THE SUPPORTIVE ROLE OF ASTROCYTES
ASTROCYTES ARE THE MOST abundant of three supportive brain cells, collectively called glia, from the Greek word for “glue.” All glia tend to be smaller than neurons but make up more than half of the volume of the brain and spinal cord. It is not known exactly how many glia cells there are, but the ratio compared with neurons is different in each region—more glia than neurons in the cortex and fewer than neurons in the cerebellum.
Astrocytes were named as such because their multiple protrusions give them a starlike appearance. They are essential for all brain function. Their arms extend to cover neuronal synapses, while their end-feet enwrap blood vessels, enabling them to easily converse with neurons and multiple other cells using signals in the blood. These connected networks of astrocytes form a tilelike scaffolding, which covers and protects all neurons and blood vessels throughout the entire brain.
To understand mental processes, brain mapping researchers generally describe circuits among neurons. A fuller understanding of these neuronal processes must also take into account all of the circuits and hubs of the astrocyte network. For instance, although fMRI (functional magnetic resonance imaging) is widely assumed to measure activity of neurons in brain research, in fact it measures the blood flow controlled by astrocytes in a brain region, with each dot of light in the image representing blood flow for tens of thousands of neurons.
It is not entirely clear how closely the fMRI brain image derived from blood flow correlates with neuronal activity. New research suggests that blood flow might increase right before neuronal activity takes place or immediately after, but not actually during the height of neuronal activity.
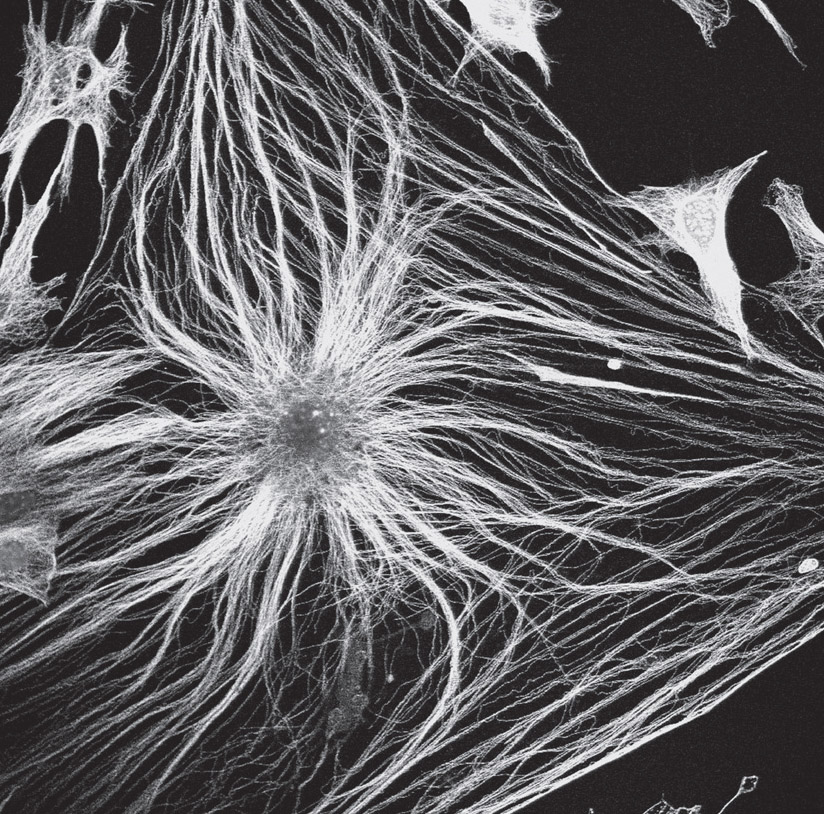
An astrocyte. Confocal light micrograph. (David Robertson, ICR/Science Source)
THE WIDE-RANGING ROLE OF ASTROCYTES IN NEURONAL FUNCTION
It is hard to exaggerate the importance of astrocytes in their support of neurons. Their signals are critical for the entire life cycle of synapses—formation, normal function, pruning of underutilized synapses, and alterations based on learning. In determining the energy needs of hungry neurons, astrocytes tell the blood vessels to open up to supply oxygen and other nutrients to these particular neurons and then signal them to close once neurons have had their fill.
Astrocyte scaffolds support ever-changing neurons that bud and shed connections each day. Each neuron can have as many as a hundred thousand synapses with other neurons, and each astrocyte protects synapses for a large number of neurons. Astrocytes control every aspect of the synapse environment, including the surrounding extracellular matrix and local chemical gradients. Astrocytes secrete growth factors for neurons to keep them healthy and digest neuron debris produced at the synapse. When neurons or axons migrate to new locations, astrocytes clear a pathway that consists of tunnels in the astrocyte scaffold.
New neurons cannot create synapses by themselves and must first have physical contact with astrocytes to “learn” how to make a synapse; this plays an important role in fetal brain development. Sticky molecules from astrocytes and neurons are then able to build the synapse and hold it together. Astrocyte signals in the fetus are also part of the process of building tight junctions for the blood-brain barrier. In addition, a large type of astrocyte in the fetus spans the entire fetal cortex. It produces stem cells for all brain cells and forms scaffolds for cells to migrate into their positions.
Signals from astrocytes, along with multiple other cells, stimulate stem cells to produce new neurons. Astrocytes maintain electric balance along the neuron’s axon, picking up potassium, which is released through channels in the membrane as part of the electrical signal along the axon. Astrocytes pick up used neurotransmitters at the synapse and recycle them.
These glia cells also send and receive large numbers of neurotransmitters, immune cytokine signals, and other factors. Astrocyte signals are different in each brain region and for each individual astrocyte. In addition, signals can be different in each of the thousands of tiny protrusions on a single astrocyte.
Types and Shapes of Astrocytes
More than a thousand types of neurons, each with a distinctive shape, have been discovered. Now, research is showing that astrocytes may be as varied, with numerous distinct types recently discovered in each brain region, with some differences too fine to be seen without the most advanced technology. A large number of “fingers,” which can be large, intermediate size, or very small, make up 90 percent of the surface area of an astrocyte.
One astrocyte type has a dozen large arms and thousands of small protrusions near synapses—each protrusion with various shapes. In memory centers, one astrocyte can connect with thousands of synapses using different-sized protrusions for each cluster of neurons.
Astrocyte protrusions move and change like amoebas. They rapidly connect with neurons to start new synapses. Protrusions vary in size, location, and the signals that are used. The smallest protrusions are thin, jagged “sheets” containing mostly stored glycogen to produce energy for neurons and ribosomes to manufacture proteins. When neuronal signals fire, these tiny processes move, as if a protrusion from an amoeba, stimulated by calcium signals inside the astrocyte. (Calcium signals are described further on the next page.)
Diverse Astrocyte Signals
Astrocytes use similar types of signals as neurons—secreted molecules, direct cell-to-cell contact, sacs filled with information molecules, and electrical synapses. They utilize all of these messaging modes in conversations among neurons, immune cells, capillaries, and other supportive brain cells.
Like cancer cells, astrocytes use sacs as a favored communication method. Sacs sent to neurons are filled with RNAs, peptides, and proteins. Information in vesicles helps to balance ions, maintain the blood-brain barrier, and alert immune cells. Sacs, created at the synapse membrane or in specialized internal cell compartments, are sent to cells that produce myelin, directing them to add more of the insulating sheath in particular sections along the axon. Messages to microglia—the glia that serve as the resident immune cells in the brain—include information about fighting microbes and pruning synapses. Messages to neurons influence the strength of axon firing and responses to stress in the entire organism. Astrocyte signals also send information in the repairing process of the neuronal network.
CALCIUM SIGNALING
Calcium is ubiquitous in cells and is vital for major cellular function. Virtually all cellular processes depend on calcium ion intracellular signaling, from muscle contraction to fertilization and nerve impulses. Signaling with calcium is widespread among all cells, although because of its complexity, it’s not widely understood. We do know, however, that it is the most adaptable and widespread type of cellular signal.
When intracellular proteins release their stores of calcium ions, signaling takes place. Calcium signaling can also take place by increasing a cell’s calcium ion concentration levels via extracellular fluid. Calcium signaling takes place in muscle cells, especially heart muscle. In addition, calcium signaling can translate the axon’s electrical signal into a molecular signal to stimulate release of neurotransmitter sacs.
The signaling derives from calcium stored in specific organelles, prominently the endoplasmic reticulum (ER), a network of membranes attached to the nucleus of a cell, and the Golgi complex, an organelle composed of layers that serve as thin pouches. The released calcium is buffered and picked up by mitochondria and other lesser-known organelles. (Signaling by these cellular compartments is discussed in section four on organelles.)
Calcium flows from stores via specialized protein tubes, with many ancillary molecules helping in the process. Calcium can also flow into the cell from outside through special channels in the outer cellular membrane. Each type of cell and each cellular compartment has multiple varied complex protein channels for movement of calcium. Also, there are calcium transporter molecules, receptors, and ancillary molecules that stimulate action. Calcium signaling is highly integrated with multiple other signaling cascades. It can control multiple processes simultaneously, which makes it difficult to study.
In the section on organelles, we describe newly discovered contact sites where organelles, such as ER and mitochondria, touch to form a signaling platform. These sites often use specific types of calcium signaling and are vital in immune cell functions.
UNCOVERING CALCIUM SIGNALING IN ASTROCYTES
Although calcium signaling is known to be incredibly important to animal development, researchers are only now starting to uncover astrocyte signaling that uses calcium fluctuations. Intracellular calcium signals have huge variations in different situations but are not yet well understood.
As electric signals travel along the axon, nearby astrocytes signal internally with calcium fluctuations, as if in response. Each arm and foot of the astrocyte has diverse compartments that communicate using calcium oscillations in various ways that are related to events in regions of nearby neurons. In addition, the central astrocyte cell body has its own independent signaling apparatus separate from the others.
Multiple types of astrocyte calcium signals have been discovered, but little is known about the details of these calcium signals. Calcium signals synchronize with neurons for each type of neurotransmitter they use. The signals vary by brain regions and activities—sensory signals, motor signals, and sympathetic and parasympathetic signals. Calcium signals appear to correlate with the triggering of receptors on neurons in the cortex and cerebellum.
Astrocytes also send large calcium spike signals unrelated to neuronal activity, especially during fetal development. These astrocyte spikes are sent in the astrocyte network across the entire brain, just as neurons can send signals in circuits across the entire brain. The functions of these widespread signals are just now being researched and may relate to coordination of structures being built across the whole brain.
In astrocytes, calcium signaling events are diverse, and details are still emerging. The events can be brief and local or extended. Extended calcium events cover large brain regions. Local activity can be related to picking up neurotransmitters in between a neuron and astrocyte. The larger overriding calcium signals can stimulate various local signals at the same time.
Signals from astrocyte central cell bodies are slow and less frequent. These appear to be related to the astrocyte opening and closing blood vessels, measured by fMRI. Specific frequencies of calcium oscillations in astrocytes trigger various responses. Small fluctuations trigger stimulatory, rather than inhibitory, neurons. Strong signals with high frequencies trigger long-lasting learning in memory centers.
NEURON-ASTROCY TE INTERACTIONS
Neuronal signals can alter astrocyte functions, including triggering astrocytes to send more energy particles to the neuron. Conversely, astrocytes can alter neuronal firing and their neurotransmitters. Previously, it was thought that astrocytes only respond to strong neuronal signals. Recent research finds many more subtle reactions to much smaller activity. Various astrocyte compartments respond constantly to multiple events, even very small events at the synapse. It is not clear how astrocytes can integrate neural activity in different places, in different timescales, in varied-size scales, and with multiple types of signals.
Diverse astrocyte signals appear to coordinate specific actions of multiple neurons. Large signals related to groups of neurons in memory regions seem to organize activity. In the cerebellum, a type of intermittent signal correlates with coordinated animal movements. In the thalamus, signals correspond to tranquilizing effects similar to psychiatric medications. In the medulla, neurons measure blood acid, carbon dioxide, and oxygen and relay this to the astrocytes, which respond with direct signals to other brain centers that regulate breathing rates. All along the spinal cord, new responses from neuronal signals that trigger astrocyte responses are being discovered.
Astrocyte signals also coordinate brain waves that consist of synchronous electrical brain activity among large numbers of neurons. This can occur because the astrocyte network is connected with most neurons in a region. These coordinated brain waves with particular frequencies communicate information between locations in the brain.
REGULATION OF BRAIN ENERGY
Perhaps regulation of brain energy is the astrocyte’s most important function. The astrocyte is the only cell in the brain that stores emergency sugar for neurons in the form of the large molecule glycogen, which is broken down to produce lactate. Astrocytes determine how much glucose is coming into the brain and how much blood flow is needed to supply energy. The brain uses 20 percent of all of the body’s energy. Eighty percent of all brain energy is used by neuronal synapses to produce and transmit neurotransmitter-filled vesicles. Astrocytes use much of the rest.
Most energy for neurons is from chemical oxidation of glucose. Recently, it was learned that neurons also use an alternative pathway 10 percent of the time—during stress or exercise. This pathway utilizes lactate, which in the brain is mostly supplied by astrocytes. In particular places, such as the retina, lactate is used exclusively instead of glucose. When neurons need food, signals trigger astrocytes to break down glycogen and produce either more sugar or lactate. Recent studies on learning show that lactate, not glucose, triggers factors for neuroplasticity.
In most cells, mitochondria regulate energy use. Neurons have large numbers of mitochondria, but they also use the alternative supply of energy from astrocytes. Astrocytes also have mitochondria but regulate their energy cycles in a different way to produce lactate. For this alternate process, astrocytes add phosphate energy particles onto molecules in alternative metabolic pathways.
Astrocytes also closely monitor energy production and consumption in other ways. Astrocyte arms surrounding synapses produce protein receptors, transporter molecules, and channels to take up neurotransmitter signals from neurons. This uptake triggers lactate and can alter neuronal behavior. Neuronal signals tell astrocytes when to break down glycogen. Particular neurons with connections to all layers of the cortex measure energy needs and signal to astrocytes for more sugar and lactate for specific layers and columns of the cortex. Another brain center sends signals over most of the brain to trigger more sugar and lactate outside of the cortex.
Astrocytes often use two-way electrical connections with neurons for rapid communication about energy. These electrical synapses stimulate nutrients for neurons even at a great distance. A recent finding is that electrical signals help move lactate to neurons that are even quite far away from the synapse.
ASTROCYTES IN DISEASE
Astrocyte signals affect multiple diseases. With brain damage, astrocytes alter their shape, number, and function to form a special type of inflammation barrier around abnormal brain tissues. This can contribute to further brain damage. The cell arrangement changes from a tiling network to a circle, with arms entangled while signaling with immune cells. Abnormal astrocytes occur in Huntington’s disease models that stimulate abnormal neuronal function. In Alzheimer’s disease, there are abnormal astrocytes near amyloid plaques. With generalized brain damage, distant astrocytes stay in place and continue to function, but they grow larger and produce more signals and products, which can further promote disease progression.
Astrocytes also interact with neurons and other cells in large, multi-cell synapses that occur with pain and inflammation syndromes, described further in chapter fourteen. Conversations in these multifaceted synapses include neurons, T cells, vascular lining cells, immune scavenger cells, and all three glia cells. Hundreds of different signals are sent simultaneously among all these cells in one large synapse. Signals from astrocytes in these synapses related to inflammation have varied time sequences, from milliseconds to seconds.
Pulsed signals affect other cells in the synapse in multiple ways. Astrocytes send energy particles as signals to the other cells in the large synapse. Signals trigger stimulation or inhibition of neural circuits. These multi-cell synapses, further described in the previous chapter and chapter fourteen, are just being researched.