This chapter was written by James Rubenstein and reviewed and updated by Dr. Adam Hartman, who is studying the ketogenic diet in animal models.
We still do not really know yet how the ketogenic diet works, despite the volume of work that has been done since the last edition of our book was published 4 years ago. Being able to answer this crucial question involves a better understanding of three factors that occur when an individual has a seizure: onset of the event; the spreading of the seizure through the brain, which determines the type of seizure that will occur; and how cessation of the seizure occurs. Starting a patient on the ketogenic diet essentially tricks the body into thinking it is maintaining a fasting state. The body is denied most carbohydrates, is given sufficient protein, and depending upon the dietary ratio chosen by the dietitian for the individual patient, is given large amounts of fat. The body quickly depletes it’s supply of easily accessed carbohydrate as it turns to fat as its alternative energy source. Fasting accelerates the process, and as fats are burned without the presence of carbohydrates, ketone bodies accumulate and can be identified and measured in the blood, urine, and cerebral spinal fluid. The simplest way to identify if an individual has a large ketone supply on board is simply to smell the fruity aroma on the individual’s breath. It is very dramatic.
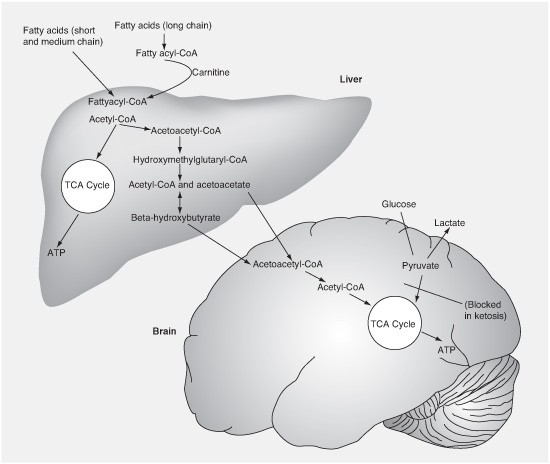
FIGURE 4.1
Breakdown of fatty acids into ketones by the liver and update by the brain for energy.
Once the ketogenic diet is established, there are ketone bodies available to cross the blood–brain barrier and enter the brain. Once they are in there, we don’t know exactly what happens, but it’s clear that there can be a significant effect on one of the three factors mentioned previously (onset, propagation, and/or cessation of the seizures).
There are three types of ketone bodies, which are water-soluble compounds produced as the byproducts of burning fat for energy instead of carbohydrate. They are called acetone, acetoacetate, and beta-hydroxybutyrate. We still do not know if any of them, or which ones, are somehow responsible for improving seizure control. They may just be indicators of the presence of some other factors or metabolic changes that we have not yet identified that are the key.
It also has been proposed that somehow the entire mystery is related to the way the body produces or uses insulin as it moves from burning primarily carbohydrate to burning primarily fat.
Because the ketone bodies are acids and cause acidosis (an increase in the amount of acid in the body), it has been proposed that the ketogenic diet works by creating an ongoing acidotic state that the body somehow compensates for, while it is also affecting one of the three factors. This was one of the first theories, but it has been largely disproven.
We know that once the body realizes that a certain level of ketones are circulating in the blood, they “spill” into the urine and can be measured there. However, that does not always seem to correlate with the actual amount of ketone bodies in the bloodstream. Levels of the ketone bodies in the blood and urine do not have a strong correlation with their levels in the brains of rodents (the brian, of course, is where the seizures are taking place) but as of now, we do not have a way to reliably measure these changes in people. Studies that have attempted to link blood (or urine) ketones with seizure control have not shown a consistent link. Many researchers believe that ketosis is a marker that indicates that your body has made the metabolic shift to be on the ketogenic diet, but they do not necessarily believe that ketosis is exactly why the diet works.
Researchers have continued to expand their work with different types of animal models. They can now evaluate the efficacy of a drug (medication) or a metabolic treatment (such as the ketogenic diet, the Modified Atkins Diet, or the Low Glycemic Index Treatment) in altering the frequency of seizures.
One of the challenges in studying how the ketogenic diet works in animals is that, surprisingly, the diet does not work well in most standard animal seizure tests. It does work in two of them in mice (the 6 Hz test, which models partial-onset seizures, and the fluorothyl test, which models generalized seizures) and one in rats (pentylenetetrazol, which identifies medicines that work in absence and myoclonic seizures). It also works in some chemical and electrical kindling models, which induce both generalized and partial seizure types.
We know that there are a number of channels (proteins that allow transport of molecules across the cell membrane) in the brain that are affected by seizures, as well as the drugs and diets used to treat them. Examples you are probably familiar with include the Ca++ (calcium), K+ (potassium), and GABA (gaba-amino-butyric acid) channels. Somehow, these channels and others may be the actual regulators of brain electrical activity, and understanding how they affect the three factors may ultimately determine how the ketogenic diet and other dietary therapies work.
Other, newer animal models to study the three factors, and hence how the ketogenic diet works, are being created by hyperthermia (drastically altering temperature), brain trauma, hypoxia (lack of oxygen), vascular occlusion (similar to a stroke), radiation exposure, chemical exposure causing brain toxicity, and genetic alterations where genes are removed or replaced, called genetically modified, transgenic, knock-out, or knock-in mice.
The animal can be studied clinically, but there are also methods for studying how brain slices and cultures of brain tissue in the variously altered models react to different treatment modalities. By pursuing a large variety of possible models, it can be expected that one or more of them will yield different details to explain the three factors and then to also explain the way both dietary and pharmacologic treatments work.
In the near future, you are likely to read more about
• the effect of chronic ketosis on brain energy reserves and mitochondria, the cell’s powerhouse;
• how ketosis alters the utilization of glutamic acid (an excitatory amino acid that promotes seizures) and GABA (an inhibitory amino acid that stops seizures);
• how the ketogenic diet alters various neurotransmitter levels, which may play a role in explaining the efficacy of the ketogenic diet not only in treating seizures but also in treating a variety of other neurological and behavioral disorders;
• the role of the diet in the levels of PUFAs (polyunsaturated fatty acids), which are known to modulate neuronal hyperexcitability;
• the role of adenosine and other neuromodulators in the mechanisms of action of the diet;
• whether fasting is the same as (or likely different than) the diet;
• the relative role of glucose stabilization versus high fats (with studies examining a drug currently called 2-DG that helps stabilize glucose and may help reduce seizures); and
• the importance of cellular metabolism-sensing pathways (in either neurons or their supporting cells) in altering sensitivity to seizures.
Unfortunately, our inability to explain exactly how the diet works has led many pediatric neurologists to remain skeptical, and some still remain quite negative about its potential usefulness. There have been studies that suggest that the ketogenic diet ranks about 14th on many physicians’ lists of effective seizure treatments, despite the known fact that if a child has failed two medications used appropriately, the ketogenic diet has a greater likelihood of achieving more than 50% seizure reduction than the next medication introduced. We tell these child neurologists that we do not know how many medications work either, initially, including Keppra® (levetiracetam), which is now one of the most popular drugs! The diet, just like many medications, probably has multiple mechanisms of action. Finally, basic scientists are working together with clinical researchers to find ways to make the diet more effective in humans.