CHAPTER 9
Reproductive Behaviour
INTRODUCTION – THE FUNCTIONAL FRAMEWORK
THE ADULT STAGE of the life cycle in British Odonata is relatively brief, lasting only a few weeks for most individuals. Each individual therefore has only a short time to pass its genes on to the next generation and so a large part of adult life is spent maximising the chances of meeting and mating. An extraordinary, complex and fascinating array of reproductive strategies has evolved in response to this strong selection pressure. Reproductive behaviour has a temporal and spatial dimension. Spring or Type 1 species (Box 16, p.167) synchronise their emergence which ensures that most of the adult population is on the wing at the same time. Reproductively mature males increase their chances of meeting females by congregating at water at the time that females are most likely to arrive to oviposit, which in Britain is usually for a few hours either side of solar noon. But, as we shall see, some males are ‘sneakers’ and adopt other strategies to meet females; this enables them to forestall their more conventional rivals. At the water’s edge, the males of many species of Anisoptera and some zygopterans stake out territories at prime oviposition sites from which they attempt to exclude rival males and so further increase their chances of reproductive success. Competition even persists during the act of mating when the male attempts to remove or displace the sperm of rivals stored within the female’s reproductive tracts before delivering his own. Once mated, the male may continue to guard the female during oviposition to prevent usurpation by other males while his own sperm retains precedence for fertilising the eggs his partner is about to lay. The challenges posed by each stage in the reproductive process have been tackled in different ways by different species. We shall view this behavioural diversity in terms of a cost-benefit analysis: a useful approach adopted by evolutionary biologists. In this chapter we shall see how this diversity has been generated.
First, it is useful to identify the essential components of the reproductive process in order to place the elements of reproductive strategy in temporal context.
The encounter
Mature males and females must meet in time and space. Both genders must be able to recognise a suitable rendezvous site. Males may become more or less attached to this site and, in order to improve their reproductive success, may attempt to exclude other conspecifics from it by using a variety of interactive repertoires. The rendezvous site thus often becomes the arena for sexual selection on males. At the rendezvous, males search for females.
- Recognition. Males and females must be able to recognise each other as conspecific to reduce costly interspecific interactions resulting from mistaken identity.
- Sperm transfer. Sperm must be transferred from the male reproductive organs to those of the female. The male may attempt to give a competitive advantage to his sperm by removing or displacing sperm received by the female from former partners (p.246).
- Guarding behaviour. Sexual selection continues to operate after mating and results in traits that males use to exclude rivals from access to their recent mates (pp.252).
- Oviposition. Fertilised eggs must be laid in a site suitable for larval survival and successful development (Chapter 3).
ENCOUNTER BETWEEN THE SEXES
The rendezvous
For reproduction to be successful it is essential that males and females of the same species meet at the same time and the same place. For most species of dragonfly the rendezvous is the oviposition site. However, for a few British species the rendezvous may occur elsewhere. For example, males of Sympetrum danae search for females during the morning away from water where and when over three-quarters of matings occur.1 Lestes sponsa has a similar strategy and pairs arrive at the water in tandem, having already copulated.2 Males of Enallagma cyathigerum may intercept females up to 800 metres from the oviposition site.3 In southern France, Peter Miller noticed that males and females of Ischnura elegans meet at the nocturnal roosting site (away from water) one hour after sunrise and begin copulating within 20 minutes.4
Males tend to arrive at the oviposition site earlier in the day than females. This allows territorial species to select and defend high-quality oviposition sites before females arrive. The rendezvous thus serves as an arena for sexual selection as a result of male-male competition, as a site for copulation, and also as an oviposition site.
The precise location of the rendezvous may be determined by the time of day. For example, as a different part of a pond receives direct sunlight and other parts become shaded, so the place defended by a male Sympetrum striolatum shifts to that part that is in full sun. Similarly, the time of day that the male is present may alter with the season. At Steve Brooks’ garden pond, male S. striolatum are present for several hours before and after solar noon during July, but by late August they appear for only about one hour preceding solar noon because for the rest of the day the pond is in shade. Even the age of the individual may determine when it is present at the rendezvous: young female Aeshna cyanea are present there earlier in the day than are older females.5
Site attachment
The males of most species of Anisoptera and also calopterygid Zygoptera show site attachment to a varying extent. They return repeatedly over a prolonged period to the same site and defend this site against incursions by conspecific males or even Odonata of different species. Within this site (the territory) the holder dominates other members of the same species and has prior access to females. The extent to which the territory is fixed in time and space varies and may depend on the number of interactions with other males and females or on exposure to sunlight. In some species, for example Libellula quadrimaculata (Fig. 111), the dominant male may tolerate subordinate males within the territory.6 The vigour with which the male defends the territory can vary with time and between species, and may depend, among other things, on the density of mature males. Territorial behaviour results in males becoming localised on a particular site and spaces them out through that site. Holding a territory becomes increasingly beneficial to the holder as the site gets more crowded and competition for females intensifies.7
The degree of site attachment is usually related to male density. Species that occur at low male density at the rendezvous (such as Aeshnidae and Cordulegastridae) typically exhibit weak site attachment, whereas those that
FIG 111. A male Libellula quadrimaculata surveys his territory from a vantage point near the edge of a weedy pond (Robert Thompson).
occur at high male density (such as Libellulidae, Corduliidae and Calopterygidae) exhibit strong site attachment. Site attachment often intensifies as the season progresses because male-male interactions and the frequency of female visits increases. Physical factors may also influence the intensity of site attachment. Those sites, such as clumps of emergent plants or discrete embayments along the shore, which provide landmarks or clearly defined boundaries, tend to intensify site attachment.8 Odonata evidently have a good topographical memory and spatial awareness: individual males of Orthetrum cancellatum (and those of the Mediterranean species Calopteryx haemorrhoidalis) remain loyal to the same site even after being absent for a few days following bad weather.9 Interactions with predators may also influence the strength of site attachment: for example, a male Calopteryx haemorrhoidalis is more likely to vacate a territory if attacked by a frog or spider early in its territorial occupancy than a male that has occupied the territory for more than three hours without being attacked and has courted females there; he may remain on site even if attacked later.10
Localisation
The first stage of site attachment is localisation. On arriving at water, the male surveys the area for its potential to provide suitable oviposition sites. Soon the male will localise at a suitable site where he establishes his territory. Cordulia aenea amurensis, the Japanese subspecies of the British Downy Emerald, will localise on a territory within two minutes of arriving at water.11 Utzeri and Dell’Anna studied localisation behaviour in Libellula depressa and found that when a newly mature male first arrives at water he does not show strong site attachment. Instead he frequently changes perches, moving around the pond, engaging with other males and searching for females.12 However, after his first successful copulation, the male guards the female during oviposition by hovering close by, and subsequently localises on the copulation site and defends this against other males. If there are no subsequent matings over the next day or two the male abandons the territory and resumes searching behaviour, only establishing another territory at the next successful copulation and oviposition site.12 In this case the male’s territory is being selected for him by the female’s choice of oviposition site, and the male has to learn which site to defend. Wandering L. depressa males are therefore likely to be either unmated and naive, or older males that have experienced low mating success. In some species the age of the male influences the degree to which he becomes localised at a specific site. Young male Leucorrhinia dubia range far and wide, but become increasingly localised at a small part of a pool as the season progresses.13 Similar behaviour was found in Orthetrum coerulescens.14 On the other hand, although site attachment initially increases in Calopteryx virgo (Fig. 112), as the flight season draws to a close males once more begin to show weaker attachment to a particular site.15
FIG 112. A male Calopteryx virgo localised on a leaf in a sunny spot overhanging a stream (Robert Thompson).
Site fidelity
Most dragonfly species remain at a single pond or group of ponds throughout the reproductive period.16 However, the length of time a particular male occupies the same territory, even if the species shows high site fidelity, can range from a few minutes to many days depending on the species or the individual. Some of the record holders, which can be said to show high site fidelity, include Calopteryx virgo (recorded intermittently for 40 days at the same territory),15 Aeshna cyanea (38 days),17 Orthetrum cancellatum (15 days)18 and Calopteryx splendens (13 days).19 While a male is holding a territory his mating success is enhanced,20 although the length of time a territory is held and the mating success this achieves must be offset against the energy costs expended in holding it.
The same male of Aeshna cyanea in Germany visited a small pond one to eight times a day on 26 days during a 33-day period, each visit lasting up to 40 minutes.17 His absences from the territory might have been due to bad weather, foraging or presence at other territories. The same territory may be shared by several males during the course of a day. A small bay at the edge of a
large woodland lake in southern England was occupied and defended almost continuously by different male Cordulia aenea (Fig. 113) from 12:38 to 17:03 hours solar time (where solar noon falls at 12:00 h) on 2 July 1994.21 The bay was left unoccupied when not illuminated by direct sunlight. During the period that the bay was occupied it was shared between seven different males, each one being present for 1-21 minutes (average 10 minutes) at a time. Three of the males appeared in the territory only once during the day, but two others occupied this or the adjacent bay twice during the day; one male reoccupied the bay on three occasions and one male was resident on four different occasions.
Attributes of a territory
The size of a territory is not necessarily related to the body size of the species occupying it or to its flight mode (i.e. whether it is a percher or flier), but there is a tendency for large fliers to have large territories and for small perchers to have small ones. Some of the largest territories recorded in British species include those of Orthetrum cancellatum which may patrol a water margin 10-50 metres in length,18 Cordulegaster boltonii which may patrol part of a stream 5-10 metres in length22 and Aeshna mixta whose territory may cover an area of 10 m2.23 The size of the territory can be modified by the physical nature of the terrain (e.g. the presence of landmarks, well-defined bays, clumps of vegetation and the density of perches). Territory size is also inversely proportional to the density of conspecific males and is related to the exposure of the site to direct sunlight. Males of Cordulia aenea patrol the edges of woodland ponds. The size of the area they patrol appears to be related to the number of males present at the pond because when a male meets another male he turns and flies in the opposite direction.21 Thus, as the number of males present at the pond increases, so the length of bank patrolled by any one individual declines. At high male densities, during the peak of the flight season and towards the middle of the day in fine weather, each territory is limited to one small bay that can be surveyed by a male hovering near the middle of the territory.
Most species of Odonata require a site to be exposed to direct sunlight before they will begin to localise on a territory, and if only a small part of a site is exposed to direct sunlight then the territory will be smaller and confined to that patch. Males of most species will leave a territory when it becomes shaded,24 although the central European Somatochlora meridionalis has been seen to accelerate through sunlit spots while patrolling shady banks.25 Some territories appear to attract more ovipositing females than do others, but this is not always related to an obvious physical attribute.26
Searching behaviour of males – fliers and perchers
At the rendezvous site a male spends much of his time in either non-aggressive flight, searching for females or, if the species shows site attachment, defending the area against other males. Searching behaviour may take the form of surveying the area from a perch, making brief forays from the perch, or patrolling the site. As we have seen, the searching behaviour of Aeshna cyanea may consist of one to eight flights of up to 40 minutes each at the same site per day.17 In contrast, Oxygastra curtisii may undertake a continuous single search for several hours.27 Searching modes used by males may be categorised into a number of types. The first is between perchers (typically Libellulidae and Zygoptera) and fliers (typically Aeshnidae, Cordulegastridae and Corduliidae) in which the male may respectively ‘sit and wait’ or patrol; this category includes species that are either localised on a particular site or non-localised. The main outcome of the patrol flight is to increase the chances of a male meeting a female. Hidenori Ubukata noted that the patrol flight of male Cordulia aenea amurensis, the Japanese subspecies of C. a. aenea, was similar to the pre-mating flight of the female of that subspecies, and that both increased the chances of meeting a mate.28
The patrol flight of a dragonfly tends to be directional (either linear or circular), steady, moderately fast and close to the water. An individual flies to and fro along the same beat or repeatedly returns to the same perch. The ambient light conditions determine the height of the patrol flight which is lowest in shade or towards dusk and higher in bright light. Sometimes the patrol flight will include bouts of hovering while the dragonfly inspects places where females may be resting or ovipositing. Such places are often in the shade, and hovering appears to improve visual discrimination29, so increasing the likelihood of the male detecting a female. The frequency of hovering bouts tends to increase as light intensity decreases. Hovering may also help to advertise the presence of the male to a female. Hovering consumes a lot of energy and tends to decrease in frequency towards the end of the period spent in the patrol flight. The patrol flight can also include elements of display, for instance when the abdomen is raised in Cordulia or Oxygastra30 or turned upwards in Calopteryx.31
Another apparent function of the patrol flight is to monitor the distribution of neighbouring conspecific males.32 Indeed, the amount of time spent patrolling by male Sympetrum striolatum increases after repeated encounters in rapid succession with other males.33 The patrol flight may also include periods of foraging, although, as already noted, these two activities are usually segregated.
The precise mode of searching behaviour may be determined by the physical nature of the habitat. After vegetation at the rendezvous had been trimmed, the patrolling behaviour of the central European corduliid Somatochlora flavomaculata changed.34 Similarly, the southeast European S. meridionalis displayed three modes of patrolling depending on the location of the rendezvous. At a shaded stream males patrolled continuously along its whole length and sunny spots were avoided; at a meadow margin shaded by trees males localised on a small shaded area and chased off intruding males; and at a group of small pools males inspected areas with overhanging trees and bushes.25 Male density may also influence patrolling behaviour (p.239).
AGGRESSIVE BEHAVIOUR
Males of species that show site attachment respond aggressively towards conspecific males, and sometimes towards other species, that invade their territory. Aggressive behaviour reduces the flight range of other conspecific males or displaces them from a defended area.35 Such aggression is directed towards intruders entering the area, but it may also take place while a male is non-contact guarding an ovipositing female. Aggressive behaviour takes different forms depending on the response of the intruder. The outcome of such disputes is often predictable: the territory holder usually wins, although under some circumstances the intruder may displace the resident male. The effect of aggressive behaviour is to space out males at the rendezvous site. Aggressive behaviour also sometimes occurs between females, which may give the winner improved access to foraging or oviposition sites (e.g. in Aeshna juncea, Anax imperator,36 Ischnura37 and Sympetrum38).
Repertoires
Even in species that do not appear to show site attachment, such as Coenagrion puella, males may engage in pursuit that can result in either or both males leaving the rendezvous.39 Such interactions can be difficult to observe and interpret. In species that show overt site attachment, aggressive behaviour is more obvious. If the defender remains perched it may take the form of a threat display (p.236), but usually a resident male takes off and attempts to drive the intruder away. Threat behaviour is most vigorous and effective near the centre of a territory and as the residence time of the male increases.40 Males are usually able to recognise conspecific males and direct such threat behaviour towards them. But males will sometimes begin to attack a female, only to modify this behaviour by forming a tandem when the female is recognised as a potential mate rather than a threat. Faulty recognition of potential mates occasionally results in the defending male forming a tandem with another male or a female of a different species.41 Such instances usually occur in conditions of low light intensity or where there has been an accumulation of sex drive after a period of low mating success.
Using high-speed film to analyse the behaviour of species of Leucorrhinia, Ilmari Pajunen established that there was a hierarchy of aggressive behavioural acts.42 This hierarchy has subsequently been witnessed in other Odonata, although the complexity of the repertoire varies. Leucorrhinia appears to occupy an intermediate position.43 Four modes of behaviour have been identified in L. caudalis, an Eastern European species closely related to L. dubia, namely approach, chase, threat and fight, although the sequence may vary and be repeated. The approach flight is rapid and direct and comes from below or from the side. This differs from the approach that a male makes to a female before forming a tandem, which is usually from above. If the two antagonists recognise each other as conspecific males, a chase follows which may extend beyond the limits of the territory. The pursuing male keeps below and behind the intruder and makes no attempt to catch him. If the intruding male turns to face his pursuer instead of fleeing, the two contestants begin threatening each other by repeatedly darting towards each other, although no contact is made. This results in a circling or spiralling interaction which punctuates the chase. Circle and spiral threats occur when both males show strong site attachment. Threat may develop into fight when one or both contestants attempt to grasp or bite the head or thorax of the opponent. Such escalating behavioural modes are exhibited more or less elaborately by many other species of Odonata. Where a species has brightly coloured patches on the face or abdomen these are often prominently displayed during the interaction, and the white legs with enlarged tibiae are conspicuously trailed by male Platycnemis pennipes when confronting other males. Threat displays and physical interactions may also be elicited by intruding tandem pairs, copulating and ovipositing pairs, or while a male is non-contact guarding an ovipositing female.
A more complex repertoire of aggressive interactions is adopted by Calopteryx virgo (Fig. 114).44 When a territorial male approaches an intruder he may engage in frontal threat, in which both males face each other, hovering and changing position to the side or rear, but maintaining the same distance apart. During this display the abdomen is arched upwards and the head and thorax are inclined. In reversed threat the territorial male slowly retreats from the intruder in a characteristic undulating flight during which the abdomen is curved upwards and the head and thorax are held horizontally. Sometimes the antagonists adopt lateral threat in which they fly side by side. Two forms of chase behaviour may occur. In the first the territorial male pursues the intruder at the same height and maintains a distance of 10-15 centimetres behind. In the second type the males face each other and wheel in a tight circle before finally breaking off in a short chase. The final type of aggressive behaviour, which may continue for 30-60 minutes, is termed rocking flight, in which both males rise and fall together, flying erratically and frequently changing direction. Fighting has not been observed in C. virgo, although occasional, accidental clashes do occur. Calopteryx splendens behaves in a similar way, but stroke frequency and orientation of the wings differ between the modes of aggressive display, and are an important component in this form of communication.45
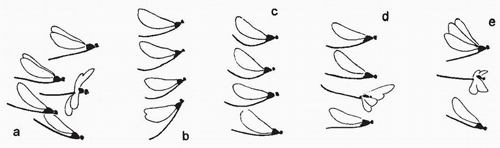
FIG 114. Representative flight postures of male Calopteryx virgo during (a) normal flight; (b) mutual frontal threat; (c) one-sided frontal threat; (d) reverse threat; and (e) rocking flight. (After Pajunen.44)
Perched male Odonata, especially Zygoptera, may react to an intruding male conspecific by flicking, clapping or lowering their wings, or by jerking, lowering, raising or curving the abdomen to expose the coloured segments. The characteristic raised abdomen of patrolling corduliids46 may serve as a form of threat or territorial declaration.
Fighting appears to be the most primitive form of inter-male interaction because it is seen in species that have the simplest interactive repertoires. Fighting is almost nonexistent in those species that have the most complex repertoires of inter-male interaction. Fighting, or even accidental clashes, can result in serious injury, especially to the legs and wings, and can be fatal. An antagonist can be forced onto the water where it may drown. Aeshna cyanea may attempt to ram an opponent17 and male Pyrrhosoma nymphula may try to force a rival into the water or mud by pushing from above.47 Leucorrhinia dubia may try to grasp his antagonist’s thorax with his legs.48 Recipients of an attack may attempt to defend themselves by stretching their fore legs above the head and thorax.49
Interspecific interactions
Whereas most aggressive interactions are between individuals of the same species, territory holders sometimes react to intruders of different species. Such a response is usually provoked by species that are similar in size and colour, for example, Anax imperator (Fig. 115) versus Aeshna juncea or Pyrrhosoma nymphula

FIG 115. A male Anax imperator cruises over his territory searching for receptive females and ready to chase intruding males (Steve Cham).
versus Ceriagrion tenellum.36 However, at high male density Cordulegaster boltonii (a large black and yellow species) has been observed to expel a perched male Orthetrum coerulescens (a smaller blue species) from its territory.50 Usually a resident male will maintain its territory when challenged by a male of a different species, although Aeshna cyanea is usually expelled by Anax imperator.36
Outcome of interactions
Almost all interactions are quickly won by the resident male, for example, in Pyrrhosoma nymphula the resident male can be victorious 97.5 per cent of the time.47 So, we may ask: under what conditions is the resident defeated? Males that have recently and frequently copulated are at a disadvantage,51 as are those that are exceptionally old or young, whereas site familiarity or proximity to the centre of the territory is an asset.52 Displacement may occur when the resident male is otherwise engaged in copulation or aggressive interactions,53 contact guarding an ovipositing female,54 or chasing a female.55
Territorial disputes escalate when there is ambiguity regarding the identity of the territory holder.56 Escalation may occur if the original territory holder is engaged in fighting or mating when the interloper arrives and has time to localise on the territory. Ambiguity may occur if the boundaries of the territory change, for example, as different parts of the rendezvous site become illuminated directly by the sun during the course of the day. If several males enter the territory simultaneously, escalation can occur.57 As the duration of the dispute increases, the chances of success of the original territory holder decline. In the North American species Calopteryx maculata, the resident male won 81.5 per cent of disputes that were resolved within ten seconds, but longer disputes could continue for several hours, leaving the resident male at a disadvantage. In prolonged disputes the contestant with the greater body size and larger energy reserves was often victorious. The greater the ratio of flight muscle weight to body weight, the greater the chances of mating success.58 However, in Pyrrhosoma nymphula size has no apparent effect on success, and resident males won 117 out of 120 observed disputes, even though the resident was smaller than the interloper on 56 occasions.47 Studies on Calopteryx splendens xanthostoma from southern Europe revealed that both contenders in an escalated dispute can exhaust 40-50 per cent of their energy reserves, so that only young pre-territorial males, which typically have a high fat content, are likely to displace a resident territorial male.20 It follows that, once a male has been displaced, he is unlikely to gain a new territory by escalated dispute because of his lowered energy reserves.
Density effects
As the density of males increases at the rendezvous site, there is an increase in the intensity of site attachment and localisation.59 Aggressive interactions tend to intensify in Aeshna cyanea60 but have been shown to decrease in Calopteryx splendens61 and Leucorrhinia dubia.48 As male density increases, the size of each defended territory decreases,62 as does the duration of occupation.17,65 The decrease in territory size under these circumstances reflects an increase in energy expended because the territory has to be defended more frequently. In these conditions males may show less inclination to leave the territory in order to guard ovipositing females or chase rivals.63 In Cordulia aenea the distance a male patrols along the edge of a pond declines as the number of males present at the pond increases (Fig. 117).64 Other density effects include an increased intensity in the guarding of ovipositing females by males of Enallagma cyathigerum66 and Sympetrum danae67 and an increased likelihood of underwater oviposition by female Calopteryx virgo.44
When all the available territories at a rendezvous are occupied there is an increase in the number of non-territorial males perched around the pond. Eventually, as the number of males continues to rise, less favoured ponds within the area come to be occupied.68 An increase in non-territorial males has been demonstrated in Calopteryx virgo,44 Libellula quadrimaculata6 and Orthetrum
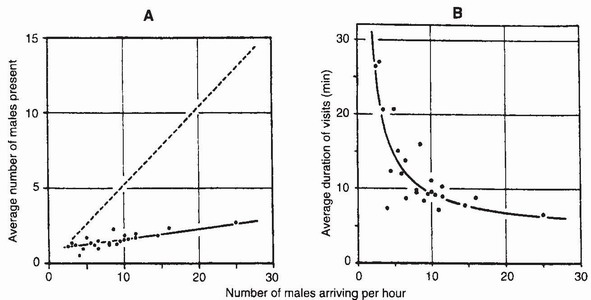
FIG 116. Two relationships that operate the feedback mechanism that regulates density of Aeshna cyanea males at water. a) the number of males at water does not increase linearly (dashed line) in proportion to the arrival rate of males; b) the average duration of visits declines abruptly as the arrival rate of males rises to ten per hour. (After Kaiser.60)
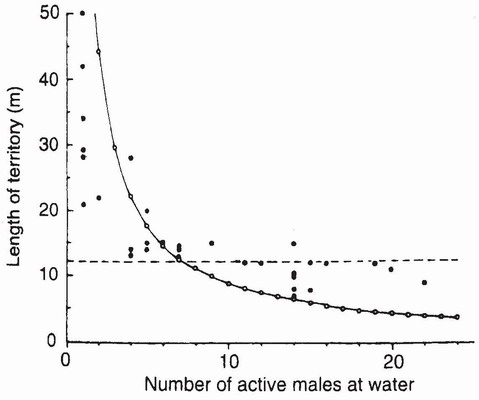
FIG 117. The quantitative relationship between the density of mature males of Cordulia aenea amurensis (the Japanese subspecies of the European C. aenea aenea) at water and territory formation. When the number of active males in the study area exceeded eight, all males became territorial, causing displacement and spacing of newcomers. The dashed line denotes the average length of territory. (After Ubukata.28)
coerulescens69 when there are no longer any territories available. The increase in non-territorial males of Cercion calamorum leads to an increase in the number of females that are intercepted on the way to the oviposition site and a decrease in the number of successful matings by territorial males at the rendezvous.70
High densities of males can eventually lead to a breakdown in territorial behaviour as the territory-defending males are overwhelmed (e.g. in Leucorrhinia dubia,.13 Calopteryx virgo72 and Enallagma72). This leads to so-called ‘swarming behaviour’ during which males en masse may pursue a single female. Swarming behaviour can also occur at low male densities if there are insufficient perches at the rendezvous or if the arrival rate of females is so high that they prove to be in excess of requirements.73
ALTERNATIVE REPRODUCTIVE BEHAVIOUR
In some cases a territorial male will tolerate the presence of other males within or close to the boundaries of his territory. The resulting groups of males may form a dominance hierarchy with a distinct ‘pecking order’, or there may be a single dominant male with an array of subordinate males that cruise the fringes of the territory. Other males may adopt a non-territorial strategy and search for females away from the rendezvous. There is a tendency for the number of territories with associated satellite and subordinate males to increase as the density of males at the rendezvous site increases.70
Dominance hierarchies
A consequence of a dominance hierarchy is that it will reduce the number and frequency of territorial disputes, which can be costly in terms of energy expended and can result in serious injuries to the contestants. A territory can be occupied by several males in which one (the ‘territorial male’) is dominant.74 The dominance hierarchy in the North American species Plathemis lydia (Plathemis is a genus closely related to Libellula), which was studied by Campanella and Wolf,75 serves as an illustration of this strategy. As the male matures he rises in the dominance hierarchy; and the higher the male’s status the closer he gets to dominating at the optimal time in the day for mating (i.e. 11:00-14:00 h). Alpha status is attained midway in reproductive life when the male is dominant during the part of the day that is optimal for mating. As the male passes his prime so does he begin to descend the hierarchy. In Libellula quadrimaculata and Orthetrum coerulescens, satellite males remain perched around the territory.76 They do not chase intruders and are not noticed by the territory holder. However, if the dominant male is removed, he is promptly replaced by a satellite.58
Shared occupancy
A territory may be occupied and defended by several males that show no strong dominance hierarchy.77 For example, subordinate male Sympetrum striolatum occupy positions just outside the territory of the dominant male. When the dominant male copulates, the subordinate that is perching closer to the territory than the other subordinates takes over the territory. Other males can be regarded as wanderers. Whereas territory holders remain perched for over 92 per cent of the time that they are at the pond, and only 4.4 per cent of them make patrol flights, wanderers spend 80 per cent of the time that they are at the pond patrolling.33
Non-territorial modes
Up to six alternative non-territorial strategies were recognised in populations of Calopteryx splendens,78 none of which included an element of courtship display. These strategies appear to represent a range of options that may be adopted by non-territorial males:
- bank lurkers grasp females as they warm up or pass by;
- pursuers chase females for up to 50 metres;
- roosting site attackers search for females as they roost either in the early morning or evening, during overcast weather or in light rain;
- sneakers prowl the edge of the territory;
- stealers attack and split tandem pairs; and
- water lurkers search oviposition sites and form tandems with females that are submerging or surfacing and may even pursue females underwater.
Many other species (including Libellula quadrimaculata,6 Sympetrum striolatum,33 Calopteryx virgo71 and Orthetrum coerulescens14) adopt non-territorial behaviour, especially during times of high male density, and any particular individual appears to be capable of switching from one strategy to another.
RECOGNITION AND VISUAL COMMUNICATION
Like humans, dragonflies are primarily ‘visual’ animals. As far as is known, they recognise the species and sex of their partners using visual cues, some of which odonatologists can readily appreciate, and some of which can be detected only by experimental analysis. For recognition to be an effective component of reproductive behaviour, it must involve an exchange of signals, the meaning of which is understood by each participant. In other words, it must involve communication, a sequential process, requiring that a mature male can recognise:
1) a mature, conspecific male (in order to try to defend a territory);
2) a mature conspecific female;
3) a receptive female;
4) and that a female can recognise a conspecific male.
In the first of these three steps it is widely accepted that recognition is primarily visual and based on clues such as flight style, size and colour (including ultraviolet reflection and optical density)79 and pattern. Humans, whose powers of visual discrimination are far inferior to those of dragonflies, employ all these cues to recognise flying dragonflies (often to species), except ultraviolet reflection for which humans lack receptors. Biologists have a tendency to adopt a reductionist approach when addressing questions of this kind, and to seek to identify discrete cues on which recognition is based. Sometimes this approach can be fruitful, but it can nevertheless be useful to bear in mind that the milieu (type of vegetation, degree of shade, speed of current, etc.) in which one dragonfly perceives another may also help it to recognise a receptive conspecific in its preferred surroundings. Often, however, the role of specific cues can be conspicuous, as when a mature male Pyrrhosoma nymphula repeatedly ‘buzzed’ a perched Libellula quadrimaculata whose thorax bore a blob of bright red paint,80 and when a male L. depressa patrolling over water tried to seize a Hornet, Vespa crabro, which in size and coloration resembles a female L. depressa.81 The use of models can demonstrate that size and posture serve as clues for intraspecific recognition.82 Intimate knowledge of the internal structure of the compound eye makes it possible to visualise the retinal image produced by different patterns, and, by this means, the essential elements of images that release reproductive behaviour can be inferred.83 Much can be learnt also about cues that release different behaviours by recording the ‘mistakes’ made by territorial males when responding to intruders,84 although care must be exercised to distinguish reproductive behaviour from actions, such as foraging, that may have a non-reproductive function. When trying to locate a potential mate, male anisopterans often seem to respond first, and from a distance of several metres, to the cue for ‘anisopteran’ and then, at closer range, to cues that require greater discrimination and that may be diagnostic for their own species. For example, a male Libellula fulva attacked every anisopteran that approached, but subsequently was able to recognise conspecifics.85 A perched, tethered female of Aeshna cyanea, with wings fluttering, attracted males of three families and six species of Anisoptera (A. cyanea, A. grandis, A. juncea, Cordulia aenea, Libellula quadrimaculata and Somatochlora metallica).86 It is noteworthy that males of the six species attracted to the tethered female Aeshna cyanea have difficulty in recognising conspecific females.87 At close range, details of the pattern on body or wings probably enable a male to decide whether or not to proceed in his attempt to secure a copulation partner. It is in the male’s interest not to form the tandem link with a heterospecific female; so it is not surprising that sometimes formation of a heterospecific tandem link is prevented by physical incompatibility between the shape of the superior abdominal appendages of the male and that of the female’s head (in Anisoptera)88 or the dorsal surface of the prothorax of the female (in Zygoptera).89 Sometimes receptive females seem to advertise their presence by flying in a conspicuous way. In his now-classic study of reproductive behaviour in two North American libellulids, Merle Jacobs90 observed that when all males of Perithemis tenera had been removed from a pond, females behaved conspicuously, visiting oviposition sites and making oviposition movements there. Similar behaviour is shown by female Sympetrum striolatum in Britain.91 This is merely an extreme example of the often distinctive flight style adopted by receptive females when they first arrive at water. On arrival at water a virgin female of the Japanese subspecies Cordulia aenea amurensis performs a brief ‘pre-mating’ flight, at a characteristic height above the water in the Pondweed (Potamogeton) zone, during which her posture differs from that of a patrolling male: her abdomen is held at a different angle and her flight is slower and more sinuous.92
Species recognition sometimes depends on details of colour pattern and morphology, as in Coenagrion puella,93 and in a habitat where illumination is poor, such as tropical rainforest, it may depend predominantly on physical contact.94 When several colour forms coexist, as in Ischnura elegans,95 it appears that males choose to mate with the commonest form of the female and that females choose to mate with the commonest colour form of the male.96 Ischnura elegans females with male-like colouring have reduced opportunities to mate and therefore also reduced costs of mating.97 The angle of approach by male I. elegans can determine their success in recognising colour forms of the female.98 The selective advantage of colour forms remains controversial.
Experiments using a small electric fan to attract males of the Japanese cordulegastrid Anotogaster sieboldii showed that they cannot initially distinguish between rotating objects and conspecific females, suggesting that it is the wing-stroke frequency of a flying female (equivalent to a rotation frequency of 20-25 Hz) that is a recognition cue for a male.99 Naoya Ishizawa suggests that there may be other species of dragonfly that respond to rotating objects.100 There may, however, be more to this response than meets the eye: A. sieboldii responds to the flickering reflection of sunlight from a small waterfall and also to an operating television set! The situation is ripe for experimentation.
Territorial male Cordulia aenea have been seen to approach tandem pairs of Pyrrhosoma nymphula from above, presumably mistaking the tandem for a conspecific female.
To resist a mating attempt by a male, female Odonata adopt characteristic ‘refusal’ postures.101 In zygopterans and aeshnids these often entail the female flying with the posterior half of the abdomen flexed ventrally at a right angle (the so-called ‘hockey-stick posture’). David Thompson102 has pointed out that only females empty of eggs are physically able to adopt this posture and that it could be of selective value to males to recognise this, and, by doing so, avoid a fruitless attempt at mating.
A matter of close relevance to a male’s reproductive success is whether he can recognise (as an individual) a female with whom he has just copulated. It would be a waste of a male’s seminal investment to expend effort and sperm by copulating again with the same female. This question is not easily answered, but the burden of evidence so far favours the inference that a male can sometimes recognise a female with whom he has just copulated, though perhaps not until he attempts tandem formation.103 Males of Calopteryx haemorrhoidalis, however, often guard non-mates.104
The means by which females recognise males as conspecific are less well understood, and in any case difficult to infer, partly because their refusal postures, which are well known,103 may be exhibited on account of a female’s physiological unreadiness for copulation and not only when she recognises that an approaching male is not a conspecific. It is much easier to interpret the behaviour of a female in species in which male courtship normally precedes copulation. Among British Odonata, courtship has been confirmed only in the two species of Calopteryx. The courting male responds to the proximity of a female by raising his abdomen and spreading his wings. If the female is receptive she alights near him whereupon he performs an aerial dance, fluttering backwards and forwards and from side to side in front of her, his wings beating with a frequency characteristic of courtship.105 If the female remains receptive (perched and immobile) the male lands on her wing tips (probably using the conspicuous pseudopterostigma as a target) and then climbs down the costal edge of her wings until he reaches her thorax, whereupon he fastens his superior abdominal appendages to her prothorax, securing her in the tandem position. If this stage is reached, the female nearly always accepts the male by curling her abdomen forwards and upwards so that its tip engages with his secondary genitalia, thus allowing copulation to proceed. It has been suggested that the male Platycnemis pennipes courts the female,106 but, even though courtship has been recorded in a tropical platycnemidid,107 it is by no means certain that the conspicuous display by the male of his flattened white tibiae serves this function in P. pennipes.108 It may only function to advertise his presence to conspecific males.
PRECOPULATORY TANDEM
The precopulatory tandem position provides the pair with relative freedom from physical interference by conspecific males. Even though a rogue male may try to grasp the female, such an attempt will be seriously impeded by the presence on the female’s head or prothorax of her partner’s appendages. So takeovers of females in tandem are rare, although the persistence of the intruding male may lead to his grasping the head or prothorax of the tandem male, resulting in a temporary and unstable threesome. Such an outcome, which lends weight to the adage that ‘two’s company but three’s a crowd’, is relatively frequent in species of Sympetrum and Corduliidae. The intruding male sometimes belongs to another species, though hardly ever to another genus.109
Soon after tandem formation the male, by flexing his abdomen downwards and forwards, transfers sperm from his genital pore, situated on the ventral surface of abdominal segment 9, to his sperm vesicle, the sperm-storage reservoir in the secondary genitalia on the ventral surface of abdominal segments 2 and 3. This action, known as intramale sperm translocation (IST), almost always occurs after precopulatory tandem formation, although in about 5 per cent of such translocations witnessed in two species of North American Enallagma IST took place before tandem formation.110 Occasionally copulation proper, entailing insemination, ensues without IST having been witnessed. In such cases the sperm vesicle may have been adequately charged before a previous copulation and so may not need replenishing. Generalisations about the time that IST occurs will always be elusive, partly because it may vary, partly because it may be extremely brief, and partly because it may have taken place out of the observer’s sight. For example, it may occur in flight and take less than a second in Orthetrum cancellatum.111
COPULATION
Copulation begins when the genitalia of the sexes interlock and the ‘wheel position’ is adopted (Figs 118 & 119). At this point a truly remarkable phenomenon occurs. Before inseminating his partner, the male uses his penis to displace any sperm in her sperm-storage organs. He may do this by hooking the sperm out of her body using recurved hooks or horns on the surface of his penis (Fig. 120) or by using his penis to push or flush the sperm into recesses in the female’s body where it is unlikely to be used when she next lays eggs. Having displaced the sperm of rivals, the copulating male then inseminates his partner, transferring his own sperm from the sperm vesicle, along a duct within the penis, to the sperm receptacle within the female from which she will draw on it to fertilise the eggs she lays during the next 24 hours or so. After that time any sperm in her body will have become mixed, and the priority enjoyed by the most recent sperm will have been lost.112 By displacing rivals’ sperm the male
will have given his own sperm priority over theirs provided that he ensures that his mate lays her complement of eggs promptly, and certainly within the next 24 hours. By achieving this, the male will improve his own fitness by winning the battle of sperm competition, ensuring that he will be the father of the forthcoming batch of eggs.
The discovery that a copulating male displaces rivals’ sperm before inseminating his mate stands as one of the most significant in the history of
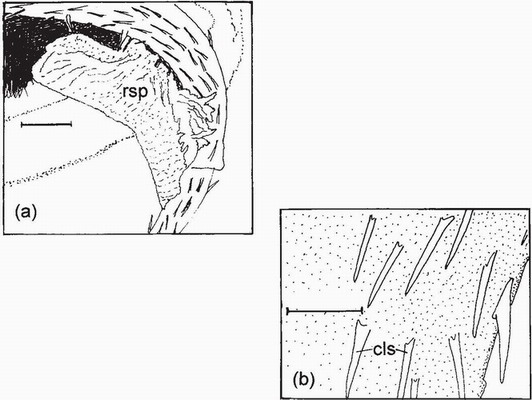
FIG 120. Drawings from scanning electron micrographs of tip of penis of the coenagrionid, Ischnura ramburii. (a) clump of rivals’ sperm (rsp) caught on the comb-like spines; and (b) comb-like spines (cls) enlarged. Scale lines (a) 20 µm; (b) 5 µm. (After Waage.113)
odonatology.114 The discovery was made by Jonathan Waage during an elegant experiment using Calopteryx maculata, a close relative in North America of the British species of Calopteryx.115 With his hypothesis of sperm displacement in mind, Waage combined rigorous field observation (observing the male’s body movements during copulation) with microanatomical examination of penis structure and the female’s sperm-storage organs, using scanning electron microscopy to reveal that:
1) before copulating, females often carry substantial amounts of sperm;
2) during copulation this sperm is progressively expelled or removed by the male before insemination; and
3) such sperm displacement is 88 to 100 per cent effective.
Waage detected clumps of sperm lodged among the backwardly pointing spines on the hornlike processes at the tip of the penis (Fig. 120). Since Waage’s discovery, which was inspired by his acquaintance with work by Geoffrey Parker on sperm competition in the Dungfly, Scathophaga stercoraria,116 sperm displacement has become a cutting-edge topic in odonatology, to which British researchers, such as the late Peter Miller117 and his former doctoral student Mike Siva-Jothy,118 have made distinguished contributions. Knowledge of sperm displacement has provided an essential and wonderfully informative context in which to interpret the reproductive behaviour of Odonata. It is evidently a prime target of natural selection. One aspect of Waage’s discovery115 has proved to be very useful for observers trying to interpret the sequence of events during copulation. Two stages can be recognised during copulation of Calopteryx, and also during the copulation of Enallagma cyathigerum119 and Ischnura elegans.120 During stage I, which is characterised by active rocking or undulatory movements of the male’s abdomen, the male is removing or displacing rivals’ sperm. During the much briefer stage II, the male’s abdomen is immobile, as he inseminates his mate. The correspondence between the movement (or immobility) of the male’s abdomen and his handling of sperm has proved to be invariable, and so the external movements serve to inform an observer of what is happening and thus obviate the need for postmortem examination. Such inference is unfortunately impracticable for those species that copulate in flight and very briefly (see below).
The duration of copulation varies widely among families. Although it also varies widely between species, certain broad patterns can be discerned. The minimum recorded duration of copulation in British Zygoptera is less than five minutes, except in the Platycnemididae. It is also less than five minutes in British Anisoptera, except in Aeshnidae and Cordulegastridae.121 The Libellulidae, in particular, complete copulation very rapidly, some species being able to do so in less than ten seconds. In Britain and elsewhere species of Libellula and Orthetrum perform at this level. Several extrinsic factors can prolong copulation.122 Among physical factors these include low light intensity, high wind and low temperature. Among biotic factors they include youth of the male, as in Orthetrum cancellatum,123 earliness of copulation in a sequence of copulations, as in Calopteryx splendens,124 disturbance by other males, as in Ischnura pumilio,125 female virginity, as in Calopteryx splendens,124 a low likelihood of encountering a female in a given site, as in Orthetrum cancellatum118 and Sympetrum danae126 and earliness in the day, as in Enallagma cyathigerum,127 Ischnura elegans,.120 Lestes sponsa128 and Sympetrum danae.126 In Sympetrum danae, in which copulation may last for 20 minutes, 95 per cent of the eggs are fertilised by the last male.129
Ischnura elegans presents a remarkable exception to the general pattern described above. In a population studied by Peter Miller (in southern France) copulation (or at least the wheel position) lasted for an average of 324 minutes120 – much longer than had been recorded for any other dragonfly. Miller hypothesised that protracted copulations might allow males to transfer more sperm or other substances to females, or they might enable males to displace more of the sperm of rivals (although in some other dragonflies most of the sperm of rivals can be displaced in relatively brief copulations). Ischnura pumilio also sometimes remains in copula for several hours, depending on the time of day.130 Aside from the needs of sperm transfer, a determining factor may well be that longer copulations in I. elegans may allow males to guard females until these are prepared to oviposit in the afternoon after 16:00h.120 Another possibility suggested by Miller is that protracted copulation in I. elegans enables the male to secure a female early in the day and so prevent takeovers by other males before the female is prepared to oviposit. According to this last hypothesis, protracted copulation is a form of guarding, enabling a male to be proactive in securing a mate and then to sequester her until she oviposits. This last hypothesis would be consistent with the knowledge that copulation in I. elegans is exceptional in being frequently interrupted by extended, inactive pauses which contribute significantly to its long duration.
Copulation duration in Odonata varies widely within and between species121,122 though correlating positively with the paternity expectation of the male,123 the extent of disturbance during copulation124 and the previous mating experience of the female.125 During stage 1 of copulation, the number of abdominal movements of the pair correlates positively with the stage’s duration.126.
Copulation duration in Orthetrum cancellatum can be distinctly bimodal, exhibiting modes at about 15 minutes and 21 seconds. The bimodality correlates with different degrees of sperm removal, long copulations resulting in almost 100 per cent removal versus 10-15 per cent removal in short copulations. The difference in copulation duration relates to the site of copulation and to the age of the male. At oviposition sites males who secure copulations are relatively old and copulate only briefly, whereas at foraging sites males who secure copulations are relatively young and copulate for long periods.127 A consequence of this difference is that males defending a territory, by copulating only briefly, increase their opportunities for mating with a greater number of females (at a favoured oviposition site) whereas males that encounter females away from favoured oviposition sites will mate with fewer females but achieve greater sperm displacement with each one.
During copulation the male can apparently displace rivals’ sperm in four distinct ways:
1) by sperm removal (physical withdrawal of stored sperm)128,
2) by sperm repositioning (packing of rivals’ sperm to sites where its use will be least likely)129,
3) by female sensory stimulation to induce sperm expulsion130, and
4) by sperm flushing (displacement of sperm using the copulating male’s sperm).131
A mated female stores sperm in the bursa copulatrix and the spermathecae, both of which receptacles are situated close to the vagina. When the male’s penis pulls out sperm, it often does so only from the bursa because the penis cannot always reach the spermathecae, which accordingly may act as a sperm cache for sperm from previous matings. By using sperm from the spermathecae to fertilise her eggs, a female may be exercising post-copulatory choice among mates.132 However, it seems that males of some Calopteryx species (such as C. haemorrhoidalis) are equal even to this ploy; during copulation the aedeagus of the penis of the male stimulates mechanoreceptive sensilla which elicit contractile activity around the spermathecae which then eject sperm, probably to the bursa where sperm can be removed by the spines on the head of the penis.133 In C. haemorrhoidalis the width of the aedeagus is positively correlated with the amount of sperm ejected by the female spermathecae. Female C. splendens adopt a characteristic post-copulatory posture during which they wipe off the sperm mass that has been received during the penultimate copulation and then ejected. This removes sperm from between the valvulae of the ovipositor and thus prevents the dislodged sperm from obstructing oviposition.133 The role of the spermathecae and associated glands in sperm storage, and subsequent fertilisation of the eggs, have been described for Pyrrhosoma nymphula by Arnold Åbro.134
The number of times a male mates in a day may be said to reflect his sex drive, which in turn appears to be hormonally driven. In Calopteryx splendens a male that has secured a copulation early in the day exhibits enhanced sexual activity thereafter during the same day.135 Such behaviour, as in Libellula depressa,136 seems also to be linked to the site where early copulations were obtained. A copulating female dragonfly is sometimes less than single-minded, feeding at the same time, for example, on another dragonfly137 or a bumble bee (Bombus).138
The male of some Anisoptera signals to his copulation partner when the copulation should terminate – Aeshna cyanea and A. mixta by wing-clapping and touching and Sympetrum striolatum by wing-lifting.139
At very high densities of males, most copulations of C. haemorrhoidalis are forced, not being preceded by courtship. Some such coerced females may have already mated. They evidently trade copulation for protection from harassment that would impede oviposition, in a strategy that Adolfo Cordero has called ‘convenience polyandry’.140
POST-COPULATORY BEHAVIOUR
As we have seen, the priority (for fertilisation) won by a copulating male through sperm displacement is time-limited because his sperm and that of previous rivals mixes completely within about 24 hours after copulation. So it is strongly in his interest that his mate should oviposit promptly after copulation and certainly within the next 24 hours. It is therefore not surprising that the male often remains with his copulation partner, ‘guarding’ her, at least until she begins to oviposit. He may do this by continuing to hold her in tandem (contact guarding), as in Chasers (Libellula spp.) and Darters (Sympetrum spp.) and many Zygoptera, or he may remain close to her, in a defensive mode, attacking any conspecific male who approaches her (non-contact guarding), as in Libellula and Sympetrum, and some species of Calopteryx.141 A male’s need to remain close to his copulation partner can be seen as critical for his conservation of energy because, in Calopteryx at least, he cannot reliably distinguish her from other females.142 In two species of European lestid, Lestes sponsa and L. virens, the importance of the male partner’s need to safeguard the paternity of the eggs about to be laid by his mate is evident from the fact that postcopulatory guarding is prolonged when there is intense interference by rival males.143
It follows that one way in which a female can sometimes choose the father of her offspring is to postpone oviposition after copulation. Although many libellulids begin to oviposit promptly after copulation, some remain perched for a while before oviposition begins. The duration of such post-copulatory rests (PCR) can vary between less than a minute and more than two hours. Among British species, Orthetrum coerulescens exhibits PCR, lasting between five seconds and about ten minutes.144 At present the biological significance of this variation is not understood, although the Millers speculated that the duration of PCR may reflect a female’s need to assess predator pressure at the oviposition site, to assess a male’s guarding capacity, or to manipulate recently received sperm, either mobilising it for fertilisation or selecting it according to the ‘quality’ of their mates.144 In this situation it is not surprising that a male Orthetrum coerulescens, having completed copulation, sometimes nudges or rams his perched mate, apparently inducing her to take off and commence oviposition.144 Such behaviour is readily explained in terms of sperm competition, but leaves one surprised that it is not more widespread. However, not all species are able to oviposit promptly after copulation. Sperm originate in the testes as ‘cloned’ lumps, or spermatodesms,145 each spermatodesm comprising several thousand sperm with their heads embedded in a mucoproteinaceous matrix.146 The female can be inseminated with spermatodesms, as in Aeshnidae and Gomphidae, with free sperm, as in Zygoptera, Corduliinae and Libellulidae, or with both, as in Cordulegastridae.147 Sperm are transferred during IST and later to the female in clusters in Zygoptera148 or spermatodesms in Aeshna cyanea.149
At one time it was thought that about two days had to elapse after insemination before sperm within a spermatodesm could be released, and that this might explain why males of Aeshnidae, Cordulegastridae and Gomphidae do not usually guard their partners after copulation. However, such an explanation is thrown into doubt by recent studies of two species of aeshnid (Anax junius and A. parthenope) that inseminate with spermatodesms. Both are exceptional among aeshnids in that the male sometimes exhibits post-copulatory guarding.150 The male enjoys exclusive paternity of the eggs next laid by the female he was most recently guarding.146 This being so, any relationship that may exist between insemination by spermatodesm, guarding and sperm precedence remains to be elucidated.
MATING SYSTEMS
The principal target for selection, both sexual selection and natural selection, is the number of offspring that an individual dragonfly can endow with his or her genes, a measure embodied in the term lifetime reproductive success (LRS) (p.94). Sexual selection is the outcome of intrasexual competition, manifest as male-male competition for copulations (and sperm priority) and in female-female competition for partners and oviposition sites. Natural selection is either interspecific (manifest in competition with other species for space or other resources) or intraspecific (arising from individual differences in fitness). The main components of LRS are the total number and genetic quality of the eggs a female lays during her lifetime, and the total number and genetic quality of eggs that a male fathers during his lifetime. For the female, LRS depends on her longevity, the number of clutches she lays per day, the number of eggs per clutch and the genetic quality of her male partners. For the male, it depends on his longevity, the number of copulations per day, the number of eggs he fathered in each copulation and the genetic quality of his female partners. One might expect both sexes to discriminate among copulation partners. Until fairly recently it was assumed that only males exercised choice of this kind, by selecting females that complied with perceived criteria for fitness. However, in theory at least, there are several ways in which a female can choose the fitness of the father of eggs she lays. To identify these requires a change in mindset, away from the old-fashioned assumption that only males take the initiative when it comes to choosing a partner. Theoretically, the choices available to females are:
1) at what time of day to visit water;
2) whether or not to copulate, and therefore
3) which male to copulate with (and for how long);
4) how many eggs to lay promptly after copulating; and
5) which partner’s sperm to use to fertilise her eggs after copulating.
To demonstrate convincingly that a female is pursuing any of these options presents formidable practical difficulties, but will nevertheless be well worth attempting. By enumerating the ways in which each sex can enhance its LRS, the investigator can visualise the different patterns according to which these are combined into strategies that form a mating system. The initiative for identifying odonate mating systems lies with Kelvin Conrad and Gordon Pritchard151 who were inspired to do so by a seminal review, based on birds.152 The system proposed by Conrad and Pritchard was subsequently modified to place greater emphasis on migration, male territoriality and guarding behaviour, the role of the primary rendezvous and the duration of copulation153 – all variables that are functionally related. The resulting six mating systems distinguished are listed in Box 22, together with examples of their British exponents.
System 6 seems to represent the most highly evolved condition in the series that leads toward increased LRS for territorial males. Most copulations are obtained by very few males, and many males never mate. It shows the greatest
BOX 22
MATING SYSTEMS ENCOUNTERED IN ODONATA (FROM CORBET)153
1. Long-range migration. Females mate when teneral, the rendezvous being the emergence site, which males control. Some at least of the females then migrate, ovipositing elsewhere after long-range displacement. The LRS of females probably correlates closely with longevity.
British example: probably Ischnura pumilio.154
2. Postponed oviposition. The rendezvous is the oviposition site, where the female typically oviposits, almost always unguarded, on a subsequent visit to water. Males typically patrol and defend a substantial area, but exhibit only slight site attachment. The type of insemination (by free sperm or by spermatodesm) may reflect the habitat.
British examples: Aeshnidae, Cordulegastridae, Corduliidae and Gomphidae.
3. Hinterland rendezvous. The rendezvous is not the oviposition site, but is functionally linked to it because the male typically escorts the female to the oviposition site, usually in tandem, either soon after copulation or beforehand, in which case copulation is postponed until the pair reaches the oviposition site. The primary rendezvous is usually away from water, being either a site where receptive females are likely to interrupt their journey to the oviposition site or the nocturnal roost. Sites used for basking, foraging and roosting all constitute a form of rendezvous frequented by both sexes for other than reproductive purposes. Most females arrive at water already in the wheel position or in tandem.
British examples: Ischnura elegans12 and Lestes sponsa.143
4. Nonterritorial; oviposition site rendezvous. The primary rendezvous is typically the oviposition site. Contact guarding occurs during oviposition. The males are not territorial but control the females’ access to the oviposition site. Oviposition is sometimes under water.
British examples: many non-territorial Zygoptera, including Coenagrion puella and Enallagma cyathigerum.
5. Long copulation. Territoriality is well developed. Males defend many, but not all, oviposition sites. Copulation typically lasts for more than 20 seconds. Post-copulatory guarding is without contact.
British examples: Calopteryx splendens and C. virgo.
6. Short copulation. Territoriality is well developed. Copulation typically lasts for less than 20 seconds. Sperm displacement is by ‘packing’, probably reflecting the very brief copulation.
British examples: species of Libellula, Orthetrum and Sympetrum.
male-to-male variation in LRS, depending on whether a male holds a territory or not, and on how much time he allocates between contact and non-contact guarding. In System 6 the male has greatest control over the female: she can postpone oviposition but eventually must return and oviposit in a territory which may well be still occupied by her most recent copulation partner. Despite the options available to the female for exercising partner choice, evolution of mating systems in dragonflies is seen as being primarily a male-driven process.147
OPPORTUNITIES FOR INVESTIGATION
The discovery that searching male Anisoptera are attracted to an electric fan (p.244) offers the prospect of analysing their responses to visual cues with simple and inexpensive equipment. Because of the great advance in our understanding of reproductive behaviour, especially since the discovery of sperm displacement, many questions now await attention. Some of these require elaborate equipment and techniques in the laboratory, but others can be tackled by skilled field observers without such aids. There is great scope for collaboration between field observers and researchers with access to laboratory facilities and specialised equipment, such as scanning electron microscopy and the means for obtaining DNA profiles. In the past, when such collaboration has been achieved, the outcome has been very informative.155 Light can be thrown on our perception of mating systems by recording the day-to-day behaviour of marked individuals, especially in regard to their mating frequencies in different places (with respect to the primary rendezvous and the identity of their copulation partners). Such information can help to elucidate the extent of female choice in determining the parentage of offspring. The availability of techniques for marking individuals and of short-focus binoculars has transformed the practicability of constructing dossiers of the day-to-day behaviour of individuals. As always, success in such ventures depends largely on the discovery of a stem habitat that facilitates field observation.
Close observation of copulating pairs may reveal the nature of signals from one sex to the other to announce the intention to separate.139 Here, access to a digital camera equipped with an automatic focus device (Appendix 2) could be especially valuable.