CHAPTER 8
Foraging in Flight
DRAGONFLIES ARE EFFICIENT predators throughout life, but it is as adults that their skill and virtuosity in this role are most conspicuous. They are the insect counterparts of the most agile raptors among birds, and their aerial supremacy never fails to thrill the human observer.
When examining the endowment that enables dragonflies to excel as aerial predators, we should beware against attributing their mastery to just one or a few features. It is the smooth integration of all their properties that makes possible the aerobatics that we so admire. The aerial agility of Odonata is by no means a recent phenomenon: Palaeozoic odonatoids showed adaptations to aerial predation on a wide size-range of prey, closely paralleling modern Odonata in shape and wing design.1 Palaeontologists regard the wings of Odonata as homologous with the leaflike gills of larvae of mayflies (Ephemeroptera), the insect order most closely related to Odonata. The development of flight was undoubtedly a key event in early insect evolution and has proved to be one of the most far-reaching in the history of the earth.2
In the opinion of Stanislav Gorb, the dragonfly wing represents an early masterpiece of evolution and the dragonfly ‘one of the most skilled pilots in the insect kingdom’.3 The wings are large and liberally endowed with properties that combine rigidity and flexibility. The nodus, a conjunction of veins halfway along the leading edge, permits elastic tension along that edge, as well as strong twisting, and can act as a shock absorber.4 The pterostigma serves as an inertial regulator, raising the critical gliding speed.5 Microstructures on the wing surface have an aerodynamic role by regulating airflow over the wings.6
Three remarkable attributes of dragonflies contribute disproportionately to their pre-eminence as aerial predators. Though present in all Odonata, these three attributes are much better developed in Anisoptera, in which wing-loading is greater7 and which in consequence excel in agility. The first is the composition of the load-bearing network of veins which contain resilin, an elastic protein3 which can store energy. Resilin enables grasshoppers and fleas to leap. These rubber-like veins of dragonflies are arranged so that the wing reacts as a whole, and in an elastic manner, to aerodynamic forces, and they enable changes in wing shape to respond appropriately.4 The second vital attribute that equips dragonflies as aerial predators is the power of sight and optical resolution. The structure and design of the compound eyes8 is almost ideal for the needs of an aerial predator. The compound eyes are large, typically occupying almost the whole of the head in aeshnids, and they possess great acuity. This is determined by the angles between adjacent units (ommatidia) that compose the compound eye (Fig. 108). The smaller this angle, the greater the distance at which small objects

FIG 108. The compound eyes of dragonflies contain a large number of facets that endow them with acute resolution. The division between the upper and lower part of the eye is clearly visible in this adult Somatochlora arctica (Robert Thompson).
can be resolved. With respect to the sizes of the units (and therefore the angles between them) the compound eye is differentiated into dorsal and ventral areas, each containing zones (the acute zones) where visual acuity is enhanced. Large, migratory aeshnids have the greatest number of facets. The North American aeshnid Anax junius can have more than 28,000 ommatidia in each compound eye,9 and boasts by far the smallest interommatidial angles among insects (0.24° in the dorsal acute zone, a value to be compared with 25-57° in some primitive, wingless insects).9 Resolution is enhanced by spectral and polarisation sensitivity.9 The properties of this remarkable compound eye enable dragonflies to make the most of their visual environment. On the other hand, each tiny lens of the compound eyes has low resolution, prompting D.E. Nilsson to comment that ‘It is only a small exaggeration to say that evolution seems to be fighting a desperate battle to improve a basically disastrous design.’9 The third remarkable structure, unique to the Odonata, is the head-arrester system, the structure and properties of which have been skilfully elucidated by Stanislav Gorb.10 This system, which comprises the organs of the head and neck, is unique among arthropods. First described in 1950 by Mittelstaedt,11 it involves fields of tiny, hair-like structures (microtrichia) on the posterior surface of the head and on the neck. The arrester ensures that the head remains immobile during foraging or tandem flight, apparently saving the head insertion from violent mechanical disturbance and at the same time stabilising the insect’s gaze (Fig. 109). It is absent in larvae. Its presence in adults probably reflects the evolutionary pressures imposed by aerial copulation and aerial predation. Though found in all Odonata, its structure differs according to family. Its ubiquity in dragonflies implies that it originated early in their evolution.12 The head-arrester system of Odonata especially Ischnura elegans and Pyrrhosoma nymphula, has been described in detail with respect to its functional morphology and ultrastructure.13
In this chapter we distinguish hunting (foraging) from processing the prey after it has been captured (feeding). The term foraging, an umbrella term to describe hunting, embraces a fascinating variety of options available to dragonflies for improving the efficiency of their food-gathering (p.217). Because dragonflies are usually large enough to enable an observer to see, and interpret, their actions, an analysis of their foraging behaviour provides a rich insight into the flexibility that a dedicated insect predator can command. It also serves to remind one of the tightrope that the adult dragonfly negotiates when having to ‘decide’ daily (indeed almost from minute to minute) how much of its energy to allocate to foraging and how much to reproduction, each of which entails an energy cost as well as an opportunity cost. To witness an aggregation of dragonflies ‘frenzy-foraging’ in flight is to be a privileged spectator at a display
FIG 109. The head-arrester system allows the orientation of the head of this male Aeshna cyanea to remain independent of that of the thorax during banked flight (Steve Cham).
of aerial virtuosity with few equals in the animal kingdom. Such vivid memories include a mercurial group of Aeshna mixta, perhaps hungry after a cross-Channel flight, catching flying ants over the lawn of a Cornish garden on an afternoon in late July. Sheer virtuosity!
FORAGING MODES
The major dichotomy that partitions foraging modes is that between midair foragers and gleaners. Midair foraging is the better-known mode, perhaps partly because it is more conspicuous. It is the mode that was being used by the Aeshna mixta just mentioned. It requires perception, and integration, of information about the movements, size and shape of the prey. When a percher, like Calopteryx, Libellula or Sympetrum, hunts from an observation post, we classify this as midair foraging because both predator and prey are in flight when the latter is captured. Gleaning, in contrast, involves the detection and capture of sessile (but living) prey. It requires perception, not of movement, but of form and perhaps colour. It also requires competent distance estimation and an ability to hover in a controlled way. Odonatology would benefit from more observations about gleaning behaviour. One reason is that this might reveal possibilities for using small Zygoptera for suppressing pests of greenhouse crops, such as aphids, by augmentative release, rather in the manner that parasitic Hymenoptera have been successfully employed for this purpose.14 This approach has potential because it is feasible (though challenging) to rear small Zygoptera in captivity15 (and easier to do so than rearing parasitic Hymenoptera) and because it avoids the need to employ synthetic broad-spectrum biocides. Moreover it is possible, but not yet confirmed, that Zygoptera can be habituated to certain kinds of prey and thus ‘taught’ to prey selectively on a target pest.
Odonata have a well-earned reputation for being generalised predators. A celebrated exception is the Neotropical family Pseudostigmatidae, species of which specialise on spiders which they pluck from webs while in flight.16 The process of capture involves an elaborate hovering routine and of course an advanced ability to recognise spiders. This should encourage observers to look for an ability to recognise prey types among other, less specialised gleaners. Occasionally Ischnura elegans has been seen to glean a spider from its web and to show deftness in avoiding entanglement.17 Indeed, ischnurans seem to show a degree of specialisation in taking prey from spiders’ webs.18 There may be more to gleaning than meets the eye.
The opportunism shown by some dragonflies is remarkable, even for so-called generalised predators. An ‘aeshnid-like dragonfly’ was seen in Holland munching on the porridge-like remains of a slug on an asphalt road.19 Another striking example of predatory versatility is illustrated by the female Enallagma cyathigerum, presumably engaged in underwater oviposition, gleaning Willow Aphids, Tuberolachnus salignus, while submerged.20 It consumed seven or eight aphids in five minutes. Ken Tennessen watched a male of the North American Enallagma exsulsans dart repeatedly towards Waterstriders, Gerris sp., that were poised on the moving water surface of a large riffle in Alabama. After about 20 unsuccessful sallies the dragonfly secured a Waterstrider and flew to the bank where it perched to consume its prey.21 Ken noted that, if the dragonfly did not surprise the gerrid and capture it immediately, she retreated and tried another one.
The dichotomy between fliers and perchers does not correspond neatly with that between midair foragers and gleaners, although most observers have the strong impression that gleaning is more prevalent among Zygoptera, which are predominantly perchers. Some perchers use the mid-air mode when foraging, but fliers seldom if ever use the gleaning mode.
On the whole, and although exceptions exist, foraging takes place away from the reproductive site. Therefore, because reproductive activity tends to centre on the warmest time of day, in Britain at least, foraging usually occurs before and after this,22 except during the prereproductive period when the immature adults do not visit the reproductive site. As soon as the ambient temperature permits spontaneous flight in the morning, and until it ceases to do so in the evening, dragonflies not engaged in reproductive activity are likely to devote time to foraging. The observant naturalist will know where best to look for dragonflies foraging in the midair mode, for example, in warm lee sites where small Diptera habitually swarm.
The structure of the compound eye of dragonflies favours the detection of movement, especially above the dragonfly. (The size and arrangement of the ommatidia on the ventral surface of the compound eye is better suited to the perception of form, rather than movement.) Thus dragonflies foraging in the midair mode almost always approach a target from below. Indeed, the swift, darting, upward movement that characterises the approach to prey is one of the hallmarks of foraging flight and a useful criterion for recognising what a dragonfly is doing, and sometimes what it is feeding on. When a dragonfly launches itself from a perch to intercept passing prey, it approaches the latter obliquely, making allowance for the distance to be travelled, the speed of its own flight and that of its target. By analysing video images of the approach flights of perchers (libellulids), Anthea Worthington and Bob Olberg are throwing light on the remarkable powers of information-processing possessed by perchers in the southern USA.23 In suitably expert hands, the ciné and video camera provide valuable tools for observing the intimate details of foraging behaviour. By this means Georg Rüppell has discovered that, when foraging from a perch, Calopteryx haemorrhoidalis uses the wings as well as the legs to improve the probability of prey capture.24
DIETS
If confined for a few hours in captivity, adult dragonflies, like the larvae, usually void faecal pellets. For an investigator with skill, experience and great patience, these can yield information about the dragonfly’s recent diet. The few such published studies have yielded useful information but, with the knowledge we now possess, it is unlikely that further analyses of faeces will justify the effort required. This is because the clear pattern that emerges is that the prey of midair foragers (and perhaps also of non-specialist gleaners) consists predominantly of small insects.25 Sometimes a dragonfly can be found with its mouth crammed with small flies (Diptera). Because insect fragments in the faeces have already been triturated and macerated during their passage through the dragonfly’s gut, they have acquired a grim uniformity of appearance that thwarts the forensic odonatologist. Often on-the-spot field observations can more readily give information about a dragonfly’s current prey. As with the detection of commensals, the use of a fine-mesh net can be rewarding, especially as sometimes a dragonfly captured foraging may still have prey between its legs or mandibles. In the now-classic inventories of prey of Odonata by Campion and Hobby, most prey items were Diptera, comprising representatives of at least eleven families, and the prey included other insects such as wasps, honeybees, bumblebees, butterflies, moths, caddisflies, alderflies, winged ants and other Odonata.25
CAPTURING, HANDLING, SUBDUING AND PROCESSING
The legs are long and well armed, especially in Anisoptera, and it is commonly supposed that they are used to grasp the prey. This is certainly so in species of Calopteryx in which the legs, liberally furnished with long spines, are positioned to form a net in front of the head during prey capture, while the densely pigmented wings are orientated so as to present a visual barrier, discouraging prey from escaping.24 Surprisingly, however, close observation has revealed that aeshnids, corduliids and lestids often, and perhaps usually, use the mouthparts alone to capture small prey.26 The legs tend to be used when capturing larger insects, but it is not always possible for the observer to identify their role precisely, because they may also be used later to subdue the prey. Odonates often carry a large prey item to the ground to consume it, perhaps making it easier to subdue. Ken Goodyear reports the remarkable observation of an adult Lestes sponsa, resting on a sprig of heather, holding a large tipulid, Tipula melanoceros, it had captured.27 The dragonfly held the prey firmly by the second abdominal segment, thus preventing the tipulid from beating its wings, and then advanced slowly into thick heather, pushing the prey before it; a few minutes later the prey was dead and being consumed. This sequence of actions comes close to tool-using! Although large prey is often brought to a perch or the ground to be consumed, small prey is often consumed while the dragonfly is in flight. When Aeshna mixta is foraging on flying ants, the shower of dismembered wings that descends shows that the ants are being processed and presumably ingested in flight. Sometimes Anisoptera foraging in a dense swarm of small Diptera may be catching prey more quickly than they can ingest it, as shown by a bolus of prey remaining between the mandibles: a male Aeshna mixta was once encountered with his mouth crammed with small black midges.28 Confronted with a situation like this, some animals, ourselves included, would arrange to store the excess against subsequent need. It seems that dragonflies sometimes hoard food in this way (Box 19, F). Benno Hinnekint has seen Ischnura elegans, Orthetrum cancellatum and Sympetrum sanguineum hoarding food regularly in the form of a meatball.29 Sympetrum sanguineum hoarded up to eight Drosophila at a time, keeping the meatball inside its mouthparts, instead of beneath them as Ischnura elegans did.
INCREASING PREDATORY EFFICIENCY
Optimal foraging theory predicts that natural selection will produce foraging behaviours that maximise the net rate of energy gain.30 Our intuition makes this outcome almost axiomatic. It would seem impossibly costly in terms of energy for dragonflies to seek prey (which typically has a clumped distribution) at random. Our qualitative experience roundly supports this intuition. Dragonflies possess several strategies for focusing their foraging behaviour in places where the predator-prey encounter rate is very high, and perhaps maximal. It lends interest to field observations to try to interpret foraging behaviour against this theoretical template. Dragonflies worldwide employ at least twelve strategies that seem to serve this purpose. Because species in the tropics are less restricted in their diel periodicity of flight than species in higher latitudes, we can witness more of these strategies in tropical species. It is worthwhile to take note of them because such strategies may appear among dragonflies in Britain during unusually warm weather, or when global warming begins to become manifest. There are three main categories of strategy (Box 19, A-C), each being expressed in several forms.
Most of the strategies listed are self-evident, but three deserve comment. Strategy B.1 is unlikely to be witnessed often in Britain because the ambient temperature near sunrise and sunset is seldom high enough to permit foraging, although it would be worth examining the flight style of occasional crepuscular foragers, such as Aeshna grandis and Anax imperator, to see if they are employing this tactic. Similar in principle is the habit of perchers, like Calopteryx species,
STRATEGIES THAT ARE ASSUMED TO INCREASE FORAGING EFFICIENCY (FROM CORBET)31
A. Foraging where prey are concentrated in space and time
A.1. At a temporary prey concentration caused by:
A.1.1. swarming of prey
A.1.2. prey aggregating at a source of attraction
A.1.3. a localised thermal
A.1.4. a lee situation.
A.2. At a persistent prey concentration caused by a light gap in forest.
B. Increasing capture success by:
B.1. facing the rising or setting sun
B.2. facing into the wind
B.3. taking prey by surprise.
C. Foraging where resting prey have been made to fly by physical disturbance caused by:
C.1. the foraging dragonfly
C.2. a large, slowly moving object.
D. Foraging for exceptionally large prey.
E. Defending a foraging site from exploitation by other predators.
F. Hoarding food.
that almost always face the sun when in the foraging mode, so that passing potential prey appears to them in silhouette. Strategy C.1, witnessed by Stephen Cham and Clive Banks,32 though not yet by us, must be exciting to watch. The observers were attracted by a rustling sound emanating from a dense patch of Stinging Nettles, Urtica dioica, partly warmed by the rising sun. The sound they heard was being made by the wings of a male Aeshna grandis brushing against the nettle stems amongst which it was flying. As the dragonfly repeatedly entered the patch, large numbers of resting chironomids were disturbed and made to fly. Each A. grandis would intermittently hover, then catch and consume a chironomid before continuing on its way. The chironomids in the patch constituted a concentrated source of potential food for the dragonfly but, being on the underside of leaves, they were relatively inaccessible for a midair forager unless they could be made to fly. The dragonfly, which was one of three conspecifics behaving similarly, continued to forage in this way for almost 20 minutes. Its behaviour was obviously strategic and not accidental. Strategy C.1 may have developed from the habit of gleaning stationary prey from tree trunks, exhibited by many species of large Anisoptera,33 when the mere act of prey capture would stimulate neighbouring prey individuals to fly. Strategy C.2 has so far been detected only in the tropics and almost exclusively among Anisoptera of the subfamily Sympetrinae.34 It occurs over open grassland and entails the foraging dragonflies assembling around large mammalian herbivores (usually cattle or antelopes) as they wander through the grass. The dragonflies are clearly using the animals as ‘beaters’ which disturb the small insects resting low amongst the grass stems during the heat of the day and thereby make them available to the midair foragers. The dragonflies are behaving very much as Cattle Egrets, Bubulcus ibis, do. The ecological context for this behaviour is that the Sympetrinae typically frequent shallow pools, often in open country where most small winged insects are likely to be resting during the day in the relatively humid environment close to the ground, making them unavailable to dragonflies foraging in the open air above them. As British summers become hotter and drier it will be interesting to see whether any of our libellulids, especially species of Sympetrum, show signs of adopting strategy C.2. The most conspicuous exponent of strategy C.2 is the Afrotropical sympetrine Brachythemis leucosticta, whose behaviour led to the discovery and analysis of this type of accompanying behaviour. It is one of the most widespread of Afrotropical libellulids and uses large, slowly moving objects, not its prey, as the stimulus for accompanying.35 This behaviour, which is characteristic of this species almost everywhere, is a stirring testament to the versatility of foraging strategies exhibited by Odonata.
Strategy E remains controversial. When Douglas St Quentin conducted his pioneer field studies on territorial behaviour he assumed that clashes between adult dragonflies over water constituted attempts to defend a foraging area.36 In general, these were probably related to reproductive, rather than foraging, behaviour, but in a few instances aggressive interactions between individuals have been reported at a foraging site.37 Examples should be sought away from water among individuals clearly in the foraging mode and showing no signs of reproductive behaviour.38
ENERGY BALANCE
Adults, like larvae, need to maintain a positive energy balance (Box 11, p.127). Adults differ from larvae in three significant respects:
1) they expend relatively much more energy on food gathering than larvae do;
2) the opportunity cost of doing so is much higher (in terms of time unavailable for reproduction); and
3) options (to forage or reproduce) are much more constrained by the weather, especially at high latitudes.
This means that each day, especially during the reproductive period, an adult is faced with decisions that have far-reaching consequences: when and for how long to forage, or to reproduce. Being free from this dilemma, prereproductive adults probably consume more food than do mature adults.39 Teneral adults of Calopteryx splendens and Erythromma najas consume less than do post-teneral immatures,40 but both Calopteryx virgo41 and Cordulia aenea42 have been seen to forage during their first day as adults. The ability of adults to survive without food is highest just after emergence and then declines steadily thereafter.43 Such a capability would help to mitigate the deleterious effects of inclement weather at the time when adults are least able to fly robustly. During the prereproductive period, adults can gain more than 100 per cent of their weight at emergence, depending on the weather44 which affects foraging opportunities to a critical extent.
The remarks above imply that foraging and reproductive activity are mutually exclusive as far as time allocation is concerned, and sometimes this appears to be so. However, a spectrum exists between two extremes: at one extreme are species that often forage while reproductively active at the rendezvous, for example, Orthetrum coerulescens;45 and at the other are those that hardly ever do so, for example, Aeshna cyanea,46 Cordulia aenea47 and Sympetrum striolatum.48 Occupying an intermediate position in this spectrum is Libellula quadrimaculata.49 Little is known about time allocation in non-territorial species. There is no clear distinction between patrol and foraging flights of Cordulegaster boltonii: a male may patrol for a whole day, during which it may perch or forage for short periods.50
The sexes differ slightly in the strategies they adopt when trying to maintain a positive energy balance, because of the different ways in which they achieve reproductive success, males by patrolling or active defence of a territory and copulation, and females by converting energy into eggs and by ovipositing. Furthermore, a bout of reproductive activity costs much more energy per unit time for a flier than for a percher,50 and so will require a proportionately larger prior investment in foraging, which is itself more energetically costly in the flier mode. One estimate, for a libellulid percher, Pachydiplax longipennis, studied in Florida by Fried and May, was that the energy expended on territorial defence
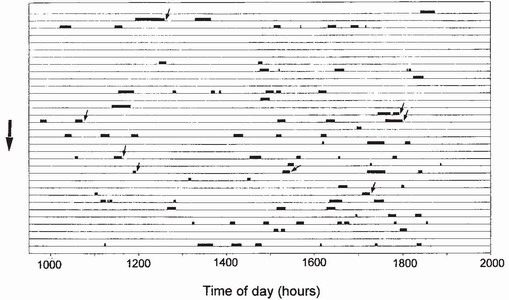
FIG 110. Times and durations of visits to a pond in Germany made by a single marked male of Aeshna cyanea from 1 August (top horizontal line) to 2 September (bottom horizontal line) 1967 inclusive. Sloping arrows mark the beginning of copulations which were completed away from water. (After Kaiser.51)
(about 150 joules per day) amounted to about 85 per cent of assimilable energy obtained per day.52 This does not seem to leave much of a safety margin if inclement weather curtails foraging activity. Indeed, Fried and May speculated that on some days during their reproductive life, individuals replenish energy reserves by reducing or eliminating their period of territorial defence and concentrating on feeding.52 It is therefore not surprising that a flier such as Aeshna cyanea interposes foraging between relatively brief visits to the rendezvous during permissive weather (Fig. 110).51
ECONOMIC IMPORTANCE
For considerably more than a century, biologists have given thought to harnessing the foraging activity of dragonflies to suppress insects that are troublesome to humans, by their numbers and/or activity. The first serious exploration of this idea surfaced in a book by Lamborn.53 This attractive volume, embellished by a fine, hand-coloured frontispiece of Anax junius, was a collection of essays on this topic submitted in response to an advertised competition. The rationale behind the competition was the need to suppress disease-carrying mosquitoes that were threatening the construction of the Panama Canal. Since then, and up to the present day, advocates of biological control of mosquitoes using dragonflies continue to present their case, sometimes marketing Anisoptera larvae to augment existing populations in places where mosquitoes are too abundant for human comfort. As long ago as the 1940s, however, a skilled observer in Florida noticed that, even when large Anisoptera had assembled to forage on swarms of stableflies, Stomoxys calcitrans, and mosquitoes (probably Aedes spp.), no noticeable reduction in prey density resulted.54 This is not surprising. As mobile, generalised predators, dragonflies are never likely to depress the numbers of one prey type sufficiently to meet comfort thresholds defined by humans. As soon as a prey type begins to fall in abundance and become more difficult to secure, the predator will switch to others that are easier to obtain. To be able to switch is a great asset for a generalised predator but a fatal drawback for a biological control agent in an open system.
The demonstration that adult Anisoptera were lowering the numbers of insect pollinators near a pond in Florida55 neither contradicts the preceding statements, nor does it demonstrate the potential of dragonflies as biological control agents. To be effective, a biological control agent must maintain its prey below an economic threshold defined by humans, which is usually very low.
However, there are situations in which dragonflies can seriously reduce the numbers of their prey. They arise when either the prey or the dragonflies are confined in a closed system. They deserve close scrutiny, if only to arrest the flow of impracticable suggestions that dragonflies in open systems have potential as biological-control agents. The first concerns predation by large Anisoptera on hives of Honey Bees, Apis mellifera.56 Although Aeshna cyanea was reported to have destroyed nearly half the Honey Bees in one district of the USSR,57 the definitive study of this phenomenon was conducted in bee yards along the Mississippi River in Louisiana in the 1940s and involved two large species of aeshnid with a proclivity for assembling close to hives: Anax junius and Coryphaeschna ingens.56 Local beekeepers called these dragonflies ‘bee-butchers’, apparently without exaggeration. If each such dragonfly has a notional consumption rate of 50 per cent of its own body weight per day, it is simple to estimate that a dragonfly feeding thus could kill 100 bees in 20 days. So a hive of 50,000 workers could be exterminated in 20 days by 500 dragonflies, or in 10 days by 1,000 dragonflies.58 It follows that the reported impact on bee yards is entirely credible. The five prerequisites for such dramatic bee mortality are listed in Box 20. The frequently encountered proposals to suppress mosquitoes by enhancing local populations of dragonflies meet none of these requirements.
The second situation in which dragonflies are effective at suppressing prey
PREREQUISITES FOR DRAGONFLIES TO BE ABLE TO DECIMATE HONEY BEES IN FLORIDA (FROM CORBET)58
- bees are spatially concentrated (in hives);
- hives are spatially concentrated (in bee yards);
- dragonflies, being highly mobile, can aggregate from a wide area;
- dragonflies can temporarily specialise on one kind of prey;
- dragonflies are large enough to capture and kill the bees; and
- the dragonflies’ flying season coincides with the time when the prey is active and vulnerable.
insects, although not an example of foraging in flight, deserves mention because of the ecological principles it illustrates. It was devised in the late 1970s by Anthony Sebastian, a medical entomologist working in Burma (now Myanmar). After exhaustive preparation, and by following a rigorous protocol, Sebastian successfully suppressed the population of the Yellow-Fever Mosquito, Aedes aegypti, in a suburb of Rangoon (now Yangon) throughout the rainy season, the time when the mosquito was transmitting dengue fever and dengue haemorrhagic fever to humans. The numbers of the mosquito were driven down to levels lower than normally attainable by application of conventional pesticides, and low enough to interrupt disease transmission during the rainy season. This situation was a special case because it involved a closed system which Sebastian was perceptive enough to recognise. He reared and released half-grown larvae of a libellulid, Crocothemis servilia, into domestic water-storage containers in which a very high proportion of the mosquito population was developing. The prerequisites for success in this case (Box 21) were restrictive, but by no means unique in tropical countries.59
A key component of this successful enterprise was that the predator-prey interaction was taking place in a closed system. Ancillary advantages of this innovative approach are that it used local resources and involved active cooperation of the local community, and its implementation did not entail use of synthetic chemical pesticides or expenditure of foreign exchange. It is occasion for regret that this discovery has not been exploited.
FACTORS THAT MADE POSSIBLE THE SUPPRESSION OF AEDES AEGYPTI IN MYANMAR BY THE AUGMENTATIVE RELEASE OF DRAGONFLY LARVAE
- ability to secure eggs of an exophytic dragonfly that is reproductively active throughout the year;
- ability to raise dragonfly larvae from eggs;
- knowledge that four F-2 larvae of Crocothemis servilia will promptly consume all mosquito larvae and pupae in a domestic water-storage container of standard size;
- concentration of a high proportion of the mosquito population as larvae and pupae in domestic water-storage containers;
- knowledge that the dragonfly larvae are able to survive for several weeks without food;
- willingness of local householders to have half-grown dragonfly larvae placed in their water-storage containers; and
- willingness of local householders to allow regular monitoring of mosquito density in their houses.
OPPORTUNITIES FOR INVESTIGATION
Qualitative information about the behaviour and prey of gleaners and the prevalence of gleaning would help to build a balanced perspective of foraging behaviour. Such observations could probably be made more effective by presenting targets of real or simulated prey for gleaners. (Coenagrionids will repeatedly try to glean a gall from a leaf)60 While such observations were in train, thought could be given to their possible application for biological control of glasshouse pests, including those in butterfly farms and other insect display houses. Also, more information is needed about the prey taken by midair foragers and about the foraging strategies they employ to secure it. In particular it would be useful to find examples among British species of strategies C.1, C.2 and E in Box 20.
The most rewarding topics probably lie in the field of ecological energetics, building on the pioneer work of Mike May and others.61 When a percher is making foraging sallies from a perch, its success rate in catching prey can be determined by a patient observer equipped with short-focus binoculars. So far it seems that success rates vary widely – from 2 to 90 per cent.62 The success rate for a single male Sympetrum striolatum was 28 per cent while making an average of 0.96 sallies per minute, the maximum duration of a single sally being 5 seconds.63 The duration of foraging-only flights of Orthetrum coerulescens varied between 1 and 11 seconds, showing an average of 2.7 seconds and a mode of 2 seconds.64
Little is known about the aggressive interaction between conspecifics at foraging sites, or ‘fights at the dinner table’ to use the terminology of Baird and May.65 Keen observers, watching individually marked adults at foraging sites, could throw light on this.
Scope also exists for enlarging the inventory of large prey taken by Odonata.