UNTIL RECENTLY, the central nervous system was considered “immune privileged,” sealed off from the rest of the body’s immune system. But recent and emerging evidence has found that the body’s peripheral immune system can cross the various barriers of the brain, with immune cells and signals entering and leaving the brain in various ways.
A surprise discovery found 500,000 T cells in cerebrospinal fluid (CSF) under normal conditions. These T cells communicate with brain cells as described in chapter three. Likewise, lymph drainage of particles from the brain was only recently discovered flowing from several fluid-filled chambers, called ventricles, to lymph nodes in the neck, where T cells can identify abnormal particles from the brain and respond.
Still, immune responses in brain tissue are different from responses in all other organs because of microglia, the resident immune brain cells detailed in chapter eleven, which are found only in the brain and spinal cord. Microglia converse with T cells and other immune cells that are outside brain tissue, providing much of the immune direction in the brain, as the T cells do in the rest of the body.
BRAIN COMPARTMENTS AND BARRIERS
Multiple types of membranes and the topography of the brain determine the various ways immunity works in the central nervous system. Three membrane layers, or meninges, cover the brain to protect the delicate tissue within. The outermost layer, called the dura mater, sits closest to the skull. It is the thickest layer and includes many blood vessels.
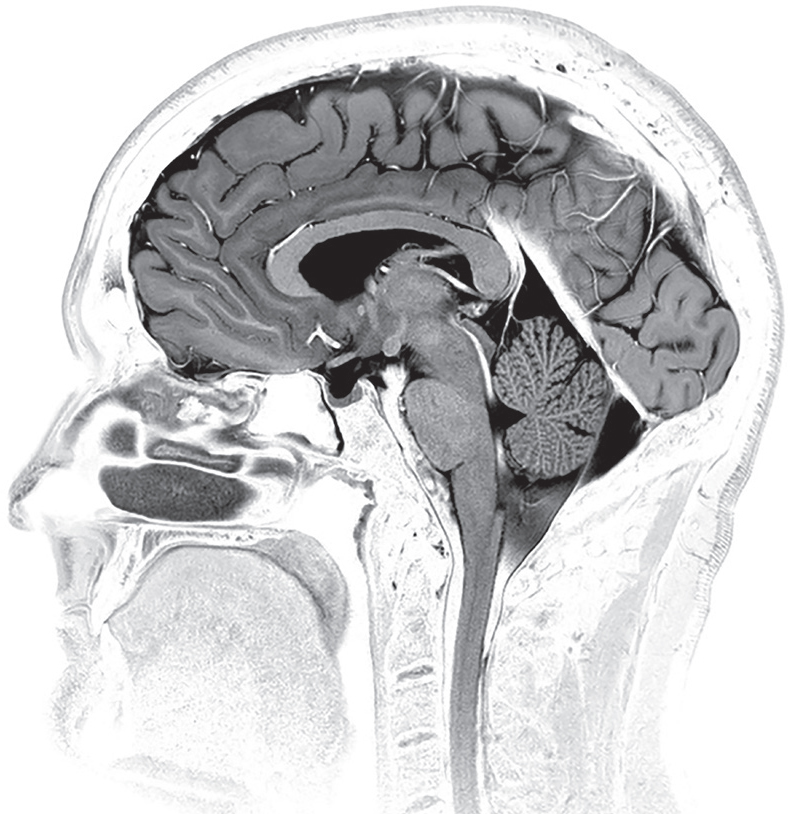
Side view of the brain showing cerebrospinal fluid mostly in black with several white streaks. Electron micrograph. (Living Art Enterprises/Science Source)
The middle layer, called the arachnoid, is a thin, weblike membrane that loosely covers the brain and spinal cord. The most inner layer, the pia, is a thin membrane composed of flattened cells, allowing the transit of molecules and immune cells. The three layers together make an elaborate barrier with varied immune properties.
The arachnoid and pia enclose the subarachnoid space, which contains the arteries, veins, and nerves and is filled with CSF. Because of this link to the blood, it is easier for microbes to get into this compartment; thus, more brain infections, called meningitis, are likely to occur.
Within the brain are also four ventricles, or interconnected cavities, where CSF is produced and stored. This clear fluid, which is also stored in the central canal of the spinal cord, protects the brain and spinal cord from trauma, provides nutrients, and removes waste. Additionally, it carries various communication signals. Multiple barriers ensure that only certain materials, cells, and foods enter brain tissues and that debris is removed. Gateway lining cells for each compartment allow signals to cross but must give permission for immune cells to travel between chambers.
Until recently, it has not been clear how immune activity occurs in the protective ventricles, where there are no microglia. Now, it has been found that these compartments have diverse immune responses based on signaling from their specialized barrier cells. Each compartment has different ways to get microbes and debris out of the brain and immune cells in. Like capillary and gut lining cells, cells inside the brain barriers also call for support during crises, such as infections and injury, using signals in the blood. All of this activity is based on conversations among a wide variety of cells.
IMMUNITY AND TRASH REMOVAL IN THE BRAIN
Traditional immune activity can occur in the CSF, but there is little in brain tissue inside the astrocyte barrier. All ordinary immune activity is normally shut down where microglia live. As in other organs, signals are sent in the blood to call for specific immune cells when trouble occurs. For these traveling cells to enter the brain, distinct conversations must occur in each ventricle.
The ways immune cells enter the brain to fight infections is different for blood vessels and CSF. Capillary lining cells in the brain allow much less material and fewer cells to pass through than in other organs. These capillaries can use active transport with small sacs for some molecules. When there are no infections, T cells don’t cross from blood via capillaries and aren’t allowed past the astrocytes. However, they can sometimes cross via small veins. When T cells are activated to fight an infection, the capillaries give them their permission to enter the brain.
Ordinary lymph drainage has been found in the most outer protective compartment and in CSF, but not in brain tissues. But, recently, an entirely new type of fluid channel in brain tissue—acting in some ways like lymphatics—has been described, with its origin between layers of cells protecting blood vessels. This current starts with a small amount of fluid escaping from pulsating arteries inside brain tissue and picks up debris between neurons and astrocytes. The flow takes the abnormal molecules out via small veins and special astrocyte water channels.
This cleaning channel has been found to be one of several ways misfolded proteins can be cleared, reducing the chance that these dysfunctional proteins will lead to degenerative brain diseases, such as Alzheimer’s. A related finding is that during sleep, neurons shrink and the channel’s flow increases, allowing greater cleanout of misfolded proteins. This phenomenon could explain the correlation between Alzheimer’s and lack of sleep.
Microglia—which play an important role in reorganizing the connections between nerve cells, fighting infections, and repairing damage—are also primarily active while we sleep. In fact, multiple events occur during sleep that affect brain circuits and the debris between them. Brain wave signals appear to trigger synchronous pulses of extracellular fluid that wash the areas between neurons to rid them of debris. As mentioned, neurons shrink, making the space between them larger and more easily accessible to cleaning. Also, neurons reduce their activity, and then the microglia get to work in pruning unnecessary synapses and remodeling the circuits.
HOW FOUR GUARDIAN CELLS WORK WITHIN THE BRAIN BARRIERS
Overall, there are two main types of barriers (with variations) that don’t allow the ordinary flow of material in the blood to enter the brain. One barrier, called the blood-brain barrier, is between blood vessels and brain tissue. The second major barrier separates blood from the CSF.
Four guardian cell types working together inside these protective barriers stay in constant communication, with signals that vary in each brain region and situation. Three of these guardian cells are the capillary lining cells, pericytes (described below), and the astrocytes that wrap their end-feet around the capillaries and pericytes. These three cell types communicate with each other using a wide range of signals. Together, they determine whether to allow cells and particles to cross from blood into brain tissues. Just as important, they decide whether to open or close blood vessels to increase or decrease blood flow to a region of neurons.
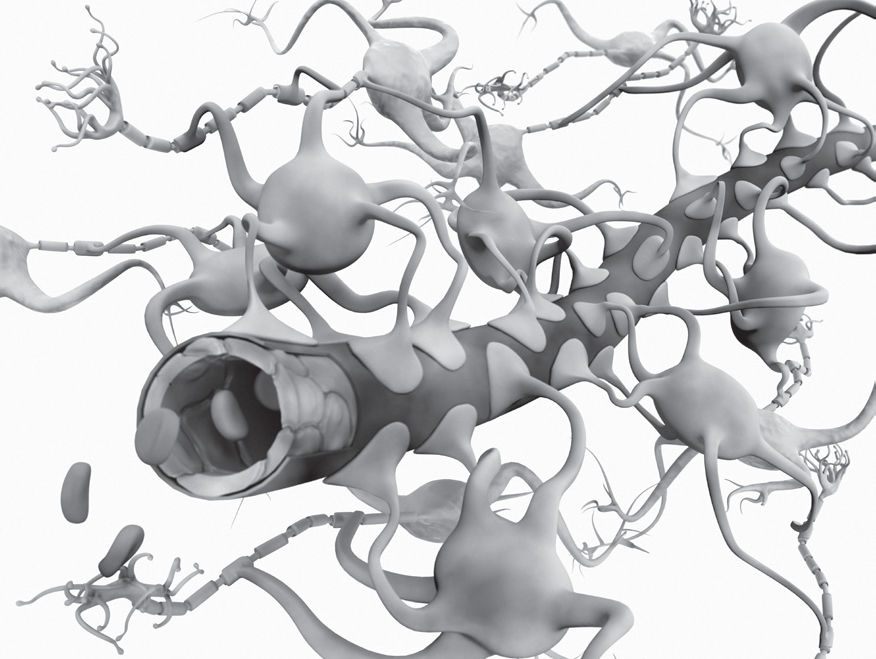
Illustration of the blood-brain barrier. Blood vessel lining cells with surrounding feet of astrocytes. Using these feet, astrocytes control blood flow by squeezing the blood vessel and as a barrier stop harmful chemicals and bacteria from reaching the brain. (Gunilla Elam/Science Source)
The fourth guardian cell, called the choroid lining cell, is integral to providing the barrier to protect CSF. These cells reside in various places lining the four ventricles. They are also situated next to specialized capillaries that filter blood as part of the cerebrospinal fluid production. Via signals among all brain cells, these choroid lining cells generate the amount and type of CSF fluid that is needed.
THE PERICYTE—A CHIEF INFLUENCER
In most organs, capillaries ultimately determine what can travel out of blood into tissue, but in the brain, pericytes take charge. As discussed in chapter four on capillaries, pericytes are the vascular cells that can contract like muscles. They wrap around capillary lining cells and are vital for the tight blood vessel barrier in the brain.
Of all cells, it is the pericyte cell—sitting in between the capillaries and astrocytes—that has the most responsibility for determining what crosses the blood-brain barrier. Pericytes have fingers, similar to astrocytes, that converse by touching nearby cells. They send signals with secreted molecules, vesicles filled with information, and electrical signals. Pericytes form a unique communication unit with capillaries, astrocytes, and neurons for multiple brain functions. They are so interconnected with capillaries that failure of one can lead to death of another.
Pericyte conversations affect blood flow in several ways. In the fetus, pericytes stabilize early brain blood vessels and determine where they will be built. With astrocytes, they talk about blood flow to neurons and regulate blood vessel tone via signals to muscles. Neurotransmitters from neurons independently cause pericytes to contract or relax the vessels.
Surprisingly, pericytes can also behave as stem cells and produce blood vessel cells, connective tissue cells, supportive brain cells, muscles, and even other types of stem cells. Pericytes can travel to new locations and produce new types of cells there as well—such as becoming a capillary lining cell when needed. They can behave like scavenger immune cells and eat debris, too. Capillaries can signal for pericytes to move into new positions and change into muscle cells and structural cells.
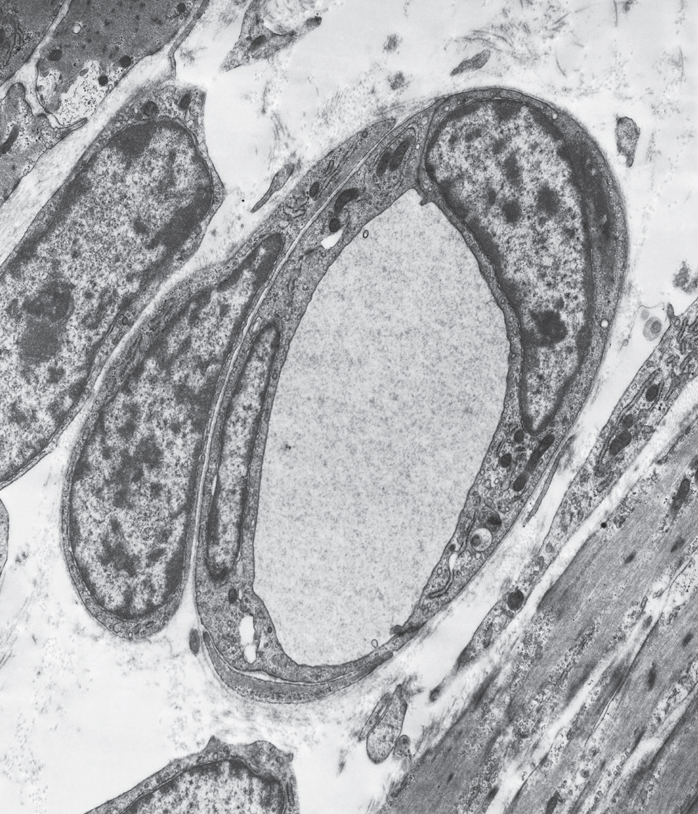
A pericyte, with a large nucleus, surrounds two capillary cells around a blood vessel. Electron micrograph. (Biophoto Associates/Science Source)
With strokes and other brain injury, pericyte signals stabilize blood vessels and help in the repair process. Pericytes travel to kill impaired capillaries and create a tight barrier to wall off damage. They send signals to enable the transport of particular vital molecules for repair, such as omega-3 fatty acids. Like capillary lining cells, pericytes use signals to call for immune cells when needed. Pericytes can also contribute to cancer growth. Defective pericyte signals can enable supportive brain cells to become cancerous. Signals from pericytes that stimulate new blood vessels can also contribute to brain cancer growth. Cancer cells, themselves, can morph into pericytes to make their own new vessels.
THE BROAD ROLE OF CHOROID LINING CELLS
Choroid lining cells (which we will call “choroid cells” subsequently for the sake of simplicity) are an integral part of the choroid plexus, a complex network that also comprises capillaries and is located in the four ventricles of the brain. The choroid plexus forms the blood-CSF barrier.
A single layer of choroid cells can determine the fate of the brain. These cells line, in various ways, each of the brain’s four ventricles and are very tightly joined to each other—eight times tighter than gut lining cells. By filtering blood, choroid cells produce CSF that protects the brain in the ventricles, smaller vessels between chambers, and along the spinal cord.
The rate of CSF production is regulated by signals from neurons to choroid cells and can be quite rapid. CSF’s function was long considered to only be a cushion to temper mechanical forces that push brain tissue into the hard skull. But now CSF is known as a rapid communication medium for signals to travel throughout the brain. Until recently, CSF was considered to be largely empty fluid. In fact, it contains proteins, fats, hormones, microRNAs, cholesterol, many different metabolites, and signals from all regions of the brain. It also contains T cells and other immune cells, as described in chapter one.
Choroid cell structures reflect their complex functions. The top of a choroid cell is in direct contact with CSF. The bottom part of these cells is near a large number of capillary blood vessels and a connective tissue barrier. Capillaries below are structured with abundant pores and a covering diaphragm that allows rapid flow of water when needed to produce CSF.
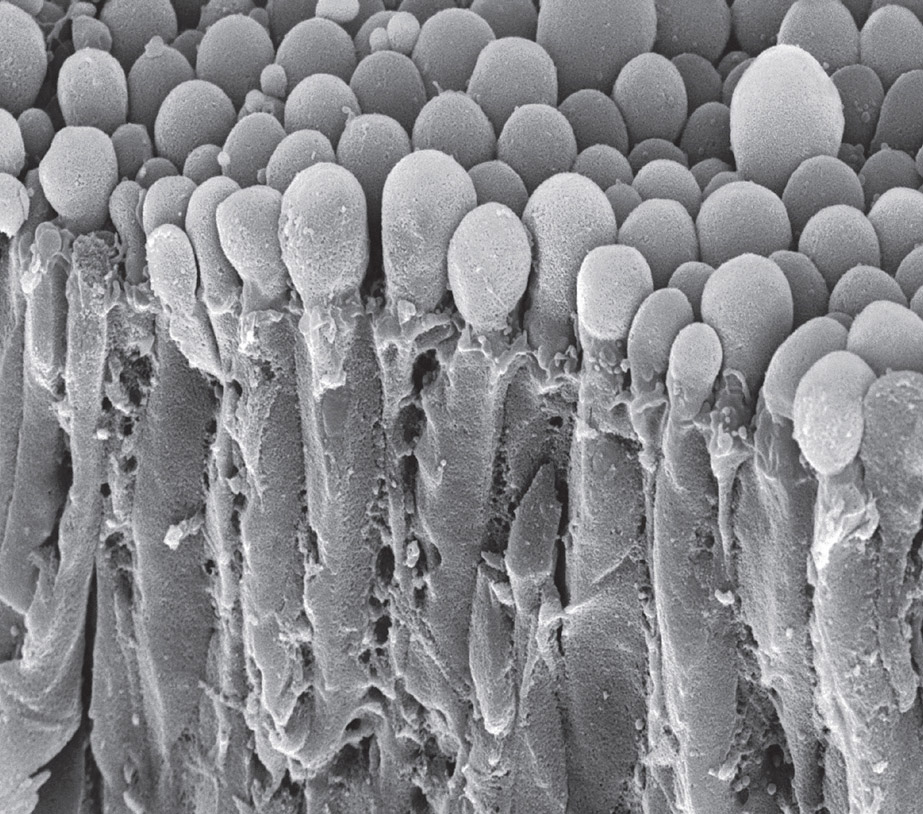
The swollen tips of choroid cells secreting cerebrospinal fluid. Electron micrograph. (Steve Gschmeissner/Science Source)
Only specific small molecules and some proteins are allowed to pass through from the blood to the CSF or from the CSF to the blood, many using the unique transport mechanisms that reside inside the choroid cells. Transporters at the top and bottom of the choroid cell actively move ions in and out of CSF—calcium, potassium, sodium, and phosphate. More elaborate transport machinery is necessary for passage of proteins and nutrients.
Like gut and skin lining cells, choroid cells gradually mature, building new skills along the way to properly do their job. The top of the mature cell near CSF develops multiple small invaginations, producing a large surface area for communication. The nucleus moves to the bottom of the cell, making room for elaborate transport machinery near the top. The tops of mature cells are filled with numerous energy-producing mitochondria and centers for the manufacture of proteins.
Choroid cells also have cilia attached to their top surfaces that face the CSF. These hairlike protrusions undulate synchronously and move water in the CSF, just as mucus is moved in the lungs with coordinated cilia on lining cells. Choroid cell cilia also serve as sensors to measure concentrations of chemicals in the CSF. Choroid cells respond to these signals by altering entry and removal of specific molecules.
In a fetus, choroid cells appear first in one of the ventricles, then sequentially in three other brain regions. Each successive choroid lining cell is distinctive and derives from stem cells that are particular for that brain region. These stem cells also produce distinct neurons for that same area. An interesting note is that capillary signals to stem cells in each region determine how many choroid cells versus how many neurons will be produced.
CHOROID CELLS PARTICIPATE IN VITAL BRAIN FUNCTIONS
Choroid cells are the center of conversations between the brain, bodily organs, and immune cells. Neurons and immune cells send signals that are relayed by choroid cells great distances throughout the CSF, brain tissues, and blood. Choroid cells also secrete hundreds of their own diverse signals to brain and immune cells. Signals can be molecules or sacs filled with receptor molecules and RNAs.
The choroid plexus responds to more than microbes and infections. Both immune cells and brain cells react to mental and emotional events, including depression and stress, and these signals also cross the plexus barrier. Such signals can trigger pain, exhaustion, and lack of appetite. Choroid cells also provide other critical brain functions. They produce signals that regulate immune surveillance of the brain, including movements of T cells and microglia.
As mentioned earlier, choroid cells are part of several interconnected systems that clean the brain of debris. Choroid cells clear debris themselves with special transport mechanisms that take molecules from the top of the cell near the CSF to the bottom near the blood vessels. They also clean out molecular garbage by directing cilia to circulate CSF fluid in particular directions. More than half of the random and dysfunctional proteins and other debris in the brain is cleared out by these various mechanisms.
Like capillaries, choroid cells participate with stem cells in producing new neurons for memory centers. These particular stem cells have large appendages sticking way out into CSF to send and receive instructions that result in production of new neurons. Once produced, immature neurons depend on thousands of signals, including those from choroid cells, to migrate to their new locations in brain memory circuits. Signals push and pull the new cells into place.
Choroid cells are important for particular brain capacities to develop. They participate in neuroplasticity processes by helping to eliminate underutilized neuronal synapses as if they are debris. Unnecessary synapses are appropriately pruned through conversations among choroid cells, astrocytes, and microglia.
When problems arise in a particular brain region, molecular signals are sent in CSF to alert other cells. Cerebral cortex cells use long protrusions extending all the way into the CSF to communicate with choroid cells via signals in the fluid. From these circulating signals, choroid cells determine exact locations of problems in distant regions of the brain. They assess the situation and call for particular immune cells. When these traveling cells arrive, choroid cells direct them to the exact part of the brain where they are needed. In a crisis, a choroid cell can open its gates and allow multiple immune cells in.
Impaired choroid cells, unfortunately, cause multiple diseases. Poor flow of CSF from problems in the choroid cell can build up pressure in the CSF, killing adjacent brain tissue. When debris is not cleared, degenerative brain diseases occur. Building or repairing particular regions of the brain, as well as fighting infections, requires constant chatter among a variety of cells, with choroid cells often serving as a master regulator.