THE MYELIN-PRODUCING OLIGODENDROCYTES
A THIRD TYPE of glial cell, which produces the axon-coating material myelin, has a name that is awkward and confusing. It was first described as a cell “with a few branches”—hence, the name oligo for “a few,” dendrite for “a branch,” and cyte for “cell”—oligodendrocyte.
Myelin is a white fatty material that is essential for neuronal function. It serves as the insulating material for optimal electrical communications among neurons. Composed of mostly lipids and some proteins, myelin forms into an insulating sheath around axons to protect the nerve endings and increase the speed of electrical communication between neurons. (Schwann cells in the peripheral nervous system outside the spinal cord also produce myelin, but their signaling mechanisms aren’t as well known.)
Manufacturing and placing layers of myelin around axons was once thought to be a simple process. Oligodendrocytes were thought to produce a simple pattern of fatty insulation along particular axons to speed up transmission of the electrical signal and not much else. It was thought that oligodendrocytes did not take part in elaborate conversations with other brain cells.
Now it has been found that myelin is placed in various complex configurations based on conversations among all brain cells, including oligodendrocytes. It has also been found that these varied myelin patterns are necessary to coordinate the speed of a variety of long signals in neural circuits all across the brain. In fact, the process of making layers of myelin has been found to be extremely complex and requires a large number of ongoing conversations among various brain cells. Also, to provide the appropriate patterns, oligodendrocytes migrate across brain tissue, at times looking like crawling amoebas.

Cross section of multiple axons with surrounding myelin sheaths. Electron micrograph. (Dennis Kunkel Microscopy/Science Source)
In neural circuits, multiple signals are received at a single synapse almost simultaneously so that rapid decisions can be made based on multiple inputs. For signals from various parts of the brain to arrive at the same moment, their velocities must be coordinated along various lengths of the axon, using particular myelin arrangements.
Myelin does not form a continuous coating along the axon. Instead, it is divided into segments that form patterns along the nerve fibers. These myelin patterns, in which the segments are separated by gaps, are constantly altered as the need arises, based on messages from neurons, astrocytes, and other cells. Also, it has been found that placing multiple layers of the precise amount of fatty insulation is one of the most challenging structural brain projects.
THE MYELIN CODE
Until the discovery of variations in myelin patterns, it was impossible to explain how the brain coordinates short, medium, and long circuits. The most recent research shows that oligodendrocyte conversations among astrocytes, stem cells, choroid lining cells (lining cells of the cerebrospinal cord described in detail in the next chapter), and capillary lining cells are critical to the management of these circuits.
There’s no doubt that producing myelin is a big job. Although not totally understood, researchers have identified a large number of elements related to myelin construction that determine varied speeds for different brain functions. These elements include myelin length along the axon, number of myelin layers, thickness of individual layers, and patterns of myelin alternating with gaps, which are the naked portions of the axon where uninsulated electrical transmission occurs.
Special gaps, called nodes, have concentrated ion channels that enable electrical signals to rapidly jump from node to node. It has been found that axon nodes send and receive signals in conversations with other cells as well, such as with astrocytes and oligodendrocytes. Like neuronal synapses, astrocytes make close contact with each axon node to enable conversations. Also, it has been found that there are other, larger regions of naked axons included in these patterns that are not nodes. It is not clear what these naked sections are used for except for sideways communication from the axon to other cells.
Laying down myelin is far more intricate than producing synapses. Producing, remodeling, and maintaining myelin occur all over the brain simultaneously. Among multiple recently discovered myelin patterns, distinct configurations have been found in columns of the cortex. Research has found axons that are deliberately unmyelinated in spots to allow communication between the neurons and local immune and blood cells. Signals from the naked axon sections include secretion of immune messages, neurotransmitters, and sacs filled with RNAs, peptides, and proteins.
The stem cells that make new oligodendrocytes produce the largest number of new cells in the brain. Based on signals, stem cells travel throughout the brain and decide where myelin is needed. These regional stem cells produce oligodendrocytes, which move to the exact spot in the brain where myelin is needed.
Like they do with migrating blood cells, capillaries communicate with traveling oligodendrocyte stem cells about their movements. As described in chapter four, capillaries give instructions to these stem cells, and they do this at three levels. The first signal tells the traveling stem cell to stay as a general type of stem cell. The second directs the stem cell to the precise location in need of new myelin. The third signal stimulates a change to a particular subtype of oligodendrocyte needed in that region. Recently, six regional variants of oligodendrocytes have been identified, and each requires different signals. As with neurons and astrocytes, there are probably more types to be discovered.
Oligodendrocytes and Myelin Signals
There are a large number of different signals between neurons and oligodendrocytes. Some determine the number of wraps of myelin needed, some the thickness, and others the width of layers. Other signals are related to the structure of the nodes. Oligodendrocytes are now known to use a wide range of neurotransmitters and growth factors to communicate with neurons. They also send and receive genetic molecules in sacs.
Some signals slow myelin production, and others stimulate the growth of more stem cells. Oligodendrocytes can send factors to nourish neurons. Ion channels that normally conduct electricity along the axon are used for communication between neurons and oligodendrocytes. Oligodendrocytes do not conduct electricity but learn about a neuron’s electrical events by sensing the channel activity.
Signals between oligodendrocytes and neurons about myelin production are usually sent from locations on the axon where there is no myelin, such as nodes and other naked sections of the axon. In each cortex layer, there are unusual patterns of multiple intermittent stretches without myelin, as well as unique signaling. Regions of the cortex with the most advanced cognitive functions have the greatest number of spotty irregular myelin patterns, and these could provide sideways communication among neurons, without using synapses, for higher-level cortex functions.
Signals to neurons are also sent from myelin itself and can determine where new axons will sprout and where new synapses will form. Recently, ongoing conversations between axons and wrapped myelin have been found at structured synapses that maintain function over time and direct remodeling of the myelin when needed. These synapses respond to neuronal activity and are altered by learning as part of neuroplasticity. At these uncommon synapses, myelin also provides energy for the neuron when needed.
Under normal circumstances, signals from neurons to oligodendrocytes are not sent at synapses where communication occurs with other neurons and with astrocytes. But when neurons are under stress from low oxygen and energy, neurotransmitters released at synapses are rerouted to the oligodendrocytes. At the same time, other stress signals from neurons go to astrocytes and blood vessel cells to send signals that stimulate production of more myelin.
Initial Node
The first unmyelinated section of the axon as it leaves the cell body acts as the master node, regulating an electrical signal that rapidly jumps over sections with myelin from node to node along the entire axon. The initial node’s particular structure determines the type of information that is sent in the signal to other nodes. Initial axon segments can vary in size by a factor of sixteen and are longer in several layers of the cortex. Also, initial segments can alter their own size and position to regulate the type of information sent.
The initial segment determines the intensity and frequency of pulses. It also produces the “shape” of the electrical wave. Shapes of waves are a complex scientific subject. In music, they determine the quality of the sound that we hear, making a piano sound different from a saxophone. It is not yet known what different shapes of axon waves mean exactly, but they are correlated with the amount and type of neurotransmitters released at the synapse.
The initial segment determines not only the type of electrical wave that will occur but also whether a signal will go backward or forward. Backward signals help modify input from other neurons and determine more about the type of signal that is eventually sent along the axon to the next neuron in the neural circuit.
COMPLEX MYELIN WRAPPING PROCESS
Making myelin sheaths starting at the initial node is an elaborate, multistep process directed by signals at every stage. Genetic networks are triggered in timed sequences for the correct placement of the first wrap and then consecutive wraps. Servicing of all wraps continues for the life of the neuron, possibly for decades.
Newly minted oligodendrocytes are instructed to make contact with a particular neuron. A handlike protrusion of the oligodendrocyte touches the axon. Then, a large multiunit factory complex is built from scaffolding proteins. Various receptors are involved in the unfolding process to make the layers of fatty myelin. Signals regulate how much protein and how much membrane are needed. They also determine where proteins are placed in the membrane so that the layers are held in place. One particular protein is stimulated by neuronal signals and is only involved in the placement of the first layer.
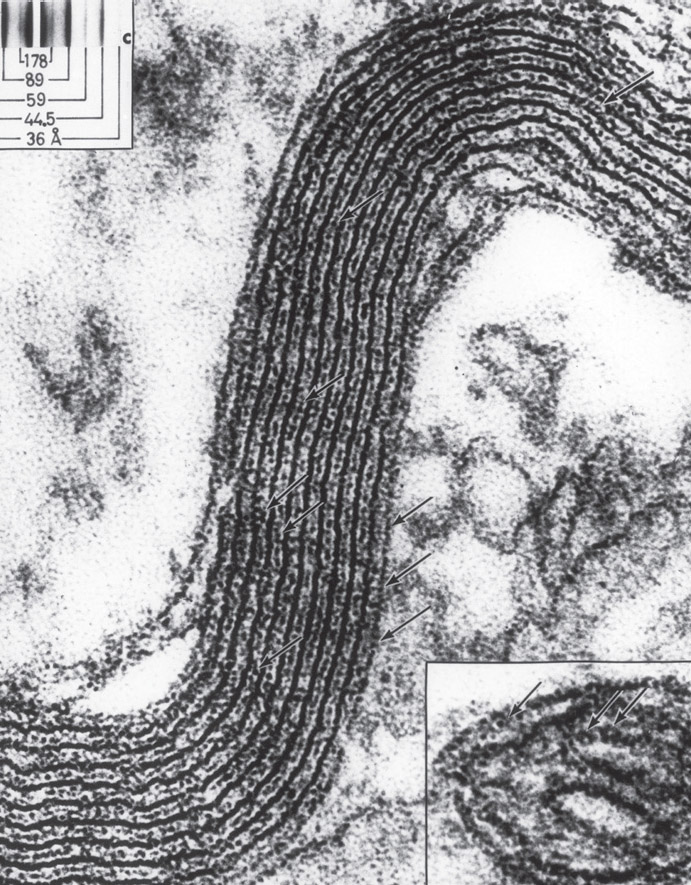
A close-up picture of a myelin sheath segment. Inset is a fold of inner membrane of a mitochondrion for comparison. Electron micrograph. (Omikron/Science Source)
Wrapping can only occur after an oligodendrocyte measures the circumference of the axon via a process in which the glial cell senses curvature. Axons have to be a certain size for myelin to be placed, and this has to be certified first before the layering starts. After the axon size is determined via signals, a spiral sheath in three dimensions is produced. A surprisingly large amount of material is involved. For example, a hundred-micrometer length of myelin with ten spiral layers uses up to 64,000 square micrometers of membrane material.
The wrapping process is not completely understood, with several competing theories about how it works. After the oligodendrocyte hand touches the axon, a leading “tongue” protrudes from the glial cell and crawls around the axon, depositing the first sheath of myelin. Then, additional layers are wrapped on top of the first. As layers are laid down, another process lengthens the existing sheaths. Oligodendrocytes put out more processes that arrange the additional layers at each spot.
Each layer of the myelin sheath attaches permanently at the fixed location where the node is built, and the layer grows from there in the other direction until it reaches the next node location. These attachments form a special region next to the node, with unique chemical and electrical features. The exact number of layers and the placement of nodes are decided in conversations.
When three layers are completed, another process called compaction starts. This squeezes together all of the layers of myelin and gradually eliminates extraneous material, making the layers of membranes smooth and closely attached for good insulation. Before compaction, wraps include two fatty membranes and material from the cell in between. When compaction finishes squeezing, it leaves multiple layers of membranes right next to each other with no cell material in between. The membranes from all layers are joined together with special proteins. These membranes are tightly connected like the sides of lining cells, resulting in multiple layers of connected fatty myelin.
Even with compaction, avenues of communication are preserved through the multiple myelin layers in order to remodel and repair the structure when needed. This communication among layers sometimes occurs for decades. Compaction begins at the outermost layers first and creates communication channels through all layers. These channels allow transport of material into the compacted areas as well as sections that are growing new layers.
Compaction is not fully understood, but it does involve counteracting forces that normally maintain a cell’s shape, such as electrostatic repulsion. Many receptors and signals regulate these wraps, including molecules that are also used to hold neuronal synapses together.
MYELIN AND LEARNING
In response to learning, neurons alter multiple synapses across the brain simultaneously—one major part of the neuroplasticity process. Via signaling, oligodendrocytes have also been found to play a critical role in this learning process by producing new myelin patterns that coordinate with these synapse alterations.
Myelin patterns are vital for movement, learning, and cognition. At birth, there is little myelin in the infant brain, such as along long motor neurons that connect the brain via spinal cord to muscles throughout the body. Without myelin on long axons connected to muscles, babies cannot move. But as myelin gradually grows down the nerves of the spinal cord, starting at the top, it produces stepwise infant milestones—vocal sounds, neck stiffening, arm movement, sitting up, standing, and then walking.
Later, higher cognitive abilities coincide with myelination of cortex centers. It is only in young adults that frontal lobes produce myelin, corresponding with maturity, judgment, and adult decision making. Because of this well-known sequence, it was extremely surprising when a myelinated region in the brain related to language was recently discovered in infants at birth. Clearly, there is more to learn about this.
In adults, myelin continues to be produced in specific parts of the brain based on activity of brain centers related to learning. Memory and learning require exact timing of synapse firing in various parts of the brain. Learning circuits are weakened if one contributing branch arrives late and strengthened if it arrives exactly within a narrow window. Myelin creates various rapid speeds. But it must create the exact velocity needed for the circuit, which is complex, because different configurations of myelin produce transmission rates that vary a thousandfold. In fact, neurons can transmit signals at three inches per second or two hundred yards per second.
It is known that myelin alterations correlate with amount of learning. Alterations of myelin have been observed in individuals as they were reading or playing musical instruments. Increasing skill correlates with increasing alterations in myelin. When mice are placed in environments with more opportunity to learn and exercise, there are great increases in oligodendrocyte stem cells. Exercise increases these stem cells in the cortex of humans as well. Some subtle changes in myelin can be seen in the memory centers within two hours in humans and other animals. Although learning is focused in certain regions, the remodeling of myelin occurs all over the brain.
MYELIN DESTRUCTION
Multiple neurological diseases occur as a result of myelin destruction, such as multiple sclerosis (MS), in which myelin is destroyed through immune hypersensitivity, or an overreaction of the immune system that can be classified as autoimmune or allergic reaction.
Immune hypersensitivity occurs in four basic ways, all of which have many unknown details. Environmental molecules can stimulate general antibody responses from immune cells, which create damaging inflammation in particular organs. Specific antibodies can attach to certain cellular molecules, causing local tissue damage. The antibody reaction can initiate large complexes of proteins that deposit in blood vessels and tissues. T cells can also mobilize attacks on particular tissues with a wide variety of signals.
Various proteins associated with myelin are targeted in a wide range of myelin diseases, but much is not known about the process. In MS, proteins are likely targeted near the myelin nodes triggered by particular signals from T cells. This process damages the myelin, making a person who has MS unable to use specific muscles. In a severe progressive type of MS, neurons are also damaged by excessive inflammation, including via the overstimulation of microglia in the brain. As more is learned about conversations of oligodendrocytes, new treatments for brain disease could be able to regrow damaged myelin.
Other diseases where myelin is attacked in hypersensitivity reactions include Guillain-Barré, a rare syndrome that can cause severe paralysis, and a range of other muscular and sensory diseases in which various neural circuits are attacked. In the future, when specific protein targets are known and specific T cell signals are discovered, treatments could be developed to directly address these and other diseases.