SIGNALING ACROSS THE SKIN LANDSCAPE
FROM A CELL’S point of view, the surface of the skin is a vast, hard, barren landscape spotted with a lattice of proteins and fatty molecules. As the organ most exposed to the external environment, the skin must defend against physical assaults, particularly from toxins and insect bites.
Just beneath the skin’s surface, there are deep follicles that regulate hair growth and harbor glands that secrete various molecules, such as salts, enzymes, and fats, as well as peptide molecules to fight microbes. Peptides are short chains of amino acids (longer chains of amino acids form into proteins). The surface of the skin is acidic, with high salt levels and lots of oxygen. Within the follicles, there is little oxygen but a lot of fat. On such an arid surface (compared to the gut and other internal organs), signals are even more important than in other organs to maintain order and avoid infections.
The landscape across the skin varies considerably—fingers, hair, armpits, face, palms. Like the gut, each local environment has particular cellular conversations to determine which microbes are friendly and which are dangerous. Varied amounts of follicles and glands create niches for specific bacteria. Yet wet, dry, and oily settings on the skin are unlike microbe-laden surfaces in the gut. Healthy skin is less than an ideal place for microbes, providing little nutrition and exposing them to ultraviolet light, which can kill or inactivate them. The skin seems so stable that it is difficult to grasp how dynamic it truly is.
SKIN STRUCTURE
The two main layers of skin are the epidermis, the outermost layer, and the dermis, which is beneath. The dermis is made up of connective tissue, blood and lymph vessels, sweat and oil glands, and hair follicles. There is also a third deeper layer of skin, called the hypodermis, which is made up of connective tissue and fat cells.
The vast majority of the top layer is made up of master lining cells called keratinocytes. These specialized lining cells, analogous to master lining cells in the gut, migrate from deeper layers to the surface while talking with immune cells, neurons, muscles, connective cells, and varied microbes, including bacteria, fungi, and viruses. Keratinocytes produce keratin, the fibrous protein that forms the structural basis of hair, nails, fur, and feathers and also protects the surface of the skin from damage or stress.
The epidermis also contains pigment cells, resident and traveling immune cells, and supportive cells that surround sensory neurons. At the bottom, capillaries provide oxygen for the cells not near the surface. These blood vessels also provide a channel for signals with other regions and a route for traveling blood cells to enter the topmost skin regions.
Cooperation among all the different kinds of cells maintains normal skin and repairs damaged skin from scrapes, cuts, ultraviolet light, and oxidative reactions. Cellular activity uses considerable energy to secrete complex lipid molecules, maintain tight junctions between cells, and avoid water loss by building a lipid protein coat. (There is much more about lipids in chapter twenty-five on membrane production.)
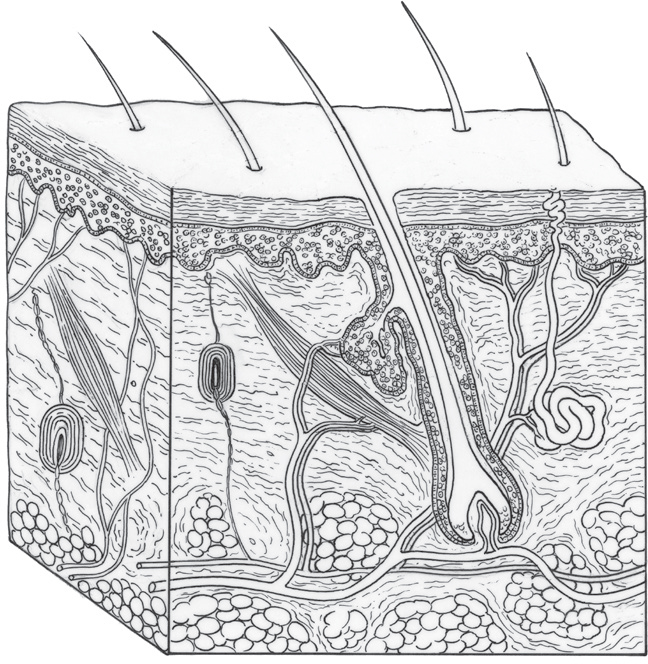
Anatomical illustration of a section through human skin, showing the epidermis, sweat gland, hair follicle, blood vessels, fat cells, and sebaceous gland. (Science Source/Science Source/Science Source)
The superficial epidermis has multiple layers, with a surface that is hard but flexible. It regulates water release and is the major barrier against external toxins, microbes, and infections. The physical barrier includes tight junctions between cells and multiple large scaffolding proteins. A chemical barrier consists of enzymes to break down toxins, fats, acids, and peptide molecules. Toxic particles secreted against multiple intruders must be eliminated after battle.
The thickness of the epidermis is quite variable—three times thicker on the palms and soles of the feet than on the eyelids. In the epidermis, oxygen diffuses from the air to feed the topmost cells. The dermis structure below consists largely of the proteins collagen, for strength, and elastin, for flexibility. Diverse types of extracellular matrix in the dermis serve as signals to immune cells to stimulate varied activities.
The subterranean dermis houses a wide range of immune cells and a large number of connective cells that secrete matrix between cells. There is also the basement membrane that separates the dermis and epidermis. Very recently, a new layer of fluid channels, called the interstitium, has been found below the skin’s surface, as well as in connective tissue throughout the body. It was not possible to observe this layer until now because previous research techniques for observing tissue always eliminated water. It remains to be seen in future research whether these channels are conduits for signals throughout the body.
The very top hard layer of skin is made of more than twenty layers of specialized keratinocytes that have shed their nuclei and are tightly joined together with sticky molecules. These cells produce fatty material for a strong, waterproof barrier that is resistant to infection and trauma. In this outermost layer, there are also large numbers of traveling T cells with diverse skills that converse with keratinocytes.
DYNAMIC CONVERSATIONS
Only recently has it become clear that skin cell conversations are as dynamic as those of gut cells and capillaries. The skin is the largest organ in the body and has the second-largest number of microbes to deal with after the gut. Skin has the most varied environments and is the most exposed. Individual keratinocytes must organize resources to defend against physical assault and deal with a wide range of microbes of all kinds, including fungi and viruses. Like the gut, skin serves as a barrier that is vital for the entire body. Increasingly, as in the gut, immune activity on the skin has been found to have ramifications in other organs throughout the body.
While gut cells talk with microbes about digestion, skin has other issues. Conversations maintain its strong surface to protect against microbial invasions and injuries. Keratinocytes must engage in various healing projects as well. Even on the barren skin, an army of immune cells can be called to rapidly respond. Keratinocytes must determine which bacteria are allowed to stay on the surface in each location along with particular immune cells. As in the gut, multiple conversations must work to inhibit immune attacks on vital friendly microbes and the skin tissue itself. Much of the communication is with friendly microbes that help protect against other, dangerous invaders.
Conversations among lining and connective cells regulate the various matrixes between cells that provide specific functions. These conversations determine the matrix for the topmost barrier where keratinocytes are fixed. Fat cells converse about matrix that provides some cushion below the surface of the skin. Surprisingly, fat cells also send signals to stop particular bacterial infections and can signal to increase the amount of brown fat, which regulates temperature, among other functions.
Via signals, connective cells produce matrix for different situations using various amounts of molecular fibers, ground substance, and extracellular fluid. Ground substance is a thick liquid, composed of large molecules, that uses variable amounts of amino acids, peptides, proteins, and sugars for different situations. In some situations, ground substance can be so thick that microbes find it hard to navigate through it.
MORE ABOUT KERATINOCYTES
Keratinocytes are born from stem cells deep within the hair follicle, or in between follicles. Like gut lining cells that gradually move up from deep crypts to the top of villi, keratinocytes move through various layers of skin cells to the surface. During this migration, they also undergo gradual maturation with a series of modifications that enable them to build more signals and receptors for complex decision making. Upon arriving at the top, some of the lining cells shed their nuclei to form the tight barrier. Signals from mature keratinocytes direct all activity in all layers and particularly protect nerves, immune cells, friendly microbes, and T cells traveling at the outermost layers of the skin.
Keratinocyte decision making includes responding to harsh conditions such as heat, cold, moisture, toxins, ultraviolet light, bruises, and cuts. Neighboring connective cells stay in close contact with these lining cells via signals. Traveling immune cells live in the skin and communicate constantly with keratinocytes about microbes, infections, and trauma. Neurons provide sensation related to touch and pain and also engage in signaling to the master lining cells about changing conditions.
Talking with Immune Cells and Microbes
Because the skin landscape is relatively desolate, keratinocytes do not build large lymph tissue, as in the gut and other organs. Instead, because of its flat nature, skin must rely on an abundance of individual traveling immune cells on the surface. To organize a large army of individual mobile cells spread over the skin, even more signals are essential than when dealing with the fixed large lymph centers of other tissues.
Keratinocyte signals, in conjunction with neurons and microbes, call for immune cells that produce low levels of chronic inflammation. This serves as protection against more severe infections by keeping active immune cells readily available. Specific immune cells work to repair the minimal damage produced by this low-level inflammation. With stress or injury, more powerful signals are sent that alter the inflammation to cope with greater problems. Keratinocyte signals can also do the opposite and stop recruitment of all traveling immune cells.
The skin also requires many more subtypes of supportive immune cells than anywhere else. Unlike in other organs, multiple subtypes of the immune cells that present material to T cells work in tandem. Some talk to each other, and others signal at different times in sequence while the suspicious particle is evaluated. When producing a particular type of protective inflammation against a fungus, for example, actions from three distinct presentation cells and multiple types of T cells take place for one process.
Microbial conversations among keratinocytes, connective cells, and immune cells have various effects. Some microbes live peacefully on the skin and only become dangerous when there are breaks in the skin, such as with insect bites and traumatic injuries. Microbial signals can gather all the cells to fight particular enemy species. With immune deficiencies, conversations among immune cells and microbes are altered, producing dangerous infections.
Microbial signals have diverse effects. In one situation, a fungus stimulates a neuron that causes pain and itching. Alternatively, fungal signals to neurons can cause painless ulcers. They can signal lining cells to produce various types of inflammation. Microbial signals to T cells can increase or decrease inflammation activity. These signals can directly trigger more T cells that chase specific enemy microbial species. Signals can also inhibit T cells that would otherwise turn against human cells and cause autoimmune disease.
Unlike in the gut, where food attracts particular microbes, there are not a lot of food-attracting microbes on the skin. Still, there are at least a million microbes every square centimeter. Various types of microbes live near glands, neurons, or immune cells. Multiple factors determine which microbes survive—skin pigment, cleaning products, temperature, moisture, and acidity.
The most complex skin region for microbes is deep in hair follicles, somewhat analogous to the crypts in the gut. The follicle is the point of entry for immune cells from the blood. The follicles that dot the skin have the most diverse immune cells and microbes. As in gut crypts, stem cells often live in deep follicles. Multiple layers of keratinocytes protect the follicle and immune cells nearby.
Directors of Communication
Among wide-ranging conversations about health and disease, keratinocytes direct all of the action. Keratinocyte signals to connective cells determine particular matrixes. Keratinocytes monitor conversations among microbes and immune cells. They trigger inflammation and instruct T cell responses. Keratinocytes must constantly temper T cell responses to avoid reactions against friendly microbes and human cells. Even when immune cells support friendly microbes and repel enemies, keratinocyte signals are the controlling factors. In fact, most immune signals to microbes are relayed through keratinocytes.
Recent research is discovering a wide variety of immune cells stimulated by keratinocyte conversations. These immune cells are produced in bone marrow, lymph tissue, or at a particular site on the skin. Like immune memory cells, keratinocytes maintain their own history of events for future reference. They remember the range of immune cells that are available for them to call for help, and even the location of the problem.
Keratinocytes also don’t just sit still when a cancer invasion starts; they behave like local police to repair damage that aberrant cells are creating. When keratinocytes come across abnormal mutated cells that could be cancerous, these master skin lining cells take dynamic action by themselves against these cells. Signals suppress the types of inflammation that can lead to cancer. Keratinocytes have now been observed surrounding invading cancer cells, shepherding them away, and repairing the damage. Because of the extensive use of signals among skin cells, the new science of signaling might have the most rapid impact on skin diseases.
MEMORY T CELLS
Twice the number of T lymphocytes live on the skin as in lymph tissue for other organs. These T cells include multiple varieties, including the largest number of memory cells of any organ in the body and diverse types of active T cells. Active T cells are the first and best defense against microbes. Particular T cells are also called from the thymus to the skin to monitor for cancer and other skin diseases. With a cut, T cells and keratinocytes produce an intense array of signals to avoid immediate severe infections.
After trauma, inflammation, or infection, T cells produce special memory cells that continue to monitor troubled sites. Memories are maintained of all exposures to dangerous microbes, toxins, trauma, and cancer. The same conditions could rapidly flare up at any moment, and these cells are ready. Memory cells retain knowledge of the exact locations on the skin where an infection occurred, the particular microbes involved, and the signals that eliminated them. Skin conditions are constantly changing, and memory cells keep up conversations with microbes that are nearby to determine whether action is needed.
Memory cells have spatial memory that allows them to understand the unique topography and landscape where a battle occurred. For example, fungi are particularly difficult to monitor. Fungus can exist as buds on the surface or as needles deep into tissues. Dangerous subterranean hyphae (the long tubular branching structures of a fungus) track into underlying skin layers and must be followed and attacked by memory cells.
Perhaps the most unusual feature of skin memory T cells is that they don’t need presentation cells that other T cells require to become killer cells. Skin memory cells can rapidly change their mode when necessary by themselves. They can produce molecules to attack microbes directly and can send immune signals to get help. They can rapidly become an army of killer cells. They have the ability to change the entire local environment.
SKIN IMMUNITY AND DISEASE
The field of immunology has recently evolved based on the discovery of these wide-ranging conversations among varied cells. Previously, most research delved into the complexities of bone marrow, lymph node communities, and the thymus. While it is difficult for research to follow small signal molecules on the skin that travel among many cells, the modern focus has changed to study conversations among multiple types of cells, including immune cells, microbes, neurons, connective cells, capillaries, and lining cells. On the skin, almost all of the action is based on these signals, especially back-and-forth communication between microbes and keratinocytes.
To fight various skin diseases, such as psoriasis or dermatitis, keratinocytes invite new types of immune cells among hundreds of possible subtypes of T cells and other white blood cells. Only mature keratinocytes have learned to produce large amounts of unique signals specific to the skin’s needs. Without these signals, the skin would be overrun with allergic responses to its wide-ranging exposure to microbes.
When keratinocytes die, molecular signals are released that activate particular immune cells, which cause inflammation. Sometimes keratinocytes kill themselves by programmed suicide in order to send signals for inflammation. Planned suicides produce vital signals for emergencies in both the gut and skin. In response, viruses and microbes produce molecules to interfere with planned cell suicide, so signals will not trigger inflammation and harm microbial colonies.
Potentially dangerous microbes most often exist on the skin without producing any problems. They can even be helpful while living peacefully. But then they can suddenly change, which often occurs from communication among multiple other species. Peaceful colonies can suddenly change to produce infections in hair follicles, cellulitis, or even severe blood infections that can go to the bone and heart. Bacteria can rapidly become dangerous “flesh-eating” varieties in a wound, mostly in those with impaired immunity from other diseases. Bacteria can also be part of chronic infections and even contribute to autoimmune disease.
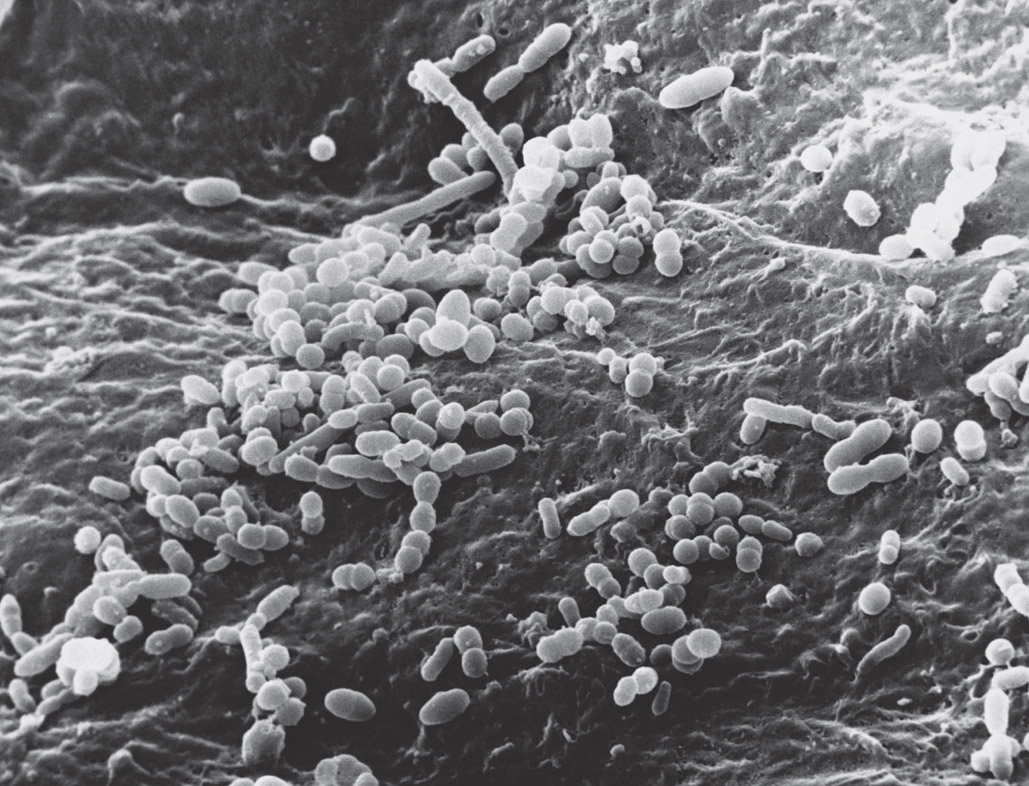
Bacteria on the surface of the skin. Electron micrograph. (David M. Phillips/Science Source)
Recently, signals were identified that incite certain bacteria—often Streptococcus pyogenes, which causes sore throat and often lives peacefully on the skin—to become the dangerous flesh-eating type. A specific toxin was found that triggered a local neuron in two ways based on natural conversations among immune cells and neurons. The first signal caused severe pain out of proportion to any signs of disease.
The second exploited the normal neuron signal for wounds with pain but without infection that inhibits immune activity. Instead of using the neuron signal for infection that calls for immune cells, the neuron was manipulated into using signals that inhibit as if there were no infection. This signal stopped neutrophils both from traveling to the site and from sending attack molecules. The result was that the bacteria had no resistance and therefore became flesh-eating microbes.