation and, considered alongside other studies, emphasises the protection they gain by occupying submerged water plants.84 Interestingly, this relationship is not necessarily reproduced among European populations of E. cyathigerum which, unlike North American populations of this species, can coexist with fish,85 although they sometimes suffer high predation as a result86 and appear to have developed (or retained) rapid swimming as an antipredation response.87 This difference between Old World and New World populations of ‘E. cyathigerum’ is less surprising now that it has been recognised that they may not have the same evolutionary history.88 It may be significant that, in two populations where larvae of E. cyathigerum in Britain were coexisting with fish (trout), the larvae were concentrated in dense swards of Shoreweed, Littorella uniflora.89
We suppose that larvae and adults of the beetle Dytiscus, and of aquatic bugs (Nepa, Notonecta, Ranatra) prey on larvae, especially Zygoptera, but there are few records. We have seen the aquatic bug Ilyocoris consuming larvae of Coenagrion puella in a garden pond.
The intensity of predation on Coenagrion puella larvae by the backswimmer Notonecta was much reduced under experimental conditions when larvae were provided with refuges in the form of artificial water plants. Presumably water plants also serve an important function for ambush foragers – as perches from which to forage. These considerations emphasise the importance to larvae of finding and defending favourable perches. They may well allow us to interpret the aggressive behaviour that Zygoptera larvae show, apparently in defence of their perches. Such behaviour is complex and stereotyped. A North American species of Ischnura exhibits more than 40 discrete behaviours when close to conspecifics,90 larvae of Pyrrhosoma nymphula exhibit 1791 and those of the diminutive Agriocnemis pygmaea 25.92 These behaviours include face-to-face staring, side-to-side swinging of the abdomen and jabbing by the caudal lamellae. Such ritualistic confrontations typically end with one adversary moving away. This behaviour must result in larvae becoming spaced out among perches, and in selection for vigour in aggressive display. In larvae of Ischnura elegans face-to-face staring leads to increased success in intraspecific larval encounters.93 (The vanquished larva in such an encounter vacates the field.) Curiously, intraspecific aggressive behaviour, which exhibits so rich a repertoire in larval Zygoptera, has seldom been recorded between larvae of Anisoptera, although large larvae of the North American Anax junius stare fixedly at each other, sometimes for many minutes at a time.94
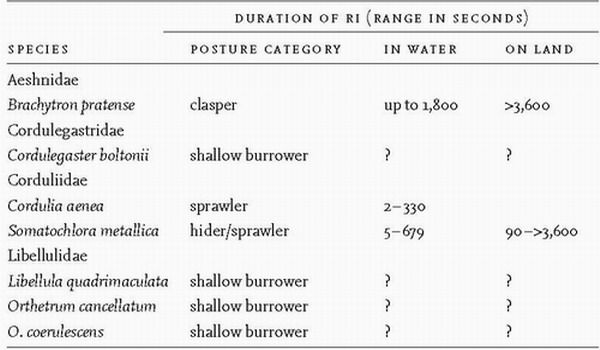
As we have seen, zygopteran larvae typically escape from predators by swimming to avoid Anisoptera and by adopting immobility to escape notice by fish. Larvae of some Anisoptera have other defensive behaviours in reserve if actually grasped or touched by a predator. These entail one of three strategies. Initially, a larva will try to wriggle free. Then, if it is a robust anisopteran such as Aeshna cyanea, it may try to pierce its attacker using the sharp caudal appendages (paraprocts) which for this action are tightly closed, forming a three-sided spike that can draw blood from a human finger.95 Also the labium or mandibles may be used to nip an attacker. Finally the larva may feign death by adopting a stiffly immobile posture which is likely to be maintained, despite further physical interference, for several to many minutes, as, for example, in Brachytron pratense.96 The hypothesis that reflex immobilisation has been selected for by the intensity of predation by fish receives support from the finding that larvae of Leucorrhinia dubia from fishless habitats in Sweden, when subjected to a simulated fish attack, exhibited no reflex immobilisation but tried to escape, whereas larvae of three other anisopteran genera feigned death under the same conditions.97 British species that sometimes feign death are listed in Table 4. This form of antipredation behaviour has been investigated thoroughly in species in Japan98 and Europe.99 This condition entails a marked prolongation of the brief immobility, lasting only a few seconds, that is often an immediate response to physical disturbance, especially in species that coexist with fish. The posture adopted (Fig. 70) tends to be characteristic of the family. Death feigning, or reflex immobilisation (RI) (or thanatosis), has so far been recorded in eleven families, all
but three of which are Anisoptera. Immobilisation tends to last longer if the larva is out of water. In species of Somatochlora, RI is exhibited by larvae in stadium 299 and so presumably occurs throughout larval life.
Death feigning has been recorded in only three families of Zygoptera, including Coenagrionidae.98 Sometimes larvae of Pyrrhosoma nymphula will remain immobile for several minutes, in a flexed position, often lying on their backs, after being disturbed.
The mere presence of a predator can entail costs for a zygopteran larva, even if it avoids being eaten. A larva of Pyrrhosoma nymphula can detect the presence nearby of a larva of Aeshna juncea by waterborne chemical stimuli, with the result that the P. nymphula larva forages less. The A. juncea larva is chemically labelled by its diet,100 a phenomenon found in some predatory fish such as Pike, Esox lucius,101 and in this way zygopteran larvae can be warned of its proximity.102 The discovery that zygopteran larvae detect some predators chemically rather than visually has far-reaching implications for our understanding of their ecology, behaviour and foraging success. One may wonder whether adults retain this sensory ability, enabling ovipositing females to detect the presence of fish.103
A zygopteran larva can increase the likelihood of escaping from a predator by voluntarily relinquishing a leg or one or more of its caudal lamellae – a process termed autotomy.104 This may allow a larva to escape to live another day, but it is not without cost. Loss of a lamella by Lestes sponsa increases the probability of its
FIG 70. Postures adopted by Somatochlora larvae during reflex immobilisation, viewed from below (a, c), above (b) and left side (d). (a, b) S. metallica, stadium F-0, length 25mm; (c) S. alpestris, stadium 2, length 1.2mm; (d) S. flavomaculata, stadium F-0, length 22mm. (After Wildermuth.99)
being eaten by conspecifics or preyed upon by the Backswimmer, Notonecta, and it also reduces hunting success by the deprived larva.105
SURVIVORSHIP
There have been several systematic studies of survivorship of larvae in the field. These have produced values for survivorship (i.e. the number of larvae surviving the larval stage as a percentage of the numbers entering it) ranging from 0.2 per cent (for the Japanese subspecies Cordulia aenea amurensis over the five-year larval period)106 to 16 per cent (for Pantala flavescens over the three-month larval period in Indiana, USA).107 Most values obtained, however, lie well below 10 per cent. The corresponding value for Pyrrhosoma nymphula, during a two-year larval period in a fish-free habitat in northern England, was 0.2 per cent.108 An unsurprising implication of such findings is that a long larval life decreases survivorship. Survivorship during the larval stage sometimes shows a disproportionate decline shortly before emergence, while larvae are undergoing metamorphosis.109 In a dense population of Anax imperator only 46 per cent of F-0 larvae survived during the month before emergence.29 In the European gomphid Onychogomphus uncatus, mortality of overwintering F-0 larvae can be 99 per cent.110 In a high-altitude population of Aeshna juncea only 2.5 per cent of hatched larvae were still alive after three years.111
The functional role of odonate larvae in aquatic ecosystems depends primarily on whether or not they coexist with fish. If fish are present, the fish are usually the top predators, and Odonata occupy an important intermediate role in the food web, preying on a wide variety of smaller invertebrates, as well as on each other. When fish are absent, the larger Anisoptera, such as aeshnids, can be the top predators and the behaviour of Zygoptera (especially) may be modified accordingly. Species-rich communities of Odonata can exhibit great stability in the relative abundance of constituent species from year to year.112 Given the complex interactions between odonate larvae and other organisms, not to mention the annual fluctuations in physical factors, such stability is remarkable, reminding us that the ways in which populations are regulated remain poorly understood. Studies of the composition and dynamics of larval odonate assemblages are few, not least because they need to be searching and long term, and to entail protracted studies of a detailed nature. Although sometimes the relative numbers of species can vary widely, the high stability of some populations that have been studied (in relatively unchanging habitats)112 indicates that regulation is taking place. The mechanism for such regulation remains obscure, although one can assume that, because it is density-dependent, it must be driven predominantly by interaction among conspecifics which, in practice, means cannibalism.
PHYSICAL FACTORS
Odonata are primarily a warm-adapted group and their abundance and species diversity diminish with increasing latitude and altitude. British Odonata constitute an impoverished, marginal, northern European fauna, subject to yet further reduction by virtue of being an island fauna.113 Within the British Isles the species diversity of Odonata decreases from south to north,114 as one would expect of a group experiencing temperature limitation. However, it is unclear at which stage or stages the limitation acts, and careful work would be needed to determine this. The limitation need not necessarily operate in the aquatic stages; it might be mainly a weather-mediated effect of inclement conditions experienced by adults. Many species of larvae can survive being frozen into blocks of ice, but the habit of moving into deeper water during winter must often enable larvae to avoid being frozen. It is unlikely that larvae in Britain ever encounter the upper temperature threshold for survival which, for species that have already been acclimatised to high temperatures in hot lentic habitats, is close to 45°C.115 Anecdotal evidence suggests that larvae of several species can survive the absence of surface water in a larval habitat.116 Such larvae have been found in minute pockets of water (e.g. Aeshna cyanea),117 in humid cavities under stones (e.g. Ischnura elegans),118 or in the dry gravel bed of a pond (e.g. Libellula depressa).119 A libellulid larva found frozen within a layer of humus in a dried-up swamp swam actively after having been thawed out in water.120 In an alpine pond in Colorado, under snow cover, a larva of Somatochlora semicircularis retained the capacity to rehydrate and then behave normally after nine months in dry moss without free water.121
Two correlated physical factors that determine habitat distribution of British species are water movement and dissolved oxygen. Species typically segregate into those inhabiting standing (lentic) and flowing (lotic) water respectively. Species of Calopteryx and Cordulegaster are almost never found in standing water, and Gomphus vulgatissimus and Platycnemis pennipes only occasionally,122 whereas many species are virtually confined to standing water. A few, such as Pyrrhosoma nymphula and Libellula quadrimaculata, can often be found in both types of habitat and, as discussed in Chapter 2, it is not uncommon for several other species to occupy both lentic and lotic habitats. No British species occupies shallow,
rapidly flowing streams with a rocky bottom, a habitat occupied by Boyeria irene, Caliaeschna microstigma and Epallage fatime in mainland Europe and by several species of dragonfly in North America. In running waters, water movement and the resulting particle-size of sediment are likely to determine the micro-distribution of burrowing larvae.123 In running waters that show wide seasonal fluctuations in depth, larvae may take refuge deep in the sediment during the season of low water.121 In standing waters, odonate larvae (especially Anisoptera) are found predominantly in shallow water less than 1 metre deep,124 but larvae of both suborders may occur in deeper water if rooted water plants are present. In New Zealand, larvae of the corduliid Procordulia grayi and the coenagrionid Xanthocnemis zealandica occur down to a depth of 19 metres (in Lake Waikaremoana) in places where there are stands of water plants.125 In the River Main, Germany, larvae of Gomphus vulgatissimus occur between depths of 0.8 and 5.2 metres, but most often between 2.5 and 3.0 metres.126 In Cordulegaster boltonii the preference of larvae for coarse sediment increases during larval development, except during F-0 when the trend is reversed. Larvae are usually totally buried except for the protruding antennae and anal pyramid (Fig. 67, p.123)..37.
Four species of Cordulegaster that occupy streams in southeast Saxony, Germany, are segregated along a stream: C. bidentata and C. insignis are confined to springs and the headwaters of their outflow streams, whereas C. boltonii and C. picta occur from the springs down to larger streams and rivulets. This complementary distribution reflects the different ways in which the two pairs of species avoid downstream drift after spates.127 Among British species Calopteryx splendens and C. virgo exhibit a complementary distribution in watercourses.
Interest has focused on the potential usefulness of Odonata as indicators of water quality and therefore on the effects on their survival of different kinds of pollution. Because pollutants seldom act singly, acute effects of individual additives are difficult or impossible to assess in the field. The main additives that pollute fresh waters are erosion products, industrial effluents, farm runoff (including pesticides) and domestic sewage. Because odonates are obligate predators, pollutants affect field populations indirectly, via the susceptibility of their prey, as well as directly, through their own exposure. One commonly used measure of organic pollution of fresh waters, including eutrophication, is the biochemical oxygen demand or BOD. This is defined as the amount of oxygen (expressed as parts per million or mg/litre) taken up by a sample during the first five days of decomposition at 20°C. Domestic sewage has a BOD of about 200 mg/litre. Work in North America has shown that only one species of Ischnura occurred where the BOD exceeded 10 mg/litre.128 It is likely that species of Ischnura are exceptionally tolerant of pollution. After a major pollution incident in April 1985, involving spillage of the organophosphate insecticide ‘Dursban 4E’ (active ingredient chlorpyrifos) into an Essex river, all species of odonate larvae were eliminated, although all had returned (presumably by recolonisation) within about 14 months.129 The first odonate species to become re-established was Ischnura elegans, which is consistent with the report that this species is often the only odonate to persist in polluted waters.130 Where organic pollutants are concerned, it may often be the heightened BOD that dominates the acute effect. Some larvae, for example Calopteryx spp.,131 possess stereotyped behavioural responses that help them to mitigate the effects of low oxygen availability in the short term, but they are still affected by the mortality of other organisms on which they depend for food. Sometimes the deleterious effects of high BOD can be offset by fortuitous human activities: Gomphus vulgatissimus in a heavily polluted lake in Germany was able to survive where turbulence caused by boat traffic increased the amount of dissolved oxygen.132
Pollutants include metals and pesticides, and their effects can be severe. For example, high concentrations of tin, such as are found in mine tailings, are associated with serious deformity among emerging adults, especially of Aeshna cyanea and Pyrrhosoma nymphula.133 Tin was used as a constituent of antifouling paints (as tributyltin) on boats until a Europe-wide ban in 1987, and was a serious pollutant in the Norfolk Broads. Apparently lead is accumulated in considerable amounts in the midgut, fat body, rectum and cuticle of exposed larvae,134 although its harmful effects, if any, in those organs are unknown. The effects of individual pesticides can only be determined from monofactorial experiments in the laboratory which may bear little relation to conditions in the field. However, by combining evidence from laboratory tests and field trials, certain provisional conclusions can be drawn about the effects on Odonata of a pollution incident. First, Odonata are often promptly affected by diminution of their prey. Second, the direct toxicity to Odonata larvae of insecticides probably follows approximately this sequence, in decreasing order of severity: organochlorines, organophosphates, rotenone, carbamates, insect growth regulators, microbials, surfactants and plant oils.135 Pyrethroids, which can cause massive invertebrate mortality for miles downstream of even a small spillage, deserve a prominent place in this list.
So, one may ask, can odonate larvae serve reliably as indicators of the type and quality of habitats? Subject to the qualifications we have recognised, it can be concluded that:
1) Odonata can be useful as bioindicators, but mainly as one among a mosaic of correlated habitat attributes that together indicate ‘water quality’ and stages of ecological succession, including degradation caused by human impact; and
2) the use of species assemblages, rather than individual species, strengthens this usefulness by rendering less fragmentary the correlation between habitat and Odonata, and by buffering that correlation against the effects of some small deficiency in the array of physical and biotic conditions needed by each species, and against intraspecific variation.136 When Odonata are enlisted as bioindicators, one must remember that they need more than pollution-free water.
OPPORTUNITIES FOR INVESTIGATION
Perhaps the greatest obstacle to progress in our understanding of the behaviour and ecology of larvae is the difficulty (often the near impossibility) of identifying to species larvae in early stadia, despite the relative paucity of the British fauna. All over the world larvae still tend to be the Cinderellas of odonatology: more effort is required to find them, they are less attractive to photographers, and they are often difficult to identify. Nevertheless, ecologically the larva is probably the most influential stage in the life cycle. In Britain, as in most temperate latitudes, it lasts far longer than the egg or adult and has intimate links with the reproductive habitat. At present larvae in the first few stadia are extremely difficult to distinguish to species. In some genera of Libellulidae and Aeshnidae, stadium-2 larvae bear characteristically shaped protuberances on the top of the head.137 If such features exist in any British species, they might prove useful for identifying early stadium larvae.
There is an urgent need for keys to earlier stadia. To rectify this will be a mammoth task, involving the raising of many species from the egg to the final larval stadium, and the search for diagnostic characters which, in early stadia, may prove elusive. Fortunately, a few species (e.g. Anax imperator, Pyrrhosoma nymphula) can be identified in all stadia by their shape, posture and colour pattern, but for the great majority of species this is impossible. Moreover, even if the information on external morphology does become available, as it has for several species of North American Enallagma, there is still no guarantee that effective keys can be constructed.138 Only for larvae of Norwegian dragonflies does a key exist which comes near to this ideal and this, the product of expert and painstaking work over many years,139 is unlikely to be duplicated elsewhere. There is, however, a way forward, available to anyone with access to simple laboratory facilities. This entails the use of cellulose acetate gel electrophoresis, the apparatus for which can be readily packed and transported for use in the field.140 The trace, or imprint, so obtained is unique to larvae (and adults) of each species, regardless of stadium. To develop this method so that it could be used routinely would probably represent the most valuable contribution that could be made at present to our knowledge of larval behaviour and ecology. An approach offering similar promise for identifying early-stadium larvae is to detect the presence of species-specific proteins in the homogenates of thoracic muscles.141
Building on the secure foundation that such an advance would provide, other topics that offer rewards for the investigator could be explored with confidence. Examples are studies of voltinism, which are needed to understand the mechanisms of seasonal regulation.
Another potentially fruitful field of study, requiring little more than an aquarium, patience and a video camera with a time-lapse facility, is the way in which some species tailor their foraging and feeding behaviour to different kinds of prey and microhabitat. This constitutes an arena where behaviour has both learnt and innate components, and where analytical studies cannot fail to be rewarding. Furthermore, as recent studies have shown,19 this approach can reveal the repertoire involved in opportunistic foraging and aggressive behaviour, which must be understood if the complex phenomenon of competition among larvae is ever going to be illuminated.
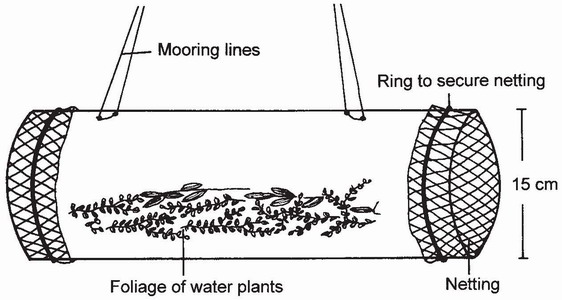
FIG 71. Design for stationary trap for collecting larvae of Zygoptera. (After Krüner143.)
Related to this topic is the need to know what kind of microhabitats larvae occupy. How do larvae of different species and different posture categories behave in simulated natural microhabitats in an aquarium? Their movements, diel activity patterns, foraging, hiding, and interactions with conspecifics of different ages are all topics worthy of further study. It would also be helpful to discover whether larvae that occupy very small bodies of water depend on terrestrial prey.
The invention of a static trap for larvae142 (Fig. 71) offers opportunities for monitoring as well as quantifying the activity of larvae in the field. We know of no tests of such a trap in Britain, but in Germany Ulrike Krüner collected larvae of Coenagrionidae and Lestidae by this means.143
To determine the prey of larvae and relate this to larval size and microhabitat would require a microscope, patience and skill, but no other specialised equipment. It can be done, without sacrificing larvae, by examining the faecal pellets they void after capture. The contents of the pellets can then be compared with other invertebrates occupying the same habitat, and, if fortune smiles on the investigator, identified.