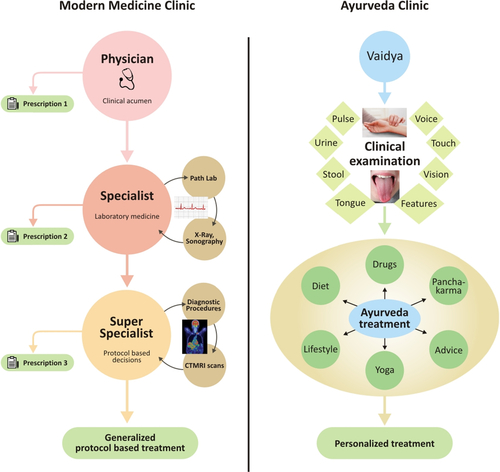
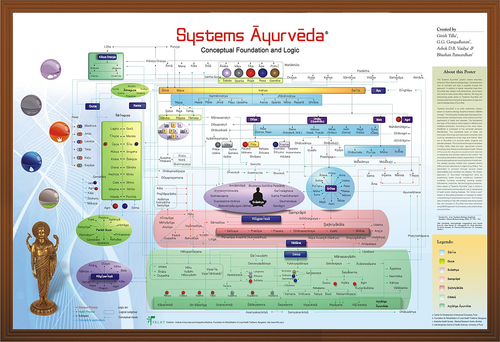
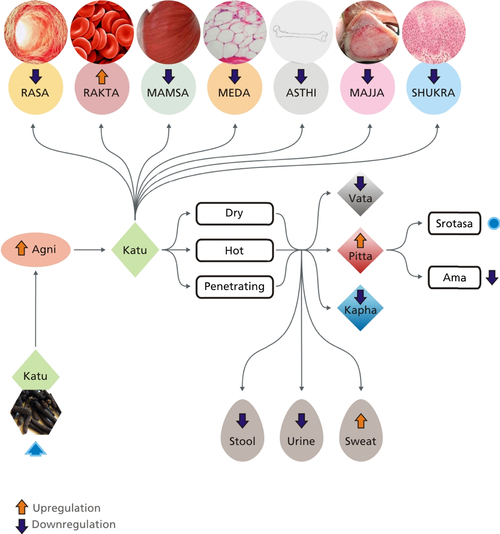
[1] Saks V, Monge C, Guzun R. Philosophical basis and some historical aspects of systems biology: from Hegel to noble – applications for bioenergetic research. Int J Mol Sci. 2009;10(3):1161–1192.
[2] Sweeney K, Kernick D. Clinical evaluation: constructing a new model for post-normal medicine. J Eval Clin Pract. 2002;8(2):131–138.
[3]
http://plato.stanford.edu/entries/reduction-biology.
[4] Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40(6):463–471.
[5]
Lobo I. http://www.nature.com/scitable/topicpage/biological-complexity-and-integrative-levels-of-organization-468.
[6]
http://ghr.nlm.nih.gov/handbook/basics/gene.
[7] Pertea M, Salzberg S.L. Between a chicken and a grape: estimating the number of human genes. Genome Biol. 2010;11(5):206. doi: 10.1186/gb-2010-11-5-206.
[8]
http://arxiv.org/abs/1312.7111.
[9]
http://www.extremetech.com/extreme/134672-harvard-cracks-dna-storage-crams-700-terabytes-of-data-into-a-single-gram.
[10]
http://www.ncbi.nlm.nih.gov/gene/statistics.
[11] Berg E.L. Systems biology in drug discovery and development. Drug Discov Today. 2014;19(2):113–1125.
[12] Gehlenborg N, O’Donoghue S.I, Baliga N.S, Goesmann A, Hibbs M.A, Kitano H, et al. Visualization of omics data for systems biology. Nat Methods. 2010;7(3 Suppl):S56–S68.
[13] Rosen R.A. Means toward a new holism. Science. 1968;161(3836):34–35.
[14] Smuts J.C. Holism and evolution. N & S Press; 1926.
[15] Kia B, Murali K, Motlagh M.R.J, Sinha S, Ditto W.L. Synthetic computation: chaos computing, logical stochastic resonance, and adaptive computing. In: International conference on theory and application in nonlinear dynamics. Springer International Publishing; 2014.
[16] Chang T.M. Semipermeable microencapsulation. Science. 1964;146(3643):524–525.
[17] Soon-Shiong P, Heintz R.E, Merideth N, Yao Q.X, Yao Z, Zheng T, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343(8903):950–951.
[18] Omenn G.S. Grand challenges and great opportunities in science, technology, and public policy. Science. 2006;314(5806):1696–1704.
[19] Gibson D.G, Glass J.I, Lartigue C, Noskov V.N, Chuang R.-Y, Algire M.A, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329(5987):52–56.
[20] Budin I, Devaraj N.K. Membrane assembly driven by a biomimetic coupling reaction. J Am Chem Soc. 2012;134(2):751–753.
[21] Annaluru N, Muller H, Mitchell L.A, Ramalingam S, Stracquadanio G, Richardson S.M, et al. Total synthesis of a functional designer eukaryotic chromosome. Science (80-) [Internet]. 2014;344(6179):55–58.
[22] Douglas T, Powell R, Savulescu J. Is the creation of artificial life morally significant? Stud Hist Philos Sci. 2013;44:688–696.
[23] Malyshev D.A, Dhami K, Lavergne T, Chen T, Dai N, Foster J.M, et al. A semi-synthetic organism with an expanded genetic alphabet. Nature. 2014;509(7500):385–388.
[24] Yates F.E. Homeokinetics/homeodynamics: a physical heuristic for life and complexity. Ecol Psychol. 2008;20:148–179.
[25] Lloyd D, Aon M.A, Cortassa S. Why homeodynamics, not homeostasis? Scientific World Journal. 2001;1:133–145.
[26] Zaloga G.P, Sager A, Black K.W, Prielipp R. Low dose calcium administration increases mortality during septic peritonitis in rats. Circ Shock. 1992;37(3):226–229.
[27] Oliveira-Filho J, Silva S.C.S, Trabuco C.C, Pedreira B.B, Sousa E.U, Bacellar A. Detrimental effect of blood pressure reduction in the first 24 hours of acute stroke onset. Neurology. 2003;61(8):1047–1051.
[28] Scheer F.A, Czeisler C.A. Melatonin, sleep, and circadian rhythms. Sleep Med Rev. 2005;9(1):5–9.
[29] Wu G.Q, Arzeno N.M, Shen L.L, Tang D.K, Zheng D.A, Zhao N.Q, et al. Chaotic signatures of heart rate variability and its power spectrum in health, aging and heart failure. PLoS One. 2009;4(2):e4323. doi: 10.1371/journal.pone.0004323.
[30] Ahn A.C, Tewari M, Poon C.S, Phillips R.S. The clinical applications of a systems approach. PLoS Med. 2006;3(7):e209.
[31] Watve M. Diabetes in a textbook. In: Doves, diplomats, and diabetes. New York: Springer; 2013.
[32] Jain P, Vig S, Datta M, Jindel D, Mathur A.K, Mathur S.K, et al. Systems biology approach reveals genome to phenome correlation in type 2 diabetes. PLoS One. 2013;8(1):e53522.
[33] Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169.
[34] Salvi S. Story of diabetes: how the world got misled into the wrong understanding of this deadly disease (Hypothesis presentation at Interdisciplinary School of Health Sciences, Savitribai Phule University of Pune). August 12, 2014.
[35] Watve M.G, Yajnik C.S. Evolutionary origins of insulin resistance: a behavioral switch hypothesis. BMC Evol Biol. 2007;7:61.
[36] Schaller S, Willmann S, Lippert J, Schaupp L, Pieber T.R, Schuppert A, et al. A generic integrated physiologically based whole-body model of the glucose-insulin-glucagon regulatory system. CPT Pharmacometrics Syst Pharmacol. 2013;2:e65.
[37] O’Rahilly S, Turner R.C, Matthews D.R. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318(19):1225–1230.
[38] Stahl M, Brandslund I. Measurement of glucose content in plasma from capillary blood in diagnosis of diabetes mellitus. Scand J Clin Lab Invest. 2003;63(6):431–440.
[39] Ahn A.C, Tewari M, Poon C.S, Phillips R.S. The limits of reductionism in medicine: could systems biology offer an alternative? PLoS Med. 2006;3(6):709–0713.
[40] Lusis A.J, Attie A.D, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9(11):819–830.
[41] Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins C.S, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329.
[42] Hood L, Price N. Demystifying disease, democratizing health care. Sci Transl Med. 2014;6(225):225ed5.
[43] Clermont G, Auffray C, Moreau Y, Rocke D.M, Dalevi D, Dubhashi D, et al. Bridging the gap between systems biology and medicine. Genome Med. 2009;1(9):88. doi: 10.1186/gm88.
[44] Meng Q, Mäkinen V.-P, Luk H, Yang X. Systems biology approaches and applications in obesity, diabetes, and cardiovascular diseases. Curr Cardiovasc Risk Rep. 2013;7(1):73–83.
[45] Le Novère N, Hucka M, Mi H, Moodie S, Schreiber F, Sorokin A, et al. The systems biology graphical notation. Nat Biotechnol. 2009;27(8):735–741.
[46] Bell I.R, Koithan M, Pincus D. Methodological implications of nonlinear dynamical systems models for whole systems of complementary and alternative medicine. Forsch Komplementärmedizin. 2012;19(1):15–21.