CHAPTER 6
Larval Development and Emergence
GROWTH
LARVAL DEVELOPMENT IS punctuated by a variable number of moults (Box 13).
The final stadium (F-0) varies little in size, any variation in the number of stadia being compensated for by the growth ratio between stadia that precede F-0. Because the number of stadia can vary, it is inappropriate to designate the degree of intermediate larval development by stadium number. The head width generally increases at a uniform rate during development, and so is a useful measure of larval size1 and is commonly used by investigators studying the rate of growth in natural populations. Body length can also be useful for those who lack the facility to measure head width, but it is subject to wider variation because the abdomen may be unusually extended before a moult (Fig. 72) and telescoped at some other times.
Species of Odonata differ with regard to their growth rate when exposed to identical conditions of temperature, daylength and food. For example, lestids usually grow more rapidly than do coenagrionids.2 Such differences correlate with the categories of lifestyle described in Table 2, p.119; for example, the higher foraging rate of Lestes sponsa correlates with a high growth rate.3 Growth rate can also be affected by variables such as temperature and prey availability, and by responses to daylength that play a crucial role in seasonal regulation of the life cycle (p.167). Growth rate of L. sponsa can be depressed by the mere presence of a predator (Aeshna cyanea), a nonlethal effect that is perhaps caused by a lowering of the assimilation efficiency and/or by raised metabolic rate,4 or perhaps by a judicious curtailment of foraging behaviour.
GROWTH AND MOULTING
Arthropods (including dragonflies), whose bodies are typically enclosed in a hard, inelastic integument, increase in size by undergoing successive moults (or ecdyses) (Fig. 72). Within a few hours after each moult, while the cuticle of the larva (or adult) remains pale and soft (i.e. while the animal is still teneral, Fig. 72), the body dimensions can increase by a factor of about 25 per cent if the larva (or adult) can swallow water (or air) to inflate the body (and wings). A short time before a moult, the epidermis separates from the cuticle of the existing stage and begins to secrete the new cuticle of the forthcoming stage. This process, termed apolysis, marks the beginning of the pharate condition in the moulting cycle. The interval between successive apolyses is termed an instar and the interval between successive moults is called a stadium. Odonata pass through 8-18 stadia (average 12.4) between the egg and adult stages, the first stadium being known as the prolarva.1 (p.107). The number of stadia can vary within a species and even among siblings originating from eggs of the same batch laid on the same day. The last few stadia are numbered backwards from the final stadium (F-0) thus: F-1, F-2, etc. During the last days of F-0, changes in morphology, physiology and behaviour take place in anticipation of the final moult (emergence) which will disclose the adult. These changes constitute metamorphosis. Some of them are visible externally and can provide a means of predicting when an individual will emerge, notably those occurring in the compound eyes, the wing sheaths and the prementum of the labium (Fig. 73).
During the last four or five stadia the rudiments of the wing sheaths become visible externally and increase in length greatly at each moult. Indeed the interstadial growth ratio of the wing sheaths is close to 1.75, compared with the corresponding value of about 1.25 for other parts of the body.1 This means that wing-sheath length usually provides a reliable criterion for recognition of the last three stadia, giving information that is essential for inferring the ways in which the life cycle is regulated (p.172). Occasionally one finds a stadium intermediate in dimension between F-1 and F-0,5 but this is very rare.
In the rest of this chapter many of the species references are to Anax
FIG 72. Larva of Aeshna cyanea. The pale, soft cuticle shows that it has recently moulted to stadium F-1 (Robert Thompson).
imperator. This is because a thorough study was made of the ecology and behaviour of this species at a stem habitat (Box 6, p.76), the Fish Pond, in southern England in the early 1950s.6 Although this study provided a great deal of information not easily obtained for other species, the temptation to regard it as representative of all species or all populations of A. imperator in Britain should of course be resisted.
METAMORPHOSIS
Changes associated with metamorphosis are conspicuous and easy to categorise in aeshnids (Fig. 73) where they can be used to track the progress of metamorphosis in a larval population and also to predict when emergence will begin. Metamorphosis in British species is almost always followed within weeks by emergence. The only known exception is Cordulegaster boltonii, a spring species, in which a few larvae in a population in southern Spain showed signs of incipient metamorphosis in autumn.7 In the latest stages of metamorphosis, normally encountered only a few days before emergence, the internal tissues of the labium
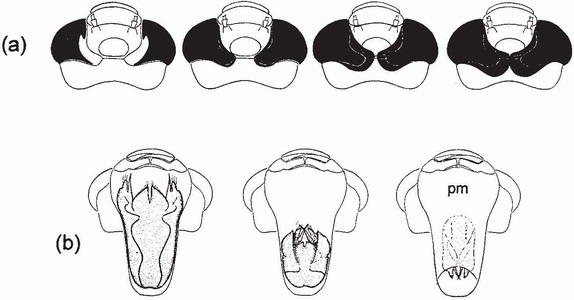
FIG 73. Heads of F-0 larvae of Anax imperator, from above, showing progressive changes associated with metamorphosis. (a) expansion of the compound eyes towards the midline (dorsal view); (b) retraction of the adult labium within the larval prementum (pm) (ventral view). (From Corbet..6)
retract within the prementum, passing across the labial suture and finishing up in the postmentum. In all species of British Odonata this progression can usually be seen, without extending the labium, using a hand lens, and can be put to good use in field studies. The easiest way to determine whether metamorphosis has begun is to note the orientation and swelling of the wing sheaths.8 Such changes can usually be detected with a simple hand lens or even the unaided eye, in Zygoptera as well as Anisoptera. Strictly speaking, metamorphosis has not been completed by the time of emergence: some of the body muscles and their nerves do not complete the transformation until several days after emergence.9
EMERGENCE
When an individual is ready to emerge its first action is to select an emergence support. Larger Anisoptera (e.g. Anax imperator) do this several days in advance, swimming towards the water margin at night.2 Zygoptera, which, weather permitting, emerge in mid-morning, swim towards the shore in daylight, possibly orientating by following a positive temperature gradient in the surface water.10 Frequently an individual will find a suitable support, usually the upright stem of an emergent water plant, before reaching the water’s edge, but, if not, it will often leave the water and clamber over the ground in search of a support. An individual of Pyrrhosoma nymphula, engaged in such an overland journey, was seen to meet a spider around the side of a grass tussock.10 Remarkably, the dragonfly whipped the tip of its abdomen forwards and jabbed its pointed caudal lamellae at the spider, which promptly withdrew. This event is noteworthy because the dragonfly, although adopting a familiar larval display posture (p.113), was at the time no longer a larva but an adult enclosed in a larval skin (i.e. a pharate adult). Anax imperator larvae sometimes travel up to 6 metres from the water’s edge before selecting an emergence support, and up to a height of 5 metres;.2 once an individual of A. imperator crossed a grassy meadow and passed several trees and a fence before emerging more than 30 metres from the nearest water.11 Cordulegaster boltonii sometimes travels far before emerging,12 F-0 exuviae having been found in situ up to 6 metres above and 4.2 metres away from a stream margin.13 Some libellulids in other countries have been seen to travel more than 40 metres before moulting.14
Once an individual has selected a position, head upwards, on an emergence support, and before starting to moult, it usually swings the abdomen and legs in a characteristically jerky manner, apparently to verify that it is securely anchored
FIG 74. Searching and testing movements of the hind legs made by a F-0 larva of Cordulia aenea while selecting an emergence site. (From Wildermuth.15)
and not too close to any object that might impede its forthcoming transformation, during which its expanding wings could be permanently damaged if they touched anything. After Cordulia aenea has ascended a potential emergence support it makes ‘searching’ movements with the fore and mid legs. Then, while stationary, it makes circling movements with the hind legs (Fig. 74). Only then does it clasp the support tightly and begin to moult. If disturbed during the testing process, it descends and chooses a new support.15 When emergence supports are in short supply, it can happen that the earliest individuals to select their emergence supports are unable to complete the final moult because individuals arriving slightly later clamber over them and use them as emergence supports. Such interference can be a major source of density-dependent mortality.2
Sometimes exuviae (usually, it seems, of Zygoptera) are found in situ but upside down16 (Fig. 75). This is unexpected and so far unexplained. In some cases, but certainly not all, such an exuvia may have become inverted (perhaps by wind) after the adult has vacated it.
When emergence has been completed, the exuvia (Fig. 76) of the F-0 larva remains on the emergence support. The value of the exuvia as a tool for quantitative ecological studies cannot be overstated. Exuviae of most British species can be determined to species17 and, except in strong wind or rain, they remain in situ for several days. They provide the most secure evidence that a generation has been successfully completed in a habitat. Also, if exhaustive
FIG 76. The collection of F-0 exuviae from around the edge of a pond is a good way of establishing which species are breeding and the size of the emerging population (Robert Thompson).
collections are made at short, regular intervals (preferably daily), exuviae reveal precisely the temporal pattern of emergence, or emergence curve, an extremely informative ecological parameter (Box 15, p.163). Additionally, they can be used to determine the size of the population at emergence. Moreover, because their sex can be determined, exuviae provide a reliable measure of the sex ratio at emergence,18 a valuable baseline for interpreting sex ratios encountered later among mature adults at the reproductive site.19 Exuvia collections are the method of choice for characterising the emergence curve of Anisoptera, but they are less suitable for Zygoptera whose exuviae are small and inconspicuous, more easily dislodged by wind and rain and sometimes telescoped to an extent that renders them very difficult to find or identify. For Zygoptera it can be more effective to record the presence of teneral adults.20 To apply this method rigorously, the investigator needs to take into account the diel periodicity of emergence so as to detect adults before they leave the emergence site on the maiden flight. Another advantage of this method is that the teneral adult can be confined for 24 hours, while the cuticle hardens, and then given an individual mark from which its exact postemergence age can be known whenever it is resighted (Appendix 2).
The successive stages of emergence are described in Box 14.
During stage 1 of emergence an anisopteran changes from employing aquatic to aerial respiration, losing the use of its rectal breathing apparatus and relying on the thoracic spiracles. During emergence, the exuvia, clinging securely to an emergence support, is usually vertical, head uppermost, or nearly so. Exceptions to this are Gomphus, which often emerges on a horizontal surface, and those individuals of other species which appear to emerge upside down.
During most of stage 2, the head, thorax and legs are out of the exuvia and the body is attached to it only by the posterior part of the abdomen, which remains inside the exuvia. The emerging individual remains immobile for a relatively long time in this position which is accordingly referred to as the resting stage. During the resting stage the head, thorax and legs hang downwards (the hanging type (Fig. 83) as in Aeshnidae, Calopterygidae, Cordulegastridae, Corduliidae and Libellulidae), or they face upwards and forwards (the upright type, (Fig. 84), as in Coenagrionidae, Lestidae and Platycnemididae), or horizontally and facing forwards, as in Gomphidae. During stage 3 the wings and abdomen attain their full size. The end of stage 4 (Fig. 82) often cannot be precisely determined, partly because takeoff in a flight-ready individual may be postponed until it receives the appropriate stimulus or until the ambient temperature permits spontaneous flight. For example, on warm nights in southern England, newly emerged adults of Anax imperator attain flight readiness well before sunrise, but do not take to the wing until dawn twilight.6 Completion of stages 1-4 may take an hour or
THE STAGES OF EMERGENCE
It is useful to distinguish four stages of emergence.
- The dragonfly is completely out of the water but the cuticle has not yet split (Fig. 77).
- The cuticle of the head and thorax has split (Fig. 78).
- The abdomen of the adult has been withdrawn from the exuvia (Fig. 79).
- The wings and abdomen are expanding. The stage ends when the abdomen and wings have attained their full size and (in Anisoptera) the wings have opened and the dragonfly is flightworthy (Figs 80, 81, 82).
FIG 77. Libellula depressa in stage 1 of emergence (Robert Thompson).
FIG 79. Libellula depressa in stage 3 of emergence (Robert Thompson).
FIG 81. Libellula depressa late in stage 4 of emergence (Robert Thompson).
FIG 82. Orthetrum coerulescens at the completion of stage 4 of emergence. The teneral adult is ready to fly (Robert Thompson).
considerably more in Britain, but can vary widely, probably depending on ambient temperature. Emergence of Gomphus flavipes in Germany took between 15 and 59 minutes.21 In Britain, emergence of Zygoptera probably takes between one and two hours (e.g. 83 minutes in Erythromma najas),22 whereas large Anisoptera probably take two to four hours (e.g. more than three hours in Aeshna juncea at high altitude23 in Japan and about three hours at night in Anax imperator6). At low latitudes emergence can be completed more rapidly: a small gomphid, Paragomphus genei, by Lake Tchad, at 13°N, emerging by day in sunlight, completed stages 1-4 in 20 minutes,24 and another gomphid, Crenigomphus renei, by Lake Albert at o-2°N, emerging after dark, did so in 30 minutes.25
In Britain many species emerge in mid-morning, but some of the largest Anisoptera leave the water close to sunset, complete the moult by about midnight and then remain on the emergence support, presumably in stage 4, until dawn
FIG 83. Cordulia aenea during the resting stage of emergence. An example of the hanging type (Steve Cham).

FIG 84. Pyrrhosoma nymphula during the resting stage of emergence. An example of the upright type (Ann Brooks).
twilight, when they take off for the first time (i.e. perform the maiden flight) (Fig. 85). The maiden flight of a large dragonfly like Anax imperator during a synchronised emergence is a memorable sight. At the first glimmer of morning light, just before the last bat returns to roost, adults begin to vibrate their wings, supposedly to warm the thoracic muscles and prepare them for flight.26 After some minutes of wing-whirring, clearly audible to the human observer, an adult walks slowly to the top of its emergence support and then, after increasing its wingbeat frequency, it becomes airborne.6 The air temperature in Britain at dawn in May and June (when A. imperator emerges) can be as low as 6-10°C and thus well below the normal threshold for spontaneous flight. One may assume that an extended period of warm-up by wing-whirring is a prerequisite for the maiden flight in species that have committed themselves to nocturnal emergence at high latitudes. It seems that this activity may be stimulated by light of a certain low intensity. To judge by observations made in southern England, an early maiden flight is of high selective value as a means of reducing predation by birds: about ten minutes after the first wave of newly emerged adults had left the emergence sites at the Fish Pond (Fig. 44, p.77), Blackbirds,
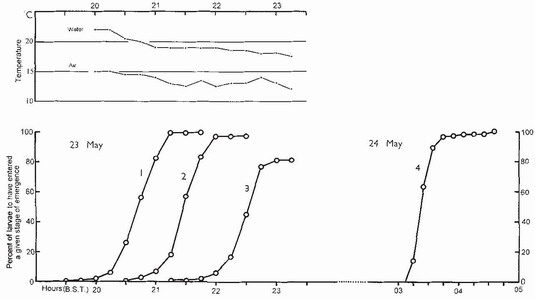
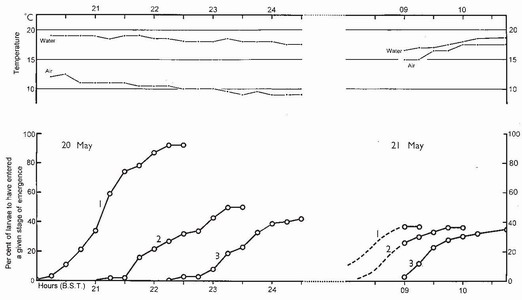
FIG 85. Normal diel periodicity of emergence of Anax imperator, illustrated by counts of larvae entering the four successive stages of emergence (Box 14). Asymptotic values for stages 1-3 differ because of mortality. The maiden flight (end of stage 4) at sunrise, for which a different sampling area was used, is much more closely synchronised than evening emergence. The air temperature (upper frame) remains permissive for emergence until midnight. (From Corbet..6)
FIG 86. Diel periodicity during divided emergence of Anax imperator. Conventions as in Fig. 74, p.149. Asymptotic values of stages 1 and 2 in the evening differ primarily because of the return to water of about 40 larvae at 22:30 h, after the air temperature had fallen below 10°C; those of stages 2 and 3 differ because of mortality. Most larvae which had returned to water emerged between 09:00 and 10:00 h the next morning, presumably when the air temperature again became permissive. (From Corbet.6)
Turdus merula, began to harvest stragglers that had failed to depart in the first tranche..6
Species of Anax, in Europe and North America, modify the emergence pattern when nights are cold (below about 10°C), thus avoiding being exposed on emergence supports, unable to fly, early the next morning. What happens then is that, at sunset, individuals leave the water as usual, but if the air temperature is below about 10°C they postpone the final moult and return to the water (which will still be warmer than the air). Having lost their ability to use jet propulsion, they swim awkwardly, waggling the abdomen from side to side, in the manner of a zygopteran larva. They then leave the water again in mid-morning the following day, when the air temperature has become permissive. This modification of the normal diel periodicity of emergence is termed divided emergence (Fig. 86) and has been observed in Anax imperator in Britain,6 in Anax junius in southern Ontario27 and Orthetrum cancellatum in northeast Spain.28 In northern Britain, in exposed sites, Aeshna caerulea emerges between noon and early afternoon.29 Such a habit probably greatly increases predation risk, this being the warmest time of the day, albeit in a cold, windswept habitat.
Recreational boating can reduce survival by causing waves, threatening the stability of emergence supports,30 and dislodging emerging adults.
Once airborne, an adult A. imperator rises to about 3 metres above any obstacle and then flies away, on a horizontal course directed away from the water; if such an adult encounters a reflective surface in its way (such as a wet sealed road) it veers away from it..6 It is not known whether such a negative response to water during the maiden flight is general among Odonata, but anecdotal observations suggest that it is. Adults within 24 hours of emergence (i.e. in a teneral condition) have not yet hardened the body and wings and so are ill-equipped to defend themselves against robust physical contact with mature conspecifics who might harass them if they remained at or near the emergence site. The maiden flight of A. imperator can vary in length between 20 and at least 200 metres.6
To leave the emergence site promptly and decisively when stage 4 has been completed must be of survival value for a teneral female. Searching males of Aeshna juncea hover low over vegetation and try to grasp teneral females at the emergence site before the latter can embark on the maiden flight.31 This is how immature females can sometimes bear copulation marks.32
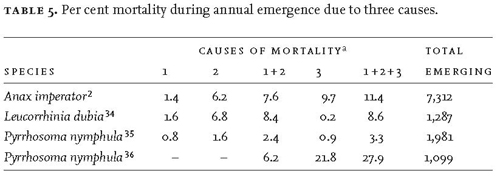
Mortality during emergence is relatively easy to study because the whole population is localised around the margin of a habitat and because the evidence of mortality is often clear. The Blackbirds that preyed heavily on Anax imperator during emergence at the Fish Pond could not have been more obliging in this regard: after collecting a teneral adult from its emergence support, a bird would take the dragonfly to a nearby ‘anvil’ and remove the wings before carrying it away to its nest.6 The anvil was a patch of bare, hardened soil in a sheltered place, and the dismembered wings remained in situ for several hours. (In this case the investigator was fortunate that ants were not taking the wing fragments away.) Wings or fragments of wings of Anisoptera can be readily assigned to fore and hind, and left and right wings. So it was straightforward to estimate from the wings that remained on the anvil the minimum number of adults involved. Such estimates yielded the values for this species shown in Table 5.
Mortality from all causes, but especially failure to moult and to expand and harden the wings, is strongly density-dependent and therefore likely to be exacerbated by the synchronisation of emergence. As might have been expected, almost all the annual (emergence) mortality of Anax imperator occurred during the first, synchronised peak of emergence. Whatever benefits may accrue from synchronised emergence, we encounter here a definite cost. In a population of Coenagrion mercuriale mortality during emergence was 4.9 per cent, the main cause being deformity,33 a condition often caused by overcrowding or wind. High mortality in one emerging population of Pyrrhosoma nymphula was due to
34 Pajunen 1962a.
35 Bennett & Mill 1993.
36 Gribbin & Thompson 1990.
Notes: a Causes: 1) failure to moult; 2) failure to expand and harden the wings and therefore to fly; 3) predation.
individuals losing their grip on Iris leaves that were too wide for them to grasp securely.37
Values in Table 5 for Pyrrhosoma nymphula (derived from different habitats in different years) show that predation by birds can vary greatly. Factors such as the location of the emergence site in relation to the feeding territories of insectivorous birds, the size and visibility of the dragonfly, its diel periodicity of emergence, and the weather at the time of the maiden flight will all influence the level of mortality during emergence. It is clear that, for some combinations of circumstances, such mortality can be heavy. In Anax imperator about 90 per cent of an annual emergence group survived emergence although, during divided emergence, almost 50 per cent of a day-group died, mainly from bird predation.6 Lestes viridis employs several strategies that greatly reduce mortality due to rain at the time of emergence: larvae can postpone emergence during rain for up to 14 hours at the emergence site, or by at least one day in the water; larvae choose emergence supports under leaves and oblique stems that provide the teneral adults with a degree of protection from rain; and during rain most larvae choose better protected sites for emergence.38 We may expect such adaptations to be found among other odonates also.
Usually neither sex emerges significantly before the other but, if either does so, protandry (earlier emergence of males) is more frequent. The disparity is liable to be increased if there is a second emergence peak, in which females can predominate.6 From a comprehensive review of exhaustive exuvia collections, it can now be said that the overall sex ratio at emergence is approximately 50 per cent males, but varies considerably among habitats and among years, males usually being slightly more numerous in Zygoptera, and females in Anisoptera.18
VOLTINISM
Determining the voltinism of a species, that is the number of generations it completes in a year, is a prerequisite for inferring the mechanisms by which seasonal regulation is achieved. It has long been recognised that, for each species, the flying season occurs at a characteristic time of year, but it is only during the last 50 years or so that odonatologists have tried to find out how this regularity comes about. The first serious attempts to study voltinism in European species were made by the Danish biologist Carl Wesenberg-Lund in the early twentieth century.39 He showed that some species regularly complete the life cycle in one year (being univoltine), whereas others required two or more years to do so, being semivoltine or partivoltine. His work was taken much further by Paul Münchberg who in the 1920s recorded the voltinism of several species in Germany.40 It was not until the early 1950s that attempts were made to determine the responses to physical factors that determined voltinism and the timing of the flying season.41
The temporal pattern of emergence, known as the emergence curve (Box 15), is disproportionately valuable for the understanding of seasonal regulation.
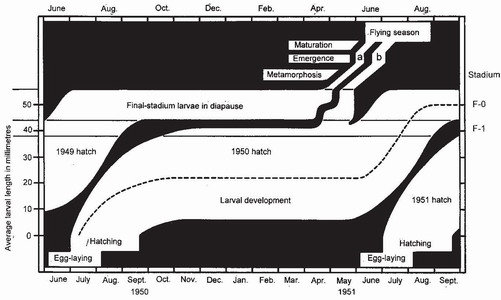
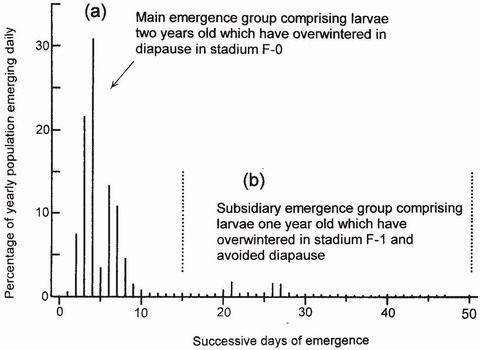
Characterisation of the emergence curve, year after year, in a population in which larval development is being monitored, makes it possible to infer when, and in what stages, developmental arrest is occurring (Fig. 88). In the population of Anax imperator studied in the Fish Pond most larvae were semivoltine, passing their second and last winter in diapause in the final stadium, but a few (sometimes about 10 per cent) grew so rapidly during their first summer that they managed to complete growth in one year, entering F-o just after their first winter and proceeding without diapause to metamorphosis and emergence.6 These precocious, univoltine individuals emerged about 25 days after the larger, semivoltine component and were responsible for a small, second peak in the emergence curve (Fig. 89) in which females predominated. The life cycle of Pyrrhosoma nymphula closely resembles that of Anax imperator, and its emergence curve also features a second peak in a similar position.5 In the populations studied, A. imperator and P. nymphula both spent their last winter in F-o in diapause and showed a highly synchronised emergence the following spring. There is a causal relationship between these two attributes. The final-stadium diapause enables laggards to catch up with more advanced larvae, in the manner of cars accumulating at traffic lights when the traffic light shows red. By early spring, diapause has been completed and all larvae are ready to respond synchronously to the rising temperature that stimulates the onset of
THE EMERGENCE CURVE
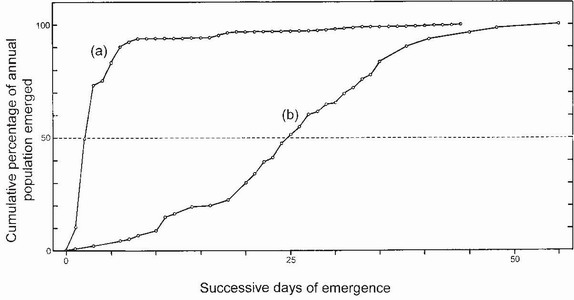
In a water body where the margin allows exhaustive collections of F-o exuviae of an identifiable species to be made daily throughout the emergence season, the exact numbers emerging daily can be recorded.6 If these numbers are plotted cumulatively, the percentage of the annual emergence that has already taken place on each day can be computed in due course. Such a plot takes the form of a curve of characteristic shape which reveals a distinct dichotomy between species that exhibit a highly synchronised emergence (spring species or Type 1 species) and those in which, by comparison, emergence is temporally dispersed (summer species or Type 2 species). The form of these emergence curves correlates closely with the mechanism of seasonal regulation used by each type of species,42 Anax imperator being a Type 1 species and Aeshna cyanea being a Type 2 species (Fig. 87). Highly synchronised emergence reflects the fact that all larvae in the senior cohort passed the previous winter in F-o and
FIG 87. Emergence curves of (a) a Type 1 species, Anax imperator, and (b) a Type 2 species, Aeshna cyanea, showing the different degrees of synchronisation. In Anax imperator 50 per cent of the annual population had emerged by the third day, whereas in Aeshna cyanea, this value was not reached until the 25th day of emergence. (After Corbet and Corbet..42)
so were all ready to respond synchronously to the rising temperature in spring when they embarked on metamorphosis together. Type 2 species, in contrast, spent their last winter in F-1 and so had to pass through stadium F-0 in spring before embarking on metamorphosis and emerging. Thus they accumulated more temporal variation and their emergence was less synchronised.
To facilitate the objective comparison of the timing of emergence between species, habitats and years, we can compare the first and last days of emergence, the day by which 50 per cent of the annual emergence has taken place (abbreviated as EM50)43 and the period within which 90 per cent of the annual emergence takes place (EM90).44 If the exuviae are retained for examination, they can be used to investigate correlations between time of emergence and sex ratio and linear dimension, both of which sometimes correlate with seasonal emergence time.45
metamorphosis. Then, like the cars being released when the traffic light turns to green, the whole population can respond simultaneously. The first peak of emergence is explosive, the EM50 value (Box 15) being reached after only three days. This causal relationship led to an ecological classification of the British dragonflies, based on the stage in which larvae spend their last winter and the degree of synchronisation of emergence.41 Because the type with the more synchronised emergence tends to emerge earlier than the other type, the two types were called spring and summer species respectively. They correspond to life cycles of Types 1 and 2 described in Box 16. Although larvae of summer species resume development in their last spring in several stadia, there are ways in which they can reduce temporal variation before emergence. One mechanism was recognised theoretically in 195746 and subsequently validated by laboratory experiment.47 It entails each successive developmental stage having a higher temperature threshold for its onset than its predecessor, so that the most advanced larvae are held back until the rising temperatures of spring reach their temperature threshold, by which time the less advanced larvae will have caught up with them. A second mechanism is the ‘light-growth effect’48 whereby the rate of development is accelerated in response to longer daylengths, so that, as the season progresses, larvae develop more and more rapidly.49 An intriguing theoretical consequence of this effect, yet to be confirmed experimentally, is that, between the spring equinox and the summer solstice, growth rate will correlate positively with latitude so that development rate in Type 2 species would enable emergence date to compensate for latitude.50 The existence of such a mechanism
FIG 88. Seasonal development of Anax imperator in a stem habitat (see Fig. 44, p.77) in southern England. The broken line approximates to the average growth rate of larvae hatching from eggs in 1950. The extent of the white areas indicates the upper and lower limits of larval size in that age group and in those hatching in 1949 and 1951. Most of an age group enter F-o in August one year after hatching, spend the winter in diapause and emerge early the following year (emergence group a), thus conforming with the typical Type 1 life cycle (Box 15). The few precocious larvae that enter F-o before 1 June forgo diapause and emerge later the same year (emergence group b), thus conforming to the typical Type 2 life cycle. (From Corbet.6)
FIG 89. The two components of the emergence curve of Anax imperator (corresponding to groups (a) and (b) in Fig. 88 when Type 1 and Type 2 life cycles respectively coexist in the same population. The lack of synchronisation among individuals in group (b), which can comprise 5-10 per cent of the annual emergence, is conspicuous. (After Corbet.51)
would help to explain how some dragonflies living close to the Arctic Circle can emerge almost as early as their southern counterparts.50
It is often possible to infer the voltinism of a population (p.162) by analysing the sizes of larvae in a sample and making allowance for the time of year, and then using the result as a baseline against which to compare further samples taken at other times of year. To take a simple example: searching for larvae immediately after the end of the emergence period can be informative. If no larvae can be found, the species is almost certainly univoltine. (If all larvae found are in the final stadium and showing signs of metamorphosis, the emergence period has clearly not finished and the species is likewise univoltine.) If smaller larvae are present, the species requires more than a year to complete a generation, and the size-distribution of the residual larvae will sometimes allow one to infer how many additional years are needed for larval development. A species that shows the last type of distribution is Cordulegaster boltonii, which evidently requires three or more years to complete a generation in Britain.52
Since 1971, meticulous research in Sweden by Ulf Norling53 has greatly increased our knowledge of the way in which responses by larvae contribute to seasonal regulation. It had been recognised since the 1960s that there are three types of life cycle among British and northern European dragonflies. This classification, recently modified and reformulated,50 remains valid, although more is now known about the larval responses that maintain the regularity of the life cycles involved. In Table 6 the British species are assigned to the three types of life cycle (Box 16).
Because not all larvae in a population develop at the same rate, it can happen that cohort-splitting takes place so that two Types of life cycle (1 and 2) may coexist within the same habitat, as in Anax imperator,6 Pyrrhosoma nymphula.5 and Coenagrion hastulatum.54 Cohort-splitting is evidently commonplace, especially in partivoltine species (i.e. those that take more than two years to develop), and those in which the oviposition season is extended.
BOX 16
LIFE CYCLES AND SEASONAL REGULATION
Dragonflies in Europe and Britain exhibit three kinds of life cycle, each being maintained by different regulatory responses. Examples among British species are listed in Table 6.
The Type 1 life cycle, typified by spring species
By spending the last winter before emergence in the final larval stadium, in diapause, such species can respond promptly and simultaneously to rising temperature in spring. So they tend to emerge early and synchronously. The eggs typically develop directly, hatching about one month or less after being laid, although some Somatochlora in Germany are facultative in this respect, developing directly if laid early in the summer, but overwintering as eggs if laid later.55 The possibility that species of Somatochlora and perhaps some libellulids show similar responses in Britain cannot be discounted.
The Type 2 life cycle, typified by summer species
Because they spend the last winter before emergence in one or more late stadia preceding F-o, such species typically emerge later than Type 1 species and with less synchronisation. The eggs of Aeshna species typically overwinter in diapause. Despite commencing growth in their last spring in more than one stadium, Type 2 species can improve their synchronisation of emergence by using a system of rising temperature thresholds that enable retarded larvae to catch up with more advanced ones56 (Fig. 90).
The Type 3 life cycle. Obligatorily univoltine species.
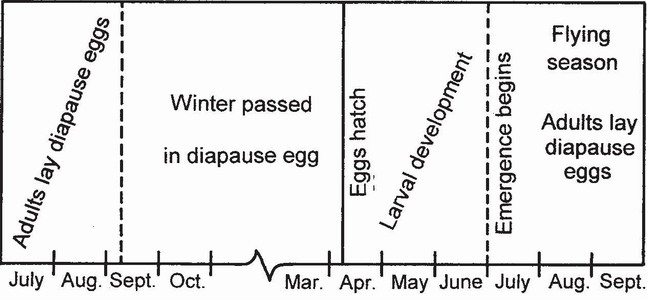
These species represent a subset of Type 2, distinguished by being invariably univoltine. They typically, but not invariably, overwinter as eggs in diapause. Larval development is completed in two or three months in spring and early summer, and adults die in late summer (Fig. 91). The larvae tend to have relatively high thermal growth coefficients.
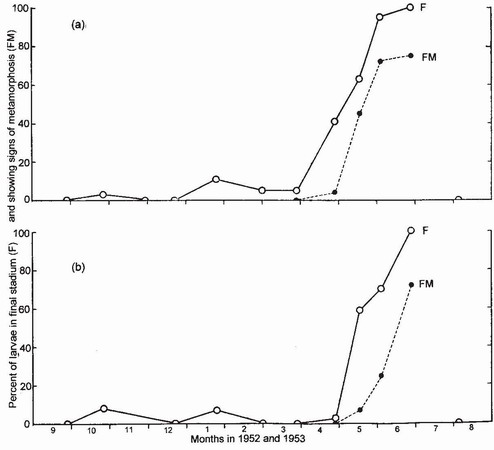
FIG 90. Larval development in two Type 2 species: (a) Coenagrion mercuriale, and (b) Ceriagrion tenellum. Both species are semivoltine. Most individuals enter F-o in spring and then proceed promptly to metamorphosis as a prelude to emergence in early summer. (From Corbet..46.)
FIG 91. The life cycle of Lestes sponsa, a Type 3 species. (After Corbet.57)
SEASONAL REGULATION
Research in Sweden by Ulf Norling60 has revealed two strands in the seasonal regulation of Odonata in temperate latitudes based on two discrete events that can both act, though at different seasons, to ensure that emergence is positioned at an appropriate time of year58 (Box 17).
It appears that, on the whole, emergence begins later at higher latitudes but, as mentioned above, observations suggest that some kind of compensating mechanism is operating such that northerly populations emerge earlier than would be expected from a latitude-temperature gradient alone..50 Another factor to be taken into account is that certain northern species (e.g. Coenagrion hastulatum) may differ from southern species (e.g. C. puella) in having a lower temperature optimum for growth.59 The situation is further complicated by the fact that the thermal optimum for growth may change seasonally, in a manner that is considered adaptive. Any latitude-compensation mechanism which entails a change in growth rate will bear a survival cost in the presence of a predator able to depress foraging activity and growth efficiency. Even so, larvae of Lestes sponsa, induced to grow rapidly, balanced this trade-off differently, by taking more risks when a predator was present. The higher predation cost was to some extent offset by the higher growth efficiency.60
In patterns of seasonal regulation we see evidence of the ways in which odonate life cycles have become adapted for survival at high latitudes. It is recognised that temperate latitudes have been colonised from regions closer to the equator; and the prevailing view is that, compared with other aquatic insects
TABLE 6. Types of life cycle found in British Odonata.a
TYPE 1 (SPRING SPECIES)
Anisoptera
Aeshnidae
Anax imperator
Brachytron pratense
Cordulegastridae
Cordulegaster boltonii
Corduliidae
Cordulia aenea
Somatochlora arcticab
S. metallicac
Gomphidae
Gomphus vulgatissimus
Libellulidae
Leucorrhinia dubia
Libellula depressa
L. fulvad
L. quadrimaculata
Orthetrum cancellatum
O. coerulescens
Zygoptera
Calopterygidae
Calopteryx virgo
Coenagrionidae
Erythromma najas
Pyrrhosoma nymphula
TYPE 2 (SUMMER SPECIES)
Anisoptera
Aeshnidae
Aeshna caeruleae
A. cyanea
A. grandis
A. juncea
Zygoptera
Calopterygidae
Calopteryx splendensf
Ceriagrion tenellum
Coenagrion mercuriale
C. hastulatum
C. puella
C. pulchellum
Enallagma cyathigerum
Ischnura elegans
I. pumiliog
TYPE 3 (OBLIGATORILY UNIVOLTINE SPECIES)
Anisoptera
Aeshnidae
Aeshna mixta
Libellulidae
Sympetrum danae
S. sanguineum
S. striolatum
Zygoptera
Lestidae
Lestes dryas
Lestes sponsa
MECHANISMS OF SEASONAL REGULATION
Strands can be identified in the larval responses that help to regulate their life cycles and the season of emergence. They operate in autumn and spring respectively.
Strand 1
Retardation of larval development in late summer and early autumn so that the larval population overwinters in an appropriate, cold-resistant stage. This process is usually accomplished by the onset of a diapause induced by a response to daylength (photoperiod). Initially long, and perhaps sometimes decreasing, photoperiods postpone entry to one or more late stadia whereupon short photoperiods prevent development from proceeding further before the onset of winter. This Strand concerns pre-diapause development and is well developed in the Type 1 life cycle in which diapause is induced in F-o; it determines the stadium and/or intrastadial stage in which the last winter will be passed. In Aeshna caerulea larvae reduce food intake as diapause starts in late summer and early autumn.64
Strand 2
The placement of emergence, in spring and early summer, early in the season favourable for adult activity and survival. This Strand concerns post-diapause development, and is achieved by responses quite different from those that characterise pre-diapause development. In this Strand, instead of being retarded (as in Strand 1), larval development is accelerated under long photoperiods. The larval response to photoperiod has evidently been reversed among larvae that have experienced a spell of low (winter) temperature and/or decreasing or short photoperiods.
(mayflies and stoneflies), the Odonata retain features of a warm-adapted group.65 Although this remains to be verified, it seems that the stages least resistant to cold are non-diapause eggs and the earliest larval stadia. Life cycles are organised in such a way that these two stages seldom or never coincide with the winter. When, as happens very occasionally, small larvae of lestids (Type 3 species) are encountered in autumn, this may be due to the anomalous premature hatching of diapause eggs, perhaps caused by the stimulus of wetting or submergence.66 Such happenings are sufficiently rare that they do not challenge the general thesis that mechanisms of seasonal regulation normally ensure that the earliest stadia are not exposed to winter temperatures.
We may expect climate change, and especially global warming (p.294), to have a marked effect on the phenology of dragonflies. In particular, because ambient temperatures in spring and early summer influence emergence date in all types of life cycle, we can expect emergence to begin earlier, resulting in a corresponding advance of the flying season (p.189). There is already evidence of such an effect. The flying seasons in northwest Germany of Coenagrion puella, Ischnura elegans, Libellula depressa, L. quadrimaculata and Pyrrhosoma nymphula started 20-28 days earlier in the 1990s than in the 1980s, when temperatures had been lower. A corresponding difference for 17 of the most widespread species in Germany was almost two weeks.67 Furthermore, a recent and significant northward range expansion has taken place in 14 European species of Mediterranean provenance accompanied in some by a spread to higher altitudes.68 Some of the routes along which such expansion takes place may reflect those along which central European species came from their postglacial refuges into central Europe.69 Experience of climate warming in Florida leads one to expect that the flying season of temperate species of Odonata will become longer and that their distribution will expand northwards.70
In Britain we can expect range extensions corresponding to those detected in Germany, together with increases in population size and colonisation of biotopes at higher altitudes.
OPPORTUNITIES FOR INVESTIGATION
For researchers with access to laboratory facilities, there is scope for determining the lower temperature threshold for survival of larvae in the earliest stadia and for determining the optimal temperature for the completion of diapause in larvae. From two Type 1 species, Anax imperator and Pyrrhosoma nymphula, there is anecdotal evidence that the final-stadium diapause has been completed by November71 and this could easily be verified by removing diapause larvae from the field each month from October onwards and exposing them to the prerequisites for metamorphosis (food and warmth).
It can always be informative to construct emergence curves by making exhaustive, daily collections of F-o exuviae.6 To achieve this the study habitat needs to be carefully selected. In particular, it would improve our understanding of seasonal regulation to obtain precise emergence curves for some of the more elusive Type 1 species, such as Cordulia aenea and Somatochlora spp. Emergence curves, especially if combined with larval samples taken from the same habitat at selected times of year, can inform about patterns of seasonal regulation. Precise characteristics of the emergence curve can also provide a reference point for inferring the duration of the prereproductive period, a feature of special interest if global warming supervenes, especially for species such as Aeshna mixta and Sympetrum striolatum (p.67), and possibly some lestids.
Gaps in our knowledge of life-cycle types and voltinism remain for several British species (Table 6, p.170). It will be rewarding to fill them and to compare the results with patterns observed for the same species in continental Europe.