WITH A HUNDRED TRILLION MICROBES in the gut, and another thousand trillion viruses hovering over them, the sum of gut microbes has been called an additional human organ. For the single layer of gut lining cells facing all of these microbes, the stakes are high. These cells must choose the best species of microbes to befriend and then work with them in multiple ways.
Collectively, microbes (mostly bacteria) in the gut have three million genes compared to twenty-four thousand in human cells. With these genes, microbes produce diverse molecules and signals, many of which are needed by humans for survival. Some microbe products become part of normal human metabolism. In many ways, humans have become completely dependent on the effects of friendly microbes in the gut. Molecules produced by gut microbe DNA affect digestion, blood vessels, weight, stress, immune functions, and bone health.
Recently, scientists identified three hundred new gut microbial products that are released into human blood. It is not yet clear what all of these molecules do, but some have important functions in human cells. Signals produced by these bacteria include well-known neurotransmitters that stimulate brain cells. Several gut microbial products influence the development of the immune system at the start of life and continue to help maintain normal immune functioning. Signals influence the building of gut villi and crypts. They instruct the placement of stem cell niches and density of blood vessels in gut tissue. Recently, it was shown that eating plant fiber attracts particular microbes that produce products to help guard against diabetes. Another study shows that people who have diverse gut microbes are less prone to atherosclerosis.
Microbial products can also contribute to disease. They can contribute to heart disease, obesity, and diabetes. Very recently, gut microbes responding to salt increases in the diet were found to cause changes in blood flow to the brain independent of the other well-known effects of salt on blood pressure. These effects of gut microbe signals included altering vessels in the brain and decreasing cognition. There is more about microbes affecting the brain in the next chapter.
Managing all these diverse microbial species and their products is a huge undertaking. Decisions are based on diverse conversations among the gut lining cells, microbes, and immune cells throughout the long gastrointestinal tract.
ATTRACTING MICROBES
Gut regions encompass hundreds of different environments that attract diverse microbes. In each region, lining cells must decide which are the best, stable, and friendly resident microbes. These varied regional gut settings also require different types of relationships between local immune cells and microbes.
Microbial settlements vary along the stomach, small intestine, appendix, and large intestine. Also, patches of microbes, as well as very large communities, thrive across the gut’s lumen. Various colonies survive in the central moving fecal stream and in layers isolated from this flow—the layer of mucus, the space between mucus and lining cells, and the deep crypts between villi. These protected regions tend to have more permanent microbe colonies.
In the small intestine, bile is released, which produces an acidic environment. Several microbe communities in this region compete for sugars but are limited by acid levels. Microbes that can survive grow rapidly. Unique genes in acid-loving microbes metabolize human food to produce a variety of molecular products, some of which can have positive and negative effects related to obesity, asthma, and the development of cancer.
The largest number of antibiotic attack molecules produced by the gut lining cells are found in the first segment of the small intestine. These antibiotics form a gradient throughout the small intestine. With fewer attack molecules at the end of the intestine, more microbial species survive, and the density of colonies increases to saturation. The large concentration of microbes continues into the large intestine.
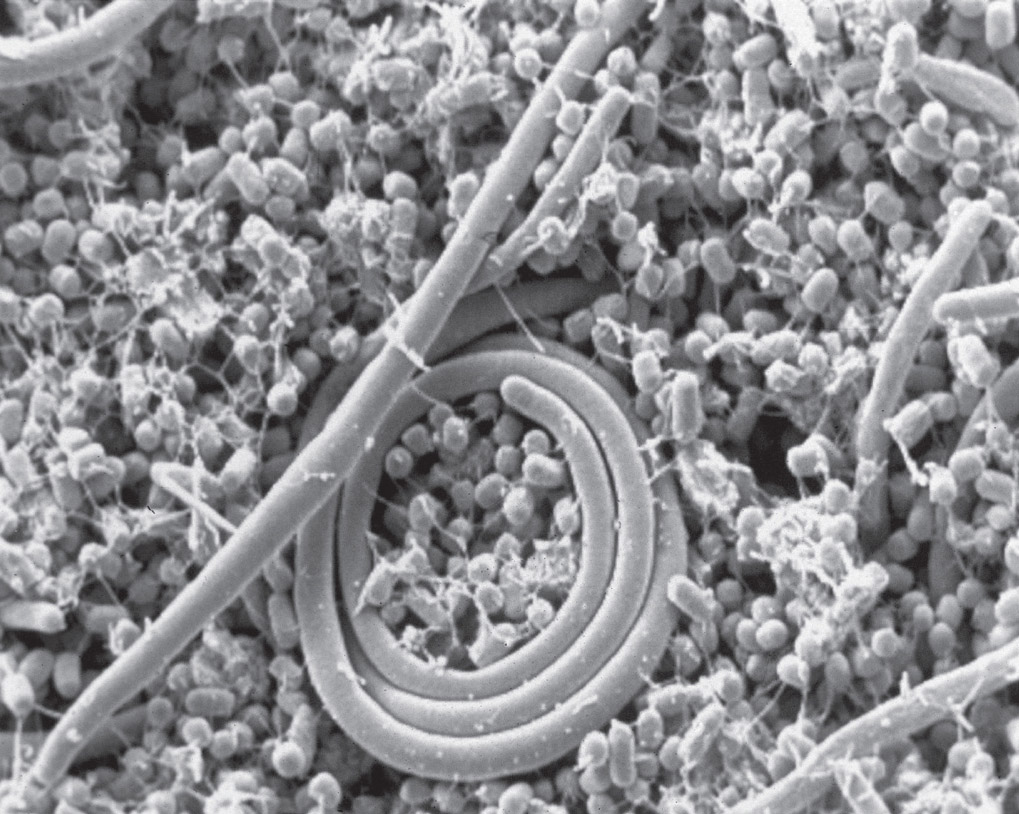
Salmonella bacteria in the gut. Electron micrograph. (USDA/Jean Guard-Petter/Science Source)
The large intestine—with more mucus, little fecal flow, and multiple creases and folds—also has multiple environments. It has the largest number of distinct microbe colonies, and the gut lining cells there produce the least amount of antibiotic molecules to kill them. The large intestine is considered to have the highest density of bacteria of any habitat on earth. Microbes in the large intestine break down plant fibers into products that are important for protection against multiple diseases, including diabetes.
Multiple factors determine stable microbe communities in each region. Survival is based on adaptation to acid and base (or alkaline, which is the opposite of acid), diet, medications, immune responses, and, most importantly, communication with master gut lining cells. Immune cells secrete molecules that help some colonies and not others. A delicate balance among various microbe communities is maintained via signaling among all gut cells. If two microbe communities are overly cooperative, they grow too large and cease to benefit each other. To be stable for a long time, nearby communities must balance cooperation with a certain amount of competition.
RESPONDING TO FOOD
Various diets encourage particular microbe species and regional gut lining cells to use diverse signals and approaches in their response to food. Patches of food lead to clusters of specific microbe colonies. When there is a lot of food, microbes grow rapidly and form large biofilms. These communities in biofilms can be more active near the lining and can become more dangerous. With less food, species separate into smaller colonies and are less dangerous.
Microbial species in the gut shift rapidly when humans eat new types of food. Diet trends can even affect long-lasting colonies that are hidden in mucus and crypts. Meat and plant foods attract distinct microbes. For example, red meat causes cardiac vessel disease based in part on the attraction of specific meat-friendly microbes.
These particular microbes eat the molecule carnitine from meat and metabolize it into a substance that enters the bloodstream from the gut. Carnitine is a family of compounds that play an essential role in energy production. The product produced by microbes that eat the meat’s carnitine then travels in the blood to the liver. There, it is altered by liver cells into a second molecule that is also released into the blood. It is the second molecule that causes plaque in blood vessels, which can lead to heart disease. This effect on heart disease has nothing to do with meat per se but is the result of behavior of specific meat-loving bacteria.
Another example is in babies, where particular microbes prefer certain sugars and milk. When solid food is introduced, other microbes form thousands of permanent communities. Dominant species start growing at the beginning of the small intestine and gradually travel downward. Eventually in adults, approximately fifty prominent species become stable for years. Following treatment with antibiotics, these stable communities reemerge after hiding in protected niches.
CONVERSATIONS AMONG MICROBES AND IMMUNE CELLS
Immune cells in the gut are educated about microbes by wide-ranging conversations with gut lining cells, capillary lining cells, neurons, and friendly microbes. Interactions with these microbes allow human cells to learn how to build unique receptors and signals for conversations with both friendly and enemy microbes. Friendly microbes collaborate with immune and lining cells on the details of digestion, as well as protection of the lining.
Microbial signals can stimulate immune signals that loosen tight junctions between lining cells to allow material into tissue below. Also, immune proteins can form a barrier on the surface of lining cells to block sugar molecules on microbe membranes that are typically used by the microbes to attach to the lining. One microbe covers its surface with human antibody molecules and uses them to attach to the lining.
Conversations among immune cells and microbes are quite varied. Signals from T cells protect specific microbial colonies. Microbes protect themselves with signals to immune cells to decrease inflammation. In the appendix environment, lymphocytes are specially educated to modulate their reactions and protect friendly bacteria. The appendix becomes a lasting reservoir of the best friendly species to replenish the intestines when necessary.
Immune signals can regulate microbe conversations, including altering competition among microbe communities. Too many cooperating microbe species cause perpetual positive feedback. This destroys important competing microbe colonies needed for stability. With signals, immune cells break up excessive feedback loops and separate the cooperating species.
Multiple diseases have strong associations with particular microbes and immune cells. One example is inflammatory bowel disease, in which there are fewer diverse microbial species. Another occurs with damage to the liver, where microbes produce toxins that cause mental confusion. Another example is particular microbes that alter bile acids to stop a threatening infection.
Conversations among microbes to avoid infections can be complex and are just now being discovered. In one finding, six different bacteria must communicate simultaneously to inhibit other dangerous bacteria in various ways at the same time.
Another example in hospital settings is preexisting friendly bacteria, chosen by the gut cells, that are able to fight off dangerous infections in some people. Other people with the same microbes are not so lucky and succumb to serious hospital-based infections. The reason for this difference is not yet understood.
LIFE IN MUCUS AND BIOFILMS
With permissive messages from lining cells, specific bacteria and viruses thrive in and around the mucous layer near lining cells. Signals from friendly bacteria stimulate more mucus for protection. In the mucous niche, viruses become friends and protectors of human cells and fight off invaders. New genes shared by viruses or bacteria secretion systems help in the adjustment of the mucous niche by generating subspecies specially adapted to the unique environment.
Mucus, with a special mix of substances that protect lining cells, is also good for friendly microbes. Enzymes in mucus kill unfriendly and competitive species. Mucus includes special nutrients, immune factors, salts, and metals that all contribute in helping friendly microbes thrive. Healthy mucus is produced by back-and-forth communication among microbes, lining cells, and immune cells.
Microbes use various techniques when dealing with mucus. Some species are able to break it down. Several can swim through the mucous gel, eating molecules as they go. However, eating mucus can backfire, stimulating excess amounts of the protein mucin, which hurts friendly bacteria. Microbe communities can swim all the way through mucus and attach to the lining-forming biofilms in deep protected crypts. For this, strong flagella motors are needed. Lining cells stay in constant communication with microbes in the mucus, and some microbes are able to use oxygen from lining cells. Nearby colonies can be protected or ignored by immune cells based on signals from lining cells and microbes.
Protective biofilms can take the place of mucus. Biofilms are much larger and thicker than mucous layers and have multiple forms. In bio-films, communication between multiple species is key in understanding whether an infection will take hold. Biofilms are engineered to allow interactions between colonies, as fully discussed in chapter fifteen.
Multiple species sharing the biofilm can allow each other to grow more dangerous. Competition among species is different in biofilms than it is in other regions. For free-floating colonies, too much cooperation is negative for both. In gut biofilms, dangerous species are more structured into specific locations and therefore can cooperate without hurting each other.
Both cooperative and competitive biofilms can produce infections in multiple ways. Several species need another colony to grow first. The second colony then becomes dangerous and kills the first. In another situation, microbe signals manipulate the immune system to alter the structural matrix that supports the biofilms, so that the two communities become separate rather than interdigitating. This can occur, for example, when one colony moves on top of the other to get more oxygen. Some colonies produce waste that kills a nearby colony. Several species like each other’s waste, and both are stimulated to grow and produce infections. Some produce molecules that protect both colonies.
Biofilm communities can cooperate in other ways. A community can grow around the other to avoid antibiotics, since one may have resistance while the other does not. In an abscess, both layers can grow bigger, but one stays distant to avoid damaging peroxide formed as waste from the other community. Also, one colony in the abscess can produce an enzyme for protection, and the other colony can inhibit the enzyme by maintaining its layers. As these signals are understood, research points to a future of more accurate probiotic treatments.
VIRAL COLLABORATIONS
It is surprising how significant viruses are for the gut environment. Some viruses form close relationships with friendly bacteria that live in mucus close to lining cells. These viruses protect the bacteria, as well as the lining cells, from the attacks of enemy bacteria.
To reach human cells in the gut lining, viruses must travel through intense acid, dangerous enzymes, dense mucus, and enemy bacteria. When they enter the gut through food and water, a complex environment bombards them—acids and bases, enzymes, molecules that kill microbes, and a vast array of bacterial communities. If viruses can penetrate mucus, often with the help of bacteria, they can reach the gut lining cells and invade tissue. Even a small number of viruses that reach human cells can stimulate an infection.
Viruses can also be involved in producing bacteria that are more dangerous to humans. Previously, it was thought that bacteria cause dangerous infections by changing from a friendly state because of immune weakness. However, pathological transformations of bacteria are often caused by conversations among human cells, bacteria, fungus, viruses, and even parasitic worms. One such signal from viruses is the transfer of antibiotic resistance molecules to microbe colonies, making them more infectious.
Bacteria can also be critical for viral survival. Multiple viruses need bacteria for their transmission to human cells. If these viruses are injected by researchers directly into the gut, they don’t survive. The virus must travel through the communities of bacteria in the mouth and gut to gain strength. One method the virus uses is attaching to bacterial surface sugars, which increases their ability to jump to human cells. Both the polio virus and norovirus (which causes vomiting and diarrhea) attach to sugars on bacteria that allow them to survive acid and heat. Then they slip into immune cells.
Immune conversations can help gut viruses either attack or protect gut lining cells. Viruses can be brought into human tissue by immune cells gathering samples of infectious microbial molecules. Messages can inhibit T cells from attacking viruses, such as herpes, which then multiply. Viruses can also be helpful to gut lining cells by stimulating immune messages to heal a break in the lining. Some viruses stimulate immune-cell responses that fight against dangerous bacteria.
A wide range of infection-producing interactions among viruses, bacteria, lining cells, and immune cells are just being discovered. One infection requires a viral mutation and, at the same time, an alteration in the gut lining cell. Another infection requires both the virus and a mutated immune cytokine signal to produce virulence. Both examples require particular bacteria as well. Another example is an infection of the large intestine that requires bacteria to be altered by viruses, as well as interactions between several other bacteria and viruses.
As the complex signals among gut cells, immune cells, and microbes are deciphered, new treatments for diseases throughout the body will be able to be developed.