States of Matter Practice Questions
-
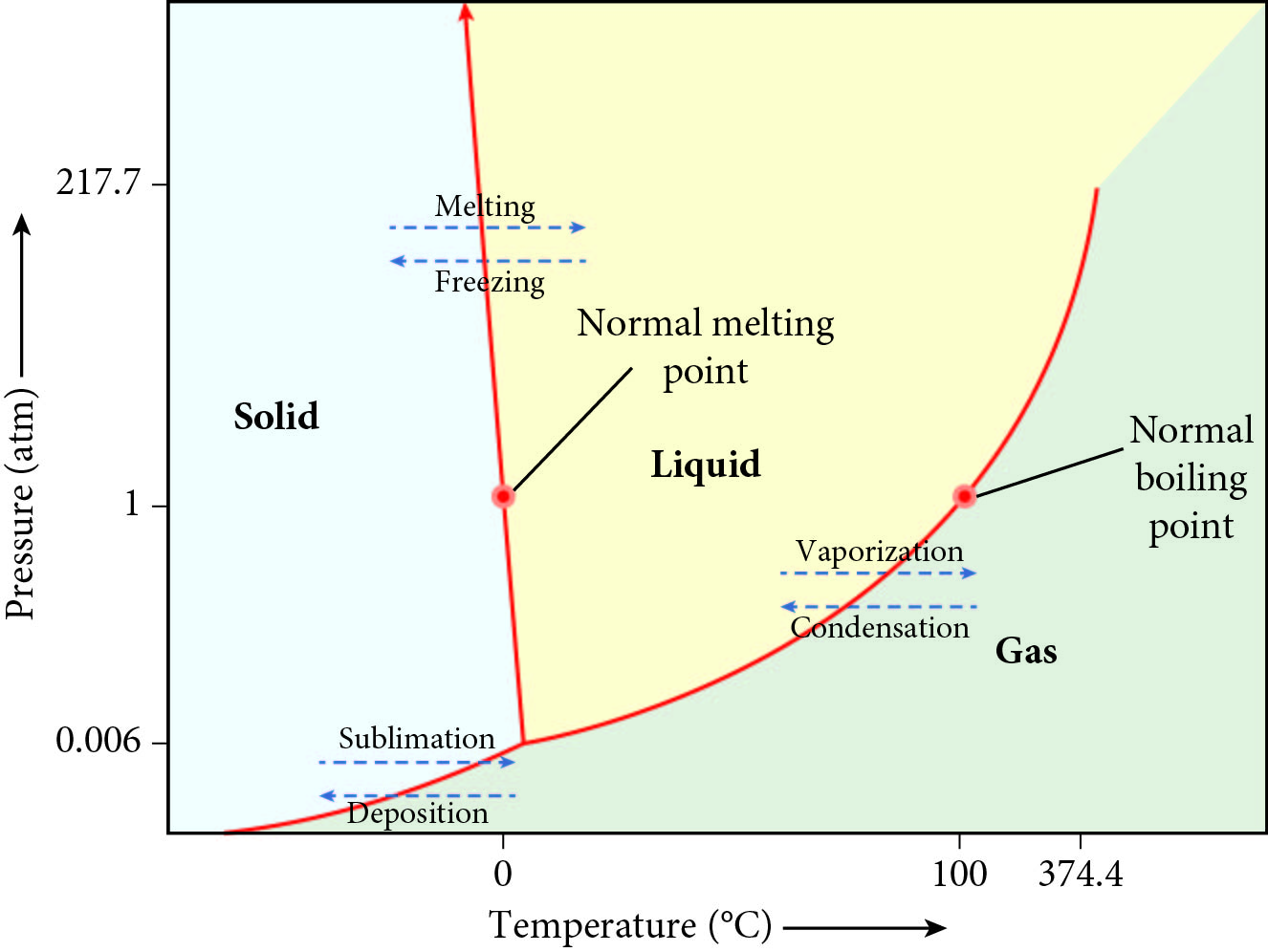
The above graph is a phase diagram for water. According to the graph, what environmental conditions allow for deposition?
- High pressure and increasing temperature
- High pressure and decreasing temperature
- Low pressure and increasing temperature
- Low pressure and decreasing temperature
-
Hydrogen has a boiling point of –253ºC. Its latent heat is 108 cal/g. What will be the heat energy change in a 10 g sample of hydrogen at –253ºC as it transitions from liquid to a gas?
- –27,320 calories
- –1080 calories
- 1080 calories
- 27,320 calories
-
At what point can a substance exist as a solid, liquid, and gas simultaneously?
- Boiling point
- Freezing point
- Triple point
- Critical point