CHAPTER 7
Adult Life
MATURATION
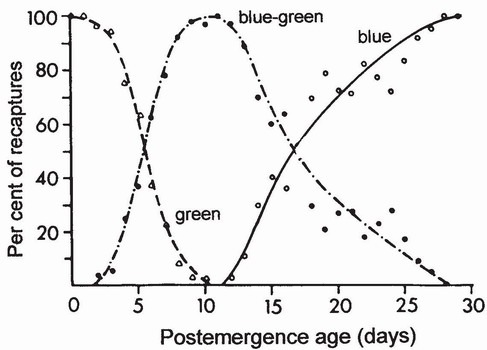
THE TENERAL ADULT is reproductively immature. After the teneral stage, which lasts about 24 hours, the adult remains immature for days or months until the gonads attain reproductive readiness. During this prereproductive or maturation period the gonads develop, the body and wings harden and acquire their mature colours, and adults come to exhibit reproductive behaviour. When the behaviour of an adult is being noted, it is important, where possible, to record its state of maturity. Diagnosis may be difficult or impossible if the body is pale and soft, but it is usually straightforward in the later stages of the maturation period. Body colour is sometimes but not invariably a reliable criterion of sexual maturity.1 For example, the gonads of male Leucorrhinia dubia in a Finnish population matured before the body coloration became ‘mature’2 but in a German population of Lestes sponsa adults achieved mature coloration after 15 days of life, but did not exhibit reproductive activity until three or four days later.3 In Ischnura elegans the green phase of the male is almost entirely a maturation phase (Fig. 92): only 4 of 709 individuals in this phase were seen mating.4 More work is needed to establish reliable (preferably external) criteria for determining the physiological age of adults during the maturation period. A suitable model for such an investigation would be I. elegans which shows an orderly, time-dependent progression of colour forms during maturation5 (Figs 92-94). To calibrate these against spermatogenesis and oocyte development would be informative.
In British species the teneral adult is not well endowed with stored fat, and the rate of maturation probably depends on the amount of food an adult can
FIG 92. Change with age of thoracic ground colour of Ischnura elegans males based on recaptures of individuals marked when teneral or green. Most males in this population became sexually mature about 11 days after emergence, although some males can mate when still green and therefore almost certainly when younger than 11 days. (After Parr.6)

FIG 93. An immature male Ischnura elegans. The thorax becomes blue when the insect is mature (Robert Thompson).



FIG 94. (a-d) Age-related colour forms of female Ischnura elegans. The form violacea (a) will develop into either the andromorph, formerly known as typica (b), or the form infuscans, and the form rufescens (c) develops into the form rufescens-obsoleta (d) (Robert Thompson).
obtain during the prereproductive period. It can be assumed that this in turn will be strongly influenced by the weather, in the form of wind, precipitation and temperature, which will determine the opportunities available for foraging. Some individuals begin to forage on their first day as adults: Wesenberg-Lund recorded Cordulia aenea doing so.7 On the other hand, Calopteryx splendens and Erythromma najas are said not to forage when ‘freshly emerged’.8 The maturation period of Ischnura pumilio (Fig. 95) lasts 6-12 days depending on the food intake.9 Ischnura elegans, which can survive in exposed habitats, has been observed to mature in 3 or 4 days in one habitat10 and in 10 or 11 days in another.11 It is common for adults to leave the reproductive site (usually also the emergence site) during the maturation period, but I. elegans appears to be an exception.12 The maturation period of Cordulia aenea amurensis (in Japan) is prolonged by cool weather.13 In a Finnish population of Leucorrhinia dubia sperm development required 10-14 days during unfavourable weather instead of the usual 4-5 days.2 The maturation period in British species usually lasts about two weeks, being slightly longer in females than males and considerably longer in large Anisoptera. In one detailed study Aeshna cyanea required at least a month to achieve maturity,14 a finding which conforms closely with information obtained over several years. In a population at 47° 23’N the average maturation period of this species over ten years was 50 days.15
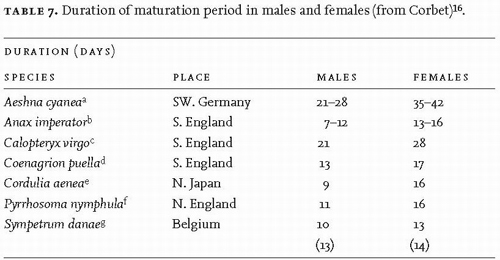
In species with a maturation period of normal (unextended) duration, males tend to mature more rapidly than females16 (Table 7).
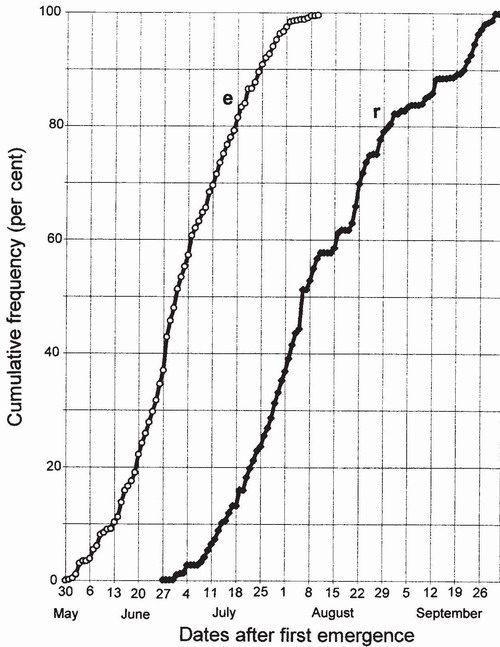
The duration of the maturation period can be estimated approximately by recording the interval between first emergence and first appearance of mature adults at water, taking note of any inclement weather that might delay maturation (by suspending foraging) during the intervening period. It can be estimated more reliably by marking newly emerged adults to indicate their date of emergence and then noting when each subsequently returns to water. Such a study is labour intensive and time-consuming, but can yield data of high quality. For a population of Aeshna cyanea in Germany (Fig. 96) such data show the maturation period to have lasted from about four weeks (for individuals emerging earlier) to about seven weeks (for those emerging later), the duration increasing gradually between these extremes.14 Whereas the minimum values
Notes:
a Kaiser (1970).
b Corbet (1957a).
c Lambert (1994).
d Banks & Thompson (1985); Thompson (1989).
e Ubukata (1974).
f Gribbin & Thompson (1990).
g Michiels & Dhondt (1989b). Values in parentheses derive from the semi-natural conditions of a field cage. The disparity between these and values derived from nature suggests that results from the field are subject to bias.
FIG 96. Duration of the prereproductive period of Aeshna cyanea throughout the emergence period, as shown by the interval between emergence (e) (N=756) and return to the emergence site (r) (N=579) of adults marked as individuals at emergence in Germany. The duration increases greatly, from about four to six weeks, as the season progresses. (After Inden-Lohmar.14)
are probably realistic, the apparent maximum values may be subject to error due to adults visiting other aquatic habitats in the meantime, and there is no obvious way of investigating this possibility.
In Britain the duration of the maturation period is not likely to play a role in seasonal regulation, but in the Mediterranean region it often does. So, with the prospect of climate change, interest attaches to reviewing some examples of Mediterranean life cycles and thus anticipating some of the changes that may one day occur among British species.
In Algeria, at about 36°N, winters are mild, summers are hot and dry, and autumn is heralded by heavy rain liable to reinstate shallow bodies of water that have become dry during the summer. In such a climate Type 3 species (Box 15, p.163), such as Aeshna mixta, Sympetrum striolatum and S. meridionale, develop rapidly in lowland lakes and pools and emerge in spring, to be faced with the early prospect of their larval habitats drying up. Immature adults leave the lowlands, ascending to woodland in nearby hills at 500-1,000 metres a.s.l., where they remain for three or more months, feeding and gradually becoming sexually mature.17 Then, in late September or early October, with the advent of the first heavy rains of autumn, they return en masse to the lowlands where they reproduce in the newly replenished ponds. In this life cycle the maturation period serves as a summer diapause, or aestivation, stage, maintaining the Type 3 life cycle by postponing reproduction until early autumn, as well as by reducing the risk of eggs being laid in ephemeral bodies of water. One wonders whether British populations of Aeshna mixta and Sympetrum striolatum, both of which are regularly augmented by migrants from continental Europe, contain individuals with a predisposition to delay maturation, perhaps in response to high summer temperature. One manifestation of this might be that these species would have an exceptionally long maturation period in Britain in years when they were exposed to high temperatures as young adults in summer. In our view, these two species will be worth monitoring closely in this regard. Although Andy McGeeney records A. mixta as having a maturation period of only seven to ten days,18 the relatively late appearance of mature adults in late July and August19 (more than a month later than Lestes sponsa which also has a Type 3 life cycle) seems to indicate a maturation period much longer than this in some years or some places. As for Sympetrum striolatum, during one very hot summer in southwest England, adults that emerged in mid-June apparently did not return to water for about two months.20 In S. striolatum, part of the wide variation in time of adult appearance may arise from the bimodal emergence caused by the existence of diapause and non-diapause eggs.21
A possible model for what may be happening in British populations of Aeshna mixta, and perhaps Lestes sponsa, or for what may come to pass as summer temperatures rise, is provided by populations of Lestes sponsa in Japan. Lestes sponsa is a Type 3 species which needs to avoid ovipositing early in the summer so that its diapause eggs do not hatch in autumn. It achieves this in Japan by increasing the duration of the maturation period in accordance with the ambient temperature in summer.22 Thus the maturation period lasts about 20 days at all latitudes between about 40 and 58°N, but lengthens progressively south of 40°N to become about 120 days near 34°N. South of 40°N a close correlation exists between the duration of the maturation period and mean annual temperature (Fig. 97). To our knowledge there is no indication that L. sponsa exhibits a long maturation period anywhere in Europe;23 on the other hand, the southernmost limit of its distribution there is 40°N,24 just the latitude at which in Japan the maturation period begins to lengthen on the north-south gradient. Tantalisingly,
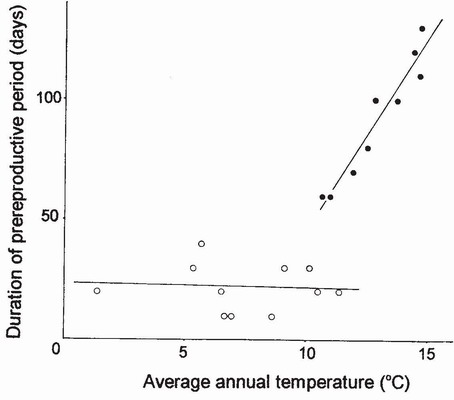
FIG 97. Relationship between duration of the prereproductive period of Lestes sponsa and mean annual temperature at 21 localities in Japan between 34° 25’ and 58°40’N. Open circles, localities where the onset of the reproductive period is not postponed; filled circles, those in which the onset is postponed. (After Uéda.22)
there is an obsolete record of L. sponsa from northeast Algeria, at about 37°N, but the species can no longer be found there.25 Two other species of Lestes in northeastern Algeria, L. barbarus and L. viridis virens, also existing there as Type 3 species, have a protracted maturation period in that area.26
In the tropics a long maturation period contributes to seasonal regulation by postponing reproduction during the dry season, which corresponds phenologic-ally to the winter of temperate latitudes. Such a suspension of development is known as siccatation, rather than hibernation (inactivity during winter) or aestivation (inactivity during the summer).
PHYSICAL FACTORS
Thermoregulation
Odonata, being insects, have been termed ‘cold-blooded’ or ‘poikilothermic’, implying that their body temperature, unlike that of ‘endotherms’, like mammals and birds, closely tracks that of their immediate surroundings. However, Heinrich has pointed out that it is more realistic to regard insects like dragonflies as facultatively endothermic because they can raise their body temperature by their own actions, for example, by wing-whirring.27 So Heinrich has proposed that dragonflies be termed heterothermic. Were dragonflies to be comprehensively poikilothermic, they would be at a great disadvantage early in the morning or during cold spells during the day because they cannot fly when cold. Fortunately for them, however, they can raise the body temperature above that of their surroundings, enabling them to maintain the thorax at a temperature high enough to permit spontaneous flight. The need to do this is ever-present in temperate latitudes and becomes acute among species that emerge during the night and (to escape from predators) need to leave their emergence supports during dawn twilight when the air temperature is close to its diel minimum. An additional need, rare in temperate regions but commonplace in the tropics, is to prevent the body overheating. Sometimes, on a hot summer’s day, one can witness British dragonflies reducing their body temperature by evaporative cooling. This involves the intermittent immersion in water during flight. Controversy has attended its physiological function because of its resemblance to exophytic oviposition. The function of this behaviour is less equivocal if a male is exhibiting it, but one cannot exclude the possibility that an adult appearing to immerse itself is drinking, as adults of Calopteryx are sometimes known to do.28 Of course, such behaviour may simultaneously serve both functions (drinking and evaporative cooling) and it would be difficult to verify this. But when a patrolling male Cordulegaster boltonii (a flier) repeatedly touches the water surface during flight on an exceptionally hot day, it can be assumed that it is drinking and/or wetting the body.
Odonates possess a suite of behaviours directed towards thermoregulation – the process of keeping the body temperature between the lower and upper limits permitting physiological function and spontaneous flight. It lends savour and interest to odonate-watching to recognise these behaviours and to infer how comfortable a dragonfly is in its current microclimate.
Dragonflies commonly roost on surfaces warmed by the setting sun, which of course face west. This places them at a thermal disadvantage the following morning when, as the sun rises in the east, the formerly insolated surfaces are shaded. To raise the thoracic temperature to a value that permits spontaneous flight (about 25-30°C), adults must initially vibrate the wings. This endothermic warming is feasible only in larger species; Zygoptera do not exhibit it. It has been closely studied by Mike May in Anax junius, the New World counterpart of Anax imperator.29 After wing-whirring has begun, the thoracic temperature rises rapidly, followed by the temperature of the head and abdomen. At an ambient temperature of about 15°C, after about 13 minutes, the temperature of the thorax (and therefore the wing muscles) peaks at about 29°C. Then the dragonfly tries to become airborne and the head temperature suddenly rises, perhaps reflecting the increased need for visual sensitivity as flight commences. Presumably the temperature differential between different parts of the body is effected by controlled circulation of the haemolymph, which, under different circumstances, can transport heat from the thorax to the abdomen to assist radiative cooling in fliers.30 Once they can fly, adults can seek out insolated surfaces where they can consolidate the warming process by basking.31 As the sun appears to circle the sky, perchers (in cool temperate latitudes) systematically move around the margin of a water body so as to remain in sunny spots.32
Thereafter they can maintain the thoracic temperature. Fliers do so by flying (which warms the thorax), and perchers by alighting on insolated surfaces or basking with the body orientated at right angles to the incident rays of the sun (ectothermic warming). By such means, adults continually adjust their behaviours through the day so as to regulate the thoracic temperature: if too warm, they seek a shaded resting site. Alternatively, if they wish to remain perched in an exposed site, they point the abdomen towards the sun so as to minimise the incident radiation on the body (minimising the body’s shadow). This position is known as the obelisk posture33 and is rarely seen in Britain. There are variations on this theme. Some Zygoptera use the wings to produce a ‘greenhouse’ effect in which the wings insulate the body from the cooling effect of winds while admitting solar energy to warm the body beneath.34 Lestes viridis places the wings together, above the abdomen, in a posture unusual for a lestid, which reflects the sun’s rays on to the abdomen, thus raising its temperature.35 A further option is used by species with blue external pigmentation. In hot, sunny weather, the body surface remains pale blue, reflecting solar energy and postponing overheating. In cool weather the blue changes to grey, absorbing energy and thus raising the body temperature. In some species the timing of this colour change is under the control of an in-built 24-hour clock (circadian rhythm) so that, when the sun rises, the body is already dark and able to absorb solar radiation more effectively.36 Female Enallagma cyathigerum typically oviposit beneath the water surface, sometimes being accompanied briefly by an attendant male who is initially predominantly blue (Fig. 98). Often, when such a male returns to the surface, his abdomen is no longer blue, but grey (Fig. 99), and so equipped to absorb the sun’s rays more effectively. Likewise, if a blue male is confined in a refrigerator for a few hours, he turns grey. Other British species that often exhibit this type of colour change include species of Aeshna and Anax imperator. The change can be so striking that, in the past, observers have sometimes

FIG 98. A mature male Enallagma cyathigerum in the blue phase (Robert Thompson).
FIG 99. An immature male Enallagma cyathigerum which when mature will become blue at normal ambient temperatures but grey when cold (Robert Thompson).
interpreted it as denoting a colour form, analogous with the colour phases of Ischnura elegans and I. pumilio (Figs 92-95, pp.176-178).
A satisfying way of documenting the changing demands of thermoregulation as the day progresses is to monitor the behaviour of individual odonates belonging to species with different temperature preferences. Dagmar Hilfert-Rüppell has done this for several species, some British, occurring near Braunschweig in central Germany.37 Many British odonatologists will know, from casual observation, that Ischnura elegans is one of the British dragonflies most tolerant of low temperature. Dagmar watched adults of I. elegans as they resumed activity in the morning. Adults often roost close to water, among fringing Carex38 or on grasses Juncus or Equisetum at a mean height of about 80 centimetres above ground. They choose a stem that matches their head width, so that they can see an approaching object and yet hide the body behind the stem, sidling around the stem as necessary to remain hidden.39 As the sun rises, they first orientate the body at right angles to the sun. Then, when warm enough to fly, they move around the pond margin, alighting intermittently in the warmest spots until warm enough to begin reproductive activity over water. Different species typically become active at different times, reflecting their differing thresholds for spontaneous flight and the options available to them for endo- and ectothermic warming.37
Although odonates use several different strategies for thermoregulation, sometimes simultaneously, body size and strategy are broadly correlated. Zygopterans and smaller anisopterans, being perchers, are predominantly ectotherms, whereas larger anisopterans, mainly but not exclusively fliers, are predominantly endotherms. A few small perchers, including libellulids and coenagrionids, appear to be thermal conformers, showing no obvious attempts at thermoregulation. Others show great versatility, behaving, at different times, as perchers, fliers or hoverers and sometimes switching from one mode to another from day to day.40 Unlike fliers, perchers (Fig. 100) have to attain the takeoff temperature by passive heat gain by basking (and so, in temperate latitudes, tend to fly only when the thoracic temperature exceeds ambient by about 7°C). The time of first morning takeoff by Sympetrum correlates better

FIG 100. A male Sympetrum sanguineum soaks up heat radiating from a stone (Robert Thompson).
with incident radiation than with ambient temperature.41 Sympetrum striolatum is a typical percher, but sometimes, in cool weather or the evening, it forages in flight, dancing up and down within a swarm of its prey, resembling a typical flier such as Aeshna mixta.
Dagmar Hilfert and Georg Rüppell examined the thermoregulatory behaviour of seven European species, comparing populations in northern Germany (52° 15’N) and southern France (43°34’N), recording the lowest ambient temperature permitting spontaneous flight.42 This value was lower in northern than in southern populations, and in both was lower in September than in May and June, reflecting the fact that thresholds can change during the life of an individual by acclimatisation. The lowest values were found in Ischnura elegans (12°C) and Orthetrum cancellatum (4.9°C).42 Both species arrived at water earlier in the day than the other species studied (Aeshna cyanea, A. mixta, Lestes sponsa, Sympetrum striolatum and S. vulgatum). Ischnura elegans and Orthetrum cancellatum were probably typical in thermoregulating through perch choice. Ischnura elegans crawled from shaded to sunlit spots and pressed its ventral surface against the warm vegetation. Orthetrum cancellatum first settled on insolated stones or patches of dry grass, sometimes holding the wings horizontally and close to the grass, thus retaining the warm air close to the thorax and reducing heat loss due to convection. As Dagmar observed, such ‘warming’ sites may feature as a proximate cue in habitat selection: O. cancellatum is known to colonise gravel pits which are typically surrounded by perch sites that facilitate thermoregulation. As the diel periodicity of oviposition indicates, females of some species probably have a lower threshold temperature for flight than do conspecific males.43 In general, Zygoptera have a lower threshold than do Anisoptera, perhaps reflecting their lower wing-beat frequency.43 As would be expected, the resting sites chosen by perchers (including O. cancellatum) are located round the perimeter of a pond according to the apparent position of the sun.32
There is a latitude gradient in the minimum takeoff temperature, which is significantly higher in a warmer climate where the temperature at which heat torpor supervenes for perchers and fliers is much higher.44 Whether or not such differences are partly genetically determined, we may expect dragonflies in Britain to differ in their temperature thresholds for activity from their conspecifics in southern Europe. One implication of this latitude gradient is that fliers should be better represented at the highest latitudes because of their greater thermoregulatory versatility. Provisional analyses support this inference.29
Flight activity
In high temperate latitudes adult Odonata are predominantly diurnal, their activity peaking close to noon. A few species (e.g. Aeshna grandis and Anax imperator) often fly during evening twilight. In A. imperator this crepuscular phase forms a discrete peak and is not merely an extension of the diurnal activity.45 Sometimes females of Aeshna cyanea visit water, apparently mainly to oviposit, in the late afternoon or early evening, well beyond the normal activity period for both sexes. This may enable them to oviposit free of male interference.
Wind and rain usually inhibit flight of British odonates. Some species, especially Ischnura elegans, sometimes fly in a rather strong wind, but perhaps choose lee situations in which to do so.46 The upper limit of wind speed permitting flight of Coenagrion puella is about 8 m/sec (28.8 km/h).47 Other Zygoptera can continue to fly into a wind of 3.5 m/sec (12.6 km/h)48 and the corresponding value for Anisoptera is up to 10 m/sec (36 km/h). In Britain rain, or a sudden drop in air temperature, promptly inhibits flight. Surprisingly, a few tropical species appear to fly mainly or only during rain, and others frequently tolerate it.49
When the sky abruptly becomes overcast, dragonflies leave the water and move to protected sites amongst vegetation, even when the coincident drop in ambient temperature is slight.50 Such behaviour can be witnessed during a total solar eclipse,51 normal activity being resumed promptly after illumination is restored.52
In some species in North America and the tropics individual members of a population exhibit, and maintain, idiosyncratic diel activity patterns, and we may anticipate such a phenomenon in British species. In Lestes disjunctus australis, for example, some males (about 33 per cent) frequented water throughout the day, others (about 32 per cent) in the morning and afternoon, with a lull near noon, and a third group (about 20 per cent) only in the late afternoon.53 Together these components made up the diel periodicity of the whole male population at water which was bimodal with peaks in morning and afternoon.
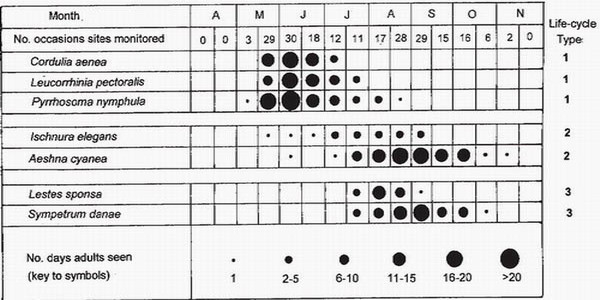
Because larval growth, metamorphosis, emergence and maturation are temperature dependent, we may expect the timing of the flying season, namely the time when reproductively mature adults are active, to reflect local climate and, in particular, the cumulative growing-degree day total. One of the most informative ways of portraying the flying season shows relative abundance (Fig. 101). A strength of this method of portrayal is that it reveals, for each species, whether the peak of numbers falls near the beginning of the flying season (as in Type 1 species, such as Pyrrhosoma nymphula) or in the middle (as in Type 2
FIG. 101. Flying seaons of dragonflies which have three types of life cycle near Zürich, Switzerland, during nine years. The top row shows the number of days on which sites were monitored in each half-month, and the bottom row an arbitrary scale of abundance based on the number of site-days on which adults were seen during each time interval. Entries in the right margin correspond to life-cycle types defined in Box 15, p.163. (After Wildermuth.54)
species, such as Enallagma cyathigerum). Within Britain, as expected, the flying season of a given species tends to be earlier in the south than the north.
Longevity
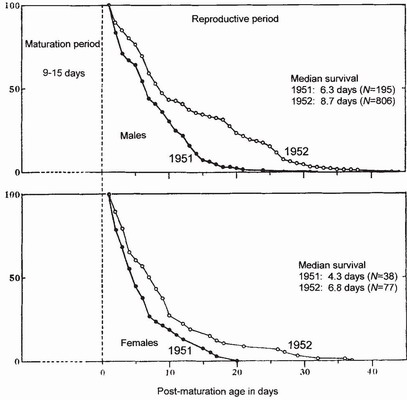
One of the first questions people ask about dragonfly biology is ‘How long do adults live?’ A rough estimate of the maximum length of life can be obtained by comparing the time elapsing between the beginning of emergence and the apparent end of the flying season. In Coenagrion puella, daily survival rate is constant and independent of size for mature adults of both sexes and the mean mature life span for males and females respectively is 5.6 and 5.4 days.55 In this species, in the population studied, daily survival rate independent of age for both sexes, being 0.83 for males and 0.82 for females. However, the asymptotic shape of the survivorship curve for Pyrrhosoma nymphula (Fig. 102) shows that the maximum length of life tells one little about the longevity of the bulk of the population. An informative value is the median or life expectancy, namely the time (or age) at which 50 per cent of the population has died. For the survivorship curves shown in Fig. 102 the median values are much less than the maxima which are (for 1951 and 1952 respectively) 32 and 44 days for males and 20 and 37 days for females. These values apply only to adults surviving the initial maturation period of 9-15 days. Like the median values, they emphasise the difference in survival (in the same population) between the two years. Because Pyrrhosoma nymphula is a Type 1 species, the peak density will come at the beginning of the flying season and the bulk of the population will grow old together.56 This being so, it was at one time hypothesised that the selective pressures for longevity would be less on Type 1 species than on species of Types 2 and 3.57 Insufficient data exist to confirm this, although some of the highest recorded values for longevity in Europe come from aeshnids with a Type 2 life cycle: Aeshna grandis in Poland and A. juncea in Germany attained post-emergence ages of at least 64 and 70 days respectively,3 including the long prereproductive period of about 35 days. Potential longevity clearly exceeds these values which depend partly on recapture probability. Post-emergence ages exceeding 80 days have been recorded for captive Calopteryx splendens, Aeshna juncea and Anax imperator.58 Excluding species in which adult life is prolonged by a diapause, it may be said that the life expectancy during the reproductive period is close to 11.5 days for Anisoptera and 7.6 days for Zygoptera and that durations for the prereproductive period are about two weeks and one week respectively, the maximum being 30 days for both suborders.59 In Coenagrion puella weight is positively correlated with male longevity,60 as it is in Sympetrum danae,61 although sometimes a finding of this kind is contradicted when a study is repeated in another season.63 The mean life
FIG 102. Survival of mature adults of Pyrrhosoma nymphula in two successive years at a stem habitat (Fig. 44, p.77). Results are based on the greatest intervals between release and recapture of marked individuals after they had returned to water, those recaptured after only one day providing the value for 100 per cent. Survival was lower in 1951, a year that featured a late spring and summer, less favourable weather and a longer prereproductive period. The median values represent the expectation of life on the first day of the reproductive period. Males lived slightly longer than females. (After Corbet.62)
span of mature adults of Coenagrion mercuriale has been estimated to be about a week.64
A continuing need is to identify morphological criteria for determining post-emergence age. A promising relationship has been found between the intensity of wing pigmentation and age in Calopteryx maculata.65 This character is used by the European C. haemorrhoidalis for mate recognition during courtship,66 during which enhanced fitness may accrue from being able to recognise the age (reproductive quality) of a potential mate.
Flight seasonality
Global warming67 is likely to change the distribution and faunal composition of Odonata in temperate latitudes.68 At present, species richness (i.e. the number of species in a given locality) in Britain is correlated positively with mean air temperature, in summer, though not in winter.69 Thirty-seven species of resident British Odonata have extended their range northwards during the past 40 years.70 Observed and anticipated effects of global warming on the phenology of British Odonata are explored on p.294 and p.295.
In northern Europe the flying season of 11 species of dragonfly now peaks earlier than it did in 1980. Pyrrhosoma nymphula (a Type 1 species) shows the strongest effect, having advanced its flight peak by 17 days. Aeshna grandis (a Type 2 species) has done so by about 14 days. For most of the 11 species the shift in flight peak is strongly correlated with increasing spring temperatures at a site, especially in May.71
Jürgen Ott, studying trends in Germany and mainland Europe, is thoroughly monitoring changes in the dragonfly fauna that are correlated with steadily rising temperatures there. Such changes include earlier seasonal emergence, more rapid larval growth, increased voltinism and change in species composition, favouring eurytopic species over ecologically sensitive (stenotopic) species: the former include Aeshna affinis, Anax parthenope, Boyeria irene, Lestes barbarus, Orthetrum brunneum and Sympetrum fonscolombii and the latter include species such as Coenagrion hastulatum, Leucorrhinia dubia, Orthetrum coerulescens and Sympetrum flaveolum.72
BIOTIC FACTORS
Adults, like larvae, are used as a resource by commensals, parasites and predators (Box 12, p.130).
Commensals
Commensals are seldom observed because they are usually so small, and probably also because they often escape when a dragonfly is netted. The benefits they gain from attachment to the dragonfly adult include transport and food. Very few observations exist of commensals of British species. One of the most interesting observations is due to Klaus Sternberg, who closely watched a male Cordulegaster boltonii in Germany.73 When the dragonfly was settled and not feeding, minute flies of the milichiid genus Desmometopa were seen on the coxae, legs and sides of the thorax, usually remaining on the thorax when the dragonfly was flying. While the dragonfly was feeding (and settled) the flies were active around the mouthparts, sucking the already masticated, fluid-covered prey and dabbing at the dragonfly’s mouthparts after it had ingested its meal. They nimbly avoided being crushed by the dragonfly’s jaws or being swept off by cleaning movements of its legs by running quickly away or by making short flights. We have already mentioned (Box 8, p.104) the scelionid wasps that are parasitoids of the eggs of endophytic Odonata and that are sometimes phoretic on ovipositing adults with the prospect of being already close to the female when eggs are laid. In the phoretic phase they are behaving like commensals.
Endoparasites
Internal parasites that are carried over from larvae to adult dragonflies (namely Protozoa, Trematoda and Cestoda) have been described on p.131. Such endoparasites are very widely distributed in adult Odonata. Infection of Calopteryx splendens xanthostoma with eugregarine Protozoa during the prereproductive period reduces the ability of adults to accumulate fat, and consequently diminishes their ability to acquire and maintain territories when they attain maturity.74 It can be assumed that most endoparasites lower the viability of their hosts, but it is difficult to identify their impact because parasitised hosts cannot readily be recognised without dissection and because parasites of different kinds may interact with one another.
Ectoparasites
Most observers of Odonata will have noticed the bright red or orange ectoparasitic mites (Acarina) attached to the ventral surface of the thorax and abdomen (especially of Zygoptera) (Fig. 103). They belong to three families of water mites, or Hydrachnida (also known as Hydrachnellae or Hydracarina). The life cycles are complex, involving parasitic and free-living phases.75 Most belong to the genus Arrenurus, at least 55 species of which parasitise Odonata, but three other genera are involved.76 Arrenurus cuspidifer, infesting Ischnura in southern France, can serve as an example. The egg, laid under water, hatches in one to three weeks, releasing a minute six-legged larva that swims freely and seeks out a final-stadium Ischnura larva, to which it attaches phoretically, favouring one at a late stage of intrastadial development. On pharate F-o larvae of Pyrrhosoma nymphula these minute mite larvae (less than 1.5 millimetres wide) attach by preference to the back of the head, in the deep groove between the head and
FIG 103. A male Ceriagrion tenellum carrying a heavy load of parasitic mites on his thorax (Robert Thompson).
thorax, this being one of the few places on the body surface immune from the grooming movements employed by the dragonfly larva.77 Some mite larvae may locate and cling to a host just after its moult to F-o; some may attach beneath the wing sheaths of Ischnura elegans78 and on the compound eyes at the bases of the antennae in Coenagrion puella.79 When viewed through a hand lens, the clustered mite larvae at this stage resemble a dusting of red pepper. As the dragonfly emerges, the mite larvae transfer to the teneral adult, very promptly, moving rapidly during stage 2 of emergence and taking up positions on the dragonfly’s body that are characteristic of the species of mite. Here they quickly penetrate the cuticle before it hardens. They may even transfer to the adult during stage 1 of emergence, entering the F-o exuvia through the ecdysial aperture to do so. Up to 30 seconds (but no longer) after the host’s ecdysis, mites will abandon an injured host, but thereafter are committed to the attachment. Having inserted the mouthparts, they then steadily imbibe the host’s haemolymph through the stylostome, a tube ensheathing the mite’s mouthparts. Over a period of several days, this feeding causes the mite’s body to swell and adopt a characteristic appearance, becoming shiny, red and almost spherical (Fig. 103). Initial loads of Arrenurus cuspidifer on Ischnura elegans can reach 150 mites per host. When the ectoparasites have attained their full size, after a period approximating to the maturation period of the host, they are ready to drop off the host’s body and enter the water. In Coenagrion puella, and probably other coenagrionids, the stimulus that induces detachment is the proximity of water:79 if a flying adult approaches to within 25 centimetres of the water surface, fully grown mites are stimulated to drop off their host. Arrenurus cuspidifer mites detach from Coenagrion hastulatum and C. puella during host oviposition, regardless of their host’s sex.80 This is consistent with the finding that males of Coenagrion mercuriale in tandem carry fewer mites than do single males.81 When a mite has detached from a dragonfly, it may leave behind it part of the stylostome and often a brownish scar.82 Once in the water, the six-legged larva, now engorged, completes its development.83
Each species of parasitic mite tends to attach to a specific part of the host’s body and to have a characteristic appearance, enabling an experienced observer to recognise the species by eye, or at least with a hand lens. Likewise, for mites that attach only at emergence, the approximate time that the mite has been attached to the immature dragonfly can be judged by the state of the mite’s engorgement and, because the host’s burden of mites becomes less with each visit to water, the number of attached mites is broadly correlated with the host’s post-emergence age.85
For most species of Arrenurus, all the mite larvae that will transfer to a host do so at the time of emergence and thereafter enlarge synchronously until they drop off. Arnold Åbro found that in Norway all Arrenurus larvae on Coenagrion hastulatum and Enallagma cyathigerum attached synchronously and only at the time of the host’s emergence, whereas on Lestes sponsa and Pyrrhosoma nymphula some mites attached at emergence but most did so later, when the hosts visited water for reproduction.84 So, in the latter two species, the mite-load actually increased with post-emergence age. This means that, for such species, the degree of engorgement of a dragonfly’s mite load (assessed by the mites’ size and surface texture) does not necessarily correlate with the host’s post-emergence age.
Absence of mites does not necessarily mean that they have already dropped off; there are usually some adult odonates that remain unparasitised. In Pyrrhosoma nymphula, a Type 1 species (Box 16, p.167) with a large, early, synchronised peak of emergence, followed about 25 days later by a small second peak,86 the onset of the second peak can be marked by an abrupt increase in the proportion of adults carrying small mites.87
Much of what is known about the effects of mite parasitism on adult Odonata derives from recent work by Jens Rolff.88 On Coenagrion puella, Arrenurus cuspidator reduces the body fat of males and reduces the fecundity of females,89 and impairs host fitness by draining nutrients,90 although parasitism does not lower ejaculate volume or mating success.89 Coenagrion puella bearing mites are more likely to disperse,91 which must have a large effect on the host’s population dynamics because in some populations of C. hastulatum and C. puella parasitism can reach 100 per cent.92
Other kinds of water mite sometimes found as ectoparasites of dragonflies belong to the Hydraphantidae and Limnocharidae. The former parasitise species of Coenagrion and Ischnura and the latter species of Anax, Enallagma, Gomphus, Lestes, Leucorrhinia and Sympetrum.76 Larvae of the Hydraphantidae and Limnocharidae are primarily terrestrial and encounter the adult dragonfly by crawling or running on the surface film of water. After attachment they engorge on the host and then transform to the nymphochrysalis, either on the host or after dropping off.
Other ectoparasites of adult Odonata, almost invariably attached to the wings, are flies of the genera Forcipomyia and Pterobosca in the (mainly Holarctic) family Ceratopogonidae. The circumstances attending their attachment are unknown. The females attach to the bases of the host’s wings, apparently without inflicting serious injury, remaining there for several days imbibing haemolymph while the parasites’ oocytes mature.93 It appears that sometimes attachment occurs close to emergence: adults of Forcipomyia paludis, recorded only on dragonflies, were found on teneral adults of Aeshna isosceles, Ceriagrion tenellum and Libellula quadrimaculata.94 Infested adults presumably suffer from the loss of haemolymph, especially if infestations are high: an adult L. quadrimaculata carried 171 Forcipomyia paludis.95
Predators
Because Anisoptera are such agile, powerful fliers, virtually the only effective predators on mature adults are birds, mainly raptors and bee-eaters, that can match or better the dragonflies’ powers of flight. Zygoptera, being weaker flyers, are vulnerable to a wider range of aerial predators (Fig. 104). In a study of Calopteryx haemorrhoidalis in southern France, the main predators were found to be wasps, Anisoptera (especially Orthetrum cancellatum) and spiders (Araneidae).96 It is likely that predation on adults of both suborders is greatest at the time of emergence, before and during the maiden flight, at roosting sites on cold mornings97 and during oviposition, especially among species that oviposit endophytically. In Britain, the Hobby, Falco subbuteo, has days when (as an
FIG 104. A Willow Warbler, Phylloscopus trochilus, proffers an adult male Ischnura elegans to its nestlings (Robert Thompson).
alternative to small passerine birds) it concentrates on Anisoptera, such as Leucorrhinia dubia and Libellula quadrimaculata, snatching them in the air in a masterly display of agility. Eleven species of Odonata have been recorded as prey of the Hobby.98 It has been estimated that a juvenile Hobby would need to consume 75-90 Aeshna mixta or 200-250 Sympetrum striolatum in order to meet its daily energy requirements.99 The celebrated ethologist, Niko Tinbergen, gave an exciting and detailed account of the behaviour of a Hobby feeding on a population of Libellula quadrimaculata in the Netherlands.100 Speeding from its nest to a source of dragonflies about 2.5 kilometres away, the bird would sweep its prey out of the air and return with it, flying at about 150 km/h. In this case the bird flew to a chosen destination to collect its prey, but sometimes falcons, like perchers among dragonflies, behave as sentinel foragers, selecting an observation perch and then darting out from it, flycatcher fashion, to intercept a passing dragonfly. We seldom witness large, overland migrations of odonates in Britain, but in the New World, where such migrations are commonplace, raptors (which may be migrating themselves at the same time and on the same flyway) often prey on aggregations of dragonflies, drifting in and out of the moving cluster of insects to assuage their hunger. Many other kinds of predators take their toll of adults, mainly when these are perched. They include insectivorous plants, such as Drosera (Fig. 105), which often capture coenagrionids, and spiders (Fig. 106) in whose webs adults may get entangled when alighting on vegetation. Adults that fall on to the water surface, often as a consequence of being defeated in a territorial clash, often fall prey to aquatic Hemiptera: Heteroptera, such as Notonecta and Nepa, foraging just beneath the surface. Adult Robber flies, Asilidae, also operate from a perch and can easily catch Zygoptera and small libellulids (such as Sympetrum) in flight.101 Among Amphibia and Reptilia, frogs and crocodiles sometimes catch mature adults at the surface of the water, although, mercifully, crocodiles do not concern us in Britain. We should also mention the toll taken sometimes on roosting adults by foliage-gleaning insectivorous bats which apparently use echolocation to detect their prey.102
Hornets, Vespa crabro, sometimes take large dragonflies as prey, attacking them in flight or when ovipositing: Steve Cham watched a Hornet kill an active male Aeshna cyanea, dismember it and return later to feed on the carcass.103 Likewise, one sunny day another active male Aeshna cyanea was caught and decapitated by a Hornet. The Hornet then removed the whole dragonfly piece

FIG 105. Zygoptera, such as this male Coenagrion puella, are often trapped by Sundew plants, Drosera (Robert Thompson).
by piece.104 On another occasion a Hornet brought down a flying female A. cyanea, stung it and then removed the exoskeleton of the thorax and consumed the contents.105 Hornets’ chances of success are greater when dragonflies are relatively inactive: ovipositing females, especially when flying in confined spaces, and roosting adults are more than usually vulnerable to attack.103 Hornets also take Zygoptera.106 Sometimes the roles of attacker and target are reversed, as when two male Libellula depressa were seen to harry a queen Hornet,107 perhaps mistaking the Hornet for a conspecific female (Fig. 107). The Common Wasp, Vespa vulgaris, may prey on emerging tenerals of Aeshna cyanea, which resist attack by wing-whirring,108 and on adult I. elegans at the emergence site.109 Other predators of Odonata include other dragonflies, usually the larger Anisoptera.
To judge from the sensory perceptions of humans, many dragonflies use camouflage, or crypsis, as a means of reducing predation risk. This they achieve by a combination of colour, pattern, posture, choice of resting site and immobility. All human dragonfly watchers will be familiar with the experience of watching a ‘patterned’ dragonfly, such as Cordulegaster boltonii or Aeshna mixta, alight and then losing sight of it almost instantly because it has become almost invisible against its chosen background. Other antipredation behaviours are brought into play when a settled adult is grasped or struck by a predator. If it can escape, it is liable to fly swiftly upwards and away, and not return to the site of the attack for an hour or more. If it is held by the attacker, it will struggle violently and may swing the abdomen dorsoventrally, even to the extent of making thrusting movements downwards and forwards, as if trying to probe the attacker with the tip of the abdomen. These resemble the precursors to stinging movements made by some bees and wasps, and can be discouraging to a human handler. Such movements may have led Dr Samuel Johnson, the celebrated lexicographer, to define dragonfly as ‘a fierce stinging fly’.110 Also, if appropriately positioned, a large anisopteran may use its formidable mandibles to try to bite its handler, even managing to puncture the skin of a human finger. Sometimes, after being

FIG 107. The female Libellula depressa resembles a Hornet, Vespa crabro (Robert Thompson).
handled, adults of both suborders may drop to the ground and exhibit a behaviour known as reflex immobilisation, thanatosis, or death feigning, remaining immobile and unresponsive to stimuli for many minutes. This has been observed in Enallagma cyathigerum and Ischnura elegans.111 Resumption of activity is usually spontaneous.
The antipredation behaviours described above are probably not predator-specific, being induced by the close approach of any unfamiliar, large object; but a North American species of Calopteryx exhibits a predator-specific response which we may expect to find in British species of this genus. A very large North American gomphid, Hagenius brevistylus, preys frequently on adults of Calopteryx maculata, which it readily captures in flight. Both species were active in a stream-side population in southwest Virginia. When female C. maculata became aware of the presence within 3 metres of a perched H. brevistylus, they remained perched and immobile, neither feeding nor wing-clapping (possibly a thermoregulatory behaviour),112 for a period exceeding two hours while the predator was present. Within 30 minutes of the latter’s departure, however, they resumed their normal activity.113 Such predator-specific avoidance behaviour is presumably innate and implies a long and close association between predator and prey, both of which inhabit running water. In Europe, in places where species of Calopteryx habitually encounter vertebrate or invertebrate predators,114 similar antipredator responses may exist.
The evolution of discrete flying seasons is often interpreted as a form of ecological partitioning which reduces competition between species assumed to be sharing one or more finite resources, such as space, food, or freedom from physical interference. To sustain such a notion it is necessary to identify a limited resource and also to observe competition taking place. Norman Moore has thrown light on this matter, showing that, among similar-sized Anisoptera sharing a habitat, a dominance hierarchy exists, such that one species regularly expels one or more others after an interspecific midair clash at a reproductive site.115 For example, at ponds he observed in southern Britain, Norman found that a male Aeshna cyanea would almost always expel a male Sympetrum sanguineum, and that a male of either Lestes sponsa or Libellula quadrimaculata would almost always expel a male Sympetrum striolatum. Notwithstanding the elevated status of L. sponsa in this hierarchy, it was usually the smaller of two competing species that was ousted. This investigation revealed a rare example of competition in action. More commonly, competition is a notional phenomenon that is accepted intuitively but can seldom be demonstrated. The dominance hierarchy maintained among dragonflies localising over a single body of water imposes a fitness cost on those that are being denied access because they are deprived of potentially valuable time at the reproductive site during favourable weather. In other words, it is a clear-cut example of interference competition. This being so, we may infer with confidence that the staggering of flying seasons has selective value.
DISPLACEMENT BY FLIGHT
Being superbly competent fliers, adult dragonflies can be expected to exhibit outstanding powers of dispersal. Evidence that they do so is widespread and convincing. A newly constructed pond is rapidly colonised by several species, even if it is several kilometres from the nearest source of immigrants. Not surprisingly, little is known about the nature of this dispersal behaviour. What we do know derives largely from an ingenious project devised by Jochen Lempert,116 working in Hamburg. He chose a small, isolated pond replete with insectivorous fish and containing a few species of dragonflies, which included Coenagrion puella and Ischnura elegans. Jochen spent many hours each day at the pond, recording the behaviour and species of arriving dragonflies. Very large numbers of adults (including 18 species of Anisoptera and 9 species of Zygoptera), mainly mature, arrived over a long period, at the remarkable rate of one every four to five minutes. Arrivals during one year included more than 1,600 Sympetrum. Adults were marked on arrival, allowing Jochen to establish that about 4 per cent stayed for two or three days and 2 per cent for four to seven days, and that more than 50 per cent of Sympetrum danae left within two hours, suggesting that some males could have visited more than one pond in a day. Some arrivals continued on their way without even descending close to the water surface, suggesting that they were detecting key properties of the habitat from a height of several metres.
The picture that emerges from these well-documented observations at the pond in Hamburg is that of a countryside overlain by a blanket of dispersing dragonflies several metres above the ground. If, as Lempert found, most of these are mature, then it is reasonable to assume that a high proportion had been expelled from a reproductive site by inter- or intra-specific competition, underlining the heavy cost of occupying a low position on the dominance hierarchy. Are members of this travelling blanket of dragonflies reducing the randomness of their paths by navigating? Certainly, as they approach a water body, their ability to detect the horizontal polarisation of light will enable them promptly to detect it and, especially near the beginning and end of the daylight period, when the sun appears low in the sky, they may use its reflection to detect ponds. Wind direction may also influence their course. We know also, from work of Klaus Sternberg in southwestern Germany,117 that large numbers of Anisoptera and Zygoptera (more than 29 and 8 species respectively), almost all mature, were found moving singly or in small groups along rivers, especially those with wooded margins. Only about 25 per cent of these species normally occupied running water. Some stopped from time to time but others (especially older aeshnids and corduliids) proceeded non-stop. Thus it appears that travelling odonates orientate visually to linear features of the landscape. It is important to emphasise that the species represented in these studies by Lempert and Sternberg were not those normally regarded as migratory (Box 18). Notwithstanding these findings, mark-recapture studies of discrete populations show that the great majority of individuals move only short distances during their lives as mature adults.118 In a population of Coenagrion mercuriale studied by David Thompson, 70 per cent of mature adults (mostly males) travelled less than 50 metres during the flying season and 85 per cent travelled less than 100 metres. A few travelled much further, covering more than 1.25 kilometres.119 Average distances travelled during a lifetime were greatest among individuals from parts of the habitat where density was least and with less pronounced underwater ledges and with deeper water.120 Indeed, molecular genetic evidence indicated that adults of this species are highly sedentary, moving very little and then only to neighbouring sites.121 Interhabitat movement of Ischnura elegans is usually very restricted, but a few individuals, both immature and mature, can cover long distances.122 Some of these must be founders of new colonies.
It is useful to distinguish between trivial and non-trivial flights. Trivial flights are relatively brief, short-range movements associated with an obvious, immediate goal, such as thermoregulation, escape, foraging or reproduction.123 Non-trivial flights, which normally play a major role in the maintenance of the life cycle, are longer, straighter, and undistracted by such immediate goals. Four kinds of non-trivial flight have been distinguished. Each performs a different ecological function (Box 18). One of these is migration, a term best used (in a zoological context) to mean spatial displacement of adults to a new reproductive site.
Non-trivial flights (at least of Types 3 and 4) can be seen as adaptations to habitat discontinuity. In Britain we are only likely to witness Types 1, 2 and occasionally (near the southern and eastern coasts) Type 4. Flights of Type 3 are associated with Mediterranean-type climates that feature long, hot summers during which aquatic habitats become unsuitable for oviposition. In Algeria Aeshna mixta and some species of Sympetrum show Type 3 flights, moving to
TYPES OF NON-TRIVIAL FLIGHT PERFORMED WITHIN A SINGLE GENERATION: A PROVISIONAL CLASSIFICATION (FROM CORBET)124
Type 1. Maiden flight
This, a one-way flight from the emergence site to the first resting site, can vary in length between 1 and at least 500 metres. It is undertaken by the teneral adult and occurs only once per generation. This flight appears to be orientated away from water. All Odonata exhibit it.
Type 2. Commuting flight
A two-way flight practised daily, weather permitting, between the roosting sites and the foraging and reproductive sites. In that each flight has an apparent goal, probably dictated by an adult’s physiological state, commuting flights share properties with trivial flights. Flights can vary in length between a few metres and more than a kilometre, as in Aeshna caerulea.125
Type 3. Seasonal-refuge flight
A two-way flight between the emergence site (or its vicinity) and a refuge that offers a benign environment in which foraging and reproductive maturation can take place during the hot, dry summer.17 A regular feature of certain species in Mediterranean climates, it has not yet been detected in Britain but may be in prospect as a consequence of global warming.
Type 4. Migration
A one-way flight between the emergence site (or its vicinity) and a new reproductive site. As far as is known, it may be obligate or facultative, and occurs only once per generation. Distances covered may extend to several thousand kilometres, depending on the strength and direction of high-level winds within which travel often takes place. Although migratory dragonflies may reach Britain, many originate in the tropics where they inhabit ephemeral pools fed by seasonal rains. The transient nature of their habitat requires that they translocate every generation, their flights being directed by rain-bearing winds. While migrating, adults roost at ground level every night, ascending on morning thermals to regain the high-level winds and descending again in the evening.31
sheltered upland sites as immatures in early summer and remaining there in diapause until summer’s end, when the first major falls of rain cause them to become reproductively mature and to return to the newly filled ponds in the lowlands.17 The same species sometimes appear in Britain as cross-Channel immigrants. We provisionally interpret this behaviour as Type 3 flights that have become diverted, rather than as genuine Type 4 flights. However, this remains an open question.
We still know very little about the nature and ecological significance of the different kinds of flight made by dragonflies. For example, the position in the provisional classification in Box 18 of the flights witnessed by Lempert115 and Sternberg116 remains unclear.
Only occasionally do long-distance migrants appear in Britain. Examples are Anax ephippiger, Pantala flavescens and Anax junius. The first two species are tropical-centred and are well known as occupants of temporary pools in semi-arid regions and as inveterate migrants, travelling long distances on weather fronts to places where rain is about to fall. Sometimes such flights become diverted so that the travellers end up in severely unsuitable destinations. For example, in 1900, a living adult of A. ephippiger turned up in Iceland126 and, in 1998, several adults of a North American species, A. junius, arrived in vigorous condition at sites in southwestern Cornwall, close to the Atlantic seaboard,127 and again, in 2003, at least one adult of this species made landfall on the coast of France near the mouth of the River Loire.128 These A. junius arrived in September, the month when this species flies south in large numbers in North America, especially along the Atlantic coast.129 Examination of wind patterns immediately before the arrival supported the inference that their transatlantic flights had been assisted, or perhaps determined, by large-scale frontal systems travelling from west to east.
The late Allen Davies, an enthusiastic and expert odonatologist, used to relate how, one misty autumn evening, he was presented with a specimen of a tropical migrant dragonfly that had arrived near his home in Pangbourne in the Thames Valley. Allen’s son, who had hitherto resolutely resisted his father’s enthusiasm for odonatology, arrived home from the railway station one evening carrying a moribund specimen of Anax ephippiger, which he had noticed lying on the pavement and had brought home out of consideration for his father. Unknown to the collector, Allen had never before encountered a British specimen of this species! This incident emphasises the generalisation that information about dragonfly migration tends to derive from disconnected, chance events which, if good fortune prevails, can be witnessed by observers who can place them in biological context.
Initial attempts to systematise records of this kind have so far met with failure. Many thousands of adult Anax junius have been marked as they begin their autumn migration south from Ontario,130 but so far not one has been recaptured, unlike adults of the Monarch Butterfly, Danaus plexippus, whose flight paths have been revealed in this way. A valuable component of success in such a venture is to know in advance the migrants’ chosen roosting sites on their journey south. These were known for Monarch Butterflies but not for Anax junius. So numbers of Monarch Butterflies first marked in Ontario were resighted further south and their migratory routes tracked. Comparable information for dragonflies is awaited. However, the outlook is promising, on two counts. First, the success of using biogeochemical markers, in the form of trace element concentrations and stable isotopes, to infer the geographical origins of migrating animals, including insects,131 offers promise for tracing the autumnal movements of Anax junius in North America, as well as the origins of those adults arriving at high latitudes each spring. This exciting technique exploits the discovery that the relative abundance of certain stable isotopes follows a distinctive pattern across the North American continent and that these values are reflected in the subsequent isotopic composition of the animals that have fed there. This method has been used successfully to infer the northerly origin, in Canada and the northeastern USA, of migrant Monarch Butterflies at their overwintering refuges in Cuba and central Mexico.132 Second, the recent discovery that a migrating Anax junius can be furnished with a microtransmitter and then tracked by land and by air for several days and hundreds of kilometres on its autumnal, southerly journey133 has opened the prospect of following adults even further, until they reach the latitudes where the species is believed to spend the northern hemisphere winter. The recent study revealed that migrating adults, tagged in September and October 2005 in New Jersey, USA, migrated, on average, every 2.9 days, making an average net advance of 58 kilometres in about six days in a generally southerly direction, and that they migrated exclusively during daytime, but only after two nights of successively lower temperatures, which were followed by cold, northerly winds that aided the dragonflies’ southward migration.
In the 1940s Aeshna mixta, the appropriately named Migrant Hawker, was known in Britain as an uncommon migrant from southern Europe,134 but it has since greatly extended its range from foundation populations in the southeast to become established in southern England southeast of a line from about Hull in the north to Bristol and Cornwall in the southwest, and its range is still expanding (p.48). Near the south coast of Cornwall, A. mixta gives the strong impression to insect watchers of being an intermittent cross-Channel migrant: many days in late July or in August will pass without any adults being sighted and then suddenly a group of immature adults will appear, foraging several metres above the ground, sometimes on a day marked also by the abrupt appearance of Clouded Yellow and Painted Lady Butterflies (Colias croceus and Pyrameis cardui). Such observations do not constitute proof of migration, but are indicative of the kind of circumstantial evidence with which naturalists often have to be content when building up an impression of the migratory proclivities of a dragonfly.
As we have seen, mark-recapture studies of small, stable populations of Zygoptera reinforce the impression that most adults stay close to their natal pond.118 Mature adults of Coenagrion mercuriale in a New Forest population dispersed less than 25 metres on average and did not colonise sites more than about 1 kilometre away.63 When such adults dispersed they tended to move along a watercourse, few being found more than 4 metres from water, and they seemed disinclined to cross areas of apparently unsuitable habitat.135 In seven species of British Zygoptera neither age nor sex correlated with the tendency to disperse.136 What we discover about dispersal of dragonflies is likely to depend on what we look for, as Lempert’s observations in Hamburg illustrate. His unique study illustrates a valuable maxim: that, by remaining watchful for a long time in one place, an informed observer can sometimes learn a great deal about animal behaviour.
OPPORTUNITIES FOR INVESTIGATION
Material in this chapter suggests several lines of investigation that could readily be pursued by naturalists in the course of their field work without recourse to specialised knowledge or equipment.
Commensals
In view of the recent, fortuitous discovery that a large anisopteran (Cordulegaster boltonii) may carry on the outside of its head, thorax and legs minute flies living as commensals and sharing its prey (see p.111), observers are encouraged to take special steps to determine the prevalence of this association. In the past, it may have been overlooked largely because such dragonflies were caught with a coarse-mesh net which allowed any commensals to escape before detection. In future the use of a fine-mesh net, coupled with observations of perched, feeding adults made with a close-focus telescope, should illuminate this area of dragonfly biology. The same techniques should throw light on the frequency with which adults of parasitoid Hymenoptera accompany endophytic Anisoptera while they are ovipositing (Box 8, p.104).
Dominance hierarchies
Research by Norman Moore has shown that dragonflies localising at a single body of water exhibit a dominance hierarchy characterised by certain species consistently expelling others after a physical clash.137 By recording the fate of adults recognisable as individuals after a clash, the careful observer can provide valuable information to confirm and extend our knowledge of dominance hierarchies, which may differ according to such factors as the type of habitat, the time of year and the presence of females. For such studies to be incisive, participating dragonflies would need to bear marks enabling them to be recognised in flight as individuals.
Dispersal patterns
The observations by Lempert at an isolated pond in Hamburg have revealed how much can be learnt about cross-country travel by dragonflies.116 For the investigator willing to devote many hours each day to recording the species of dragonflies arriving at an isolated pond, and their behaviour on arrival, a study of this kind is certain to be fruitful. Likewise, Sternberg’s finding that many species use watercourses as flyways when dispersing117 suggests that systematic observations by streams and rivers could be informative. Where dragonfly dispersal is concerned, we know so little that hard data of almost any kind are certain to enlarge our perspectives.
Until recently, attempts to track dragonflies dispersing from the larval habitat have been frustrated by the lack of a method for marking F-o larvae with a label that remains identifiable in the adult after emergence. Until now, the only method available has been to collect tenerals at the emergence site, retain them until the cuticle has hardened and then apply an external mark. Mike and Marion Parr have shown that one can safely mark tenerals of Anisoptera by applying a date-specific coding of small paint spots on the wings138 but Carlo Utzeri139 found that longevity can be reduced, in Anisoptera, according to the number and position of the paint spots applied for identification. So, besides being labour-intensive, marking can impair a dragonfly’s survival. An alternative approach, to render a larva or adult radioactive, requires expensive equipment and special safeguards.