VIRUSES ARE SO MUCH SMALLER than bacteria that observing their complex behavior has only recently become possible. The first bacteria signal for making group decisions was discovered decades ago. The first signal among a community of viruses was just discovered in 2017.
The signal was discovered in a type of bacteriophage. Also simply called a phage, it is a virus that infects, replicates in, or travels with bacteria and archaea. They are the largest class of viruses on earth. The signal, derived from a peptide, is called arbitrium, which is Latin for “arbitration,” named so because it determines whether to kill the host bacterium or keep it alive to serve its needs.
In the few years since the signal’s discovery, an increasing number of signals from a wide variety of viruses have been discovered, prompting a new field of sociovirology to emerge. Signaling has been found among the viruses that cause hepatitis, polio, measles, and flu. It has been found, too, that multiple types of viruses can understand one another’s signaling, much like different bacterial species communicate together in the gut or in a biofilm. Also, it appears that viruses sometimes cooperate and sometimes go it alone.
Researchers have found that viral communication is based on signals that somehow trigger receptors on the receiving viruses. For example, a phage viral signal, consisting of a molecule with six amino acids, is expressed when the virus first enters a bacterium. As more viruses enter, there are more of these signals. With a large number of viral signals, the community of viruses is alerted to slow down in order not to kill the bacterium that is supplying the machinery for multiplication.
Some researchers now think that certain viral signals can change the DNA of bacteria, thereby altering bacterial function. Very recently, it’s been discovered that fifteen types of phage viruses produce certain signals that are only used among their own colonies to alter bacterial behavior.
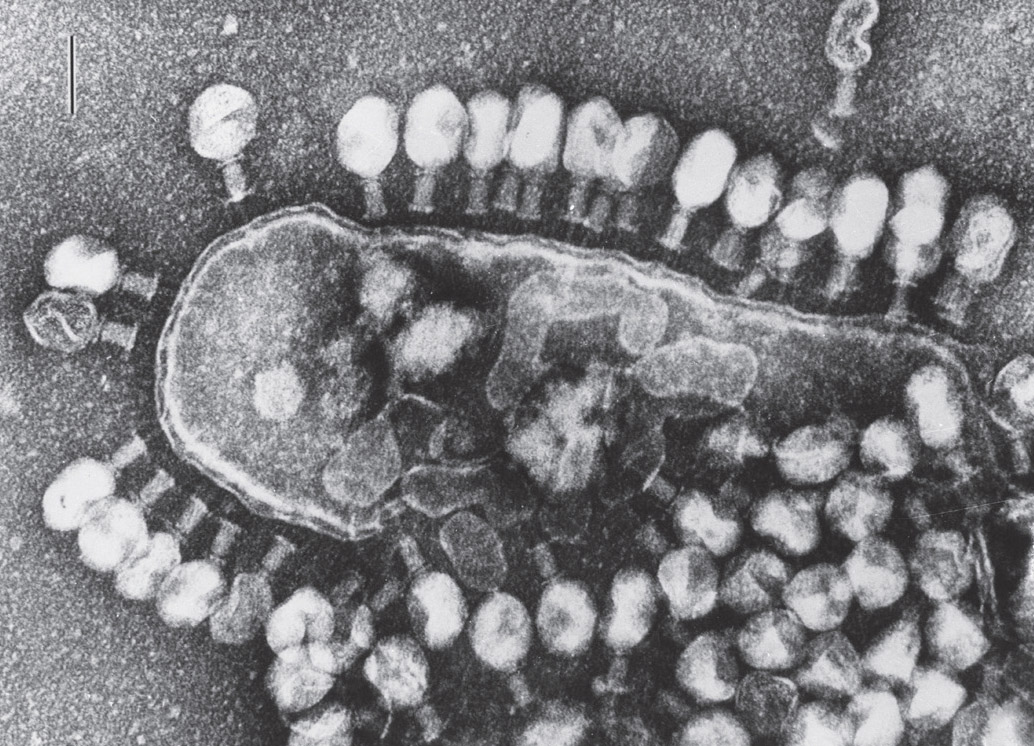
After signaling amongst themselves that this bacterium is no longer necessary, phage viruses destroy it. Electron micrograph. (Lee D. Simon/Science Source)
These viruses read the signals of the bacterial communities they surround to determine when to attack and when to reproduce. The signals also seem to affect when a bacterium will hibernate or remain active. In their efforts to adapt these signals to fight infections, scientists have now been able to engineer phage viruses to attack particular bacteria.
Bacteria are also known to use viruses as signals or use them as the purveyor of signals, although finding exact viral signals for the most part has been elusive. Viruses can transport signals among bacteria, such as genetic material for antibiotic resistance. Or, bacteria can produce their own viruses that contain messages and transmit them with secretion systems. Transfer of genetic material among bacteria is so widespread that it is hard to perfectly define species and trace their evolution.
EVADING CRISPR
In researching how bacteria defend against viruses, it was discovered that each species of bacteria has evolved elaborate “immune” systems to identify and destroy viruses. One of these systems, originally found in bacteria, was modified to become the important research tool called CRISPR-Cas9, whereby strands of cellular DNA can be accurately cut, edited, and inserted by scientists. CRISPR (which stands for “clustered regularly interspaced short palindromic repeats” in the DNA code) is part of a natural system that enables bacteria to identify viruses by their genes and attack them. Scientists have adapted CRISPR-Cas9 as an accurate gene-editing tool, which they are now beginning to use in the fight against cancer and other diseases.
There are currently more than thirty varied CRISPR-Cas-like systems identified in bacteria, so there is variation in how they work. Some cut DNA from viruses that have DNA. Others attack RNA viruses by first using reverse transcription and changing the viral RNA into DNA. The enzyme for this step is stored with the CRISPR-Cas system in bacterial genes.
CRISPR-Cas9 works basically in the following way: Bacterial DNA has patterns of multiple, simple, short, repetitive codes that use palindromes—a code that is the same read backward and forward. Bacteria use these repeated patterns as placeholders for storing pieces of viral genetic material in between the repeats, as if in a file cabinet, so they can find them later on. Stored cuts are either from viral DNA or viral RNA transcribed to DNA. The particular technique works because a protein (Cas9) is able to use this system to help identify a specific point in the viral genes and accurately cut it, thereby disabling the virus.
When a virus enters a bacterium, the bacterium cuts a piece of the virus’s DNA (or DNA reverse transcribed from viral RNA) and places it in the repeats. This enables the bacterium to remember the virus and later identify it. If the virus invades again, the bacterium cuts pieces of the invading virus genetic material and uses the stored cuts to identify the new invader. The bacterium then uses an enzyme to find the identical spot of genetic material on the invading virus and cut it, disabling the virus.
From the multiple varied, natural bacterial systems now being discovered, other editing tools are being developed by scientists. All of them appear to use a way to identify pieces of DNA that are placed in repeated patterns. They also use an enzyme to accurately cut DNA by using the DNA already enmeshed in the pattern.
Very recently, in research pursuing signals in viruses, it has been found that viruses have developed ways to fight back against the bacterial CRISPR defense systems. Researchers are now in the process of deciphering a complex communication system in which viruses work together against the central bacterial CRISPR defense system.
A form of altruism appears to occur in the viral community related to CRISPR. Several viruses must first attack the bacterial CRISPR defense system. They are killed in the process, but their attack produces a molecule that is then used by other members of the viral community against the bacterial CRISPR system. One group of viruses, therefore, depends on another group to die for overall viral survival.
Other viruses cooperate in different ways. With the virus that causes polio, viral members collaborate to increase their offenses on human cells by sticking together and exchanging molecules with one another for a more effective raid against their host cells. In an infection caused by a virus in a mouse, the viral members share molecules in sacs sent between cells while evading immune cells. In these sacs, the virus is more effective in causing infection. In fact, many different viruses use sacs to send signals and to travel while inside the human body. These viruses include Zika, hepatitis, norovirus, and the varicella-zoster virus (the virus that causes chicken pox).
When scientists are able to better understand these viral signals, treatments could be developed to use viruses in different ways to kill infectious bacteria. Some strategies might include using multiple cooperating viruses to protect human cells and helpful bacteria while eliminating dangerous bacteria and perhaps even cancer. Finding the signals to start and stop hibernation will help in the treatment for many diseases.
COMPLICATED AND INTRIGUING
No one really knows how viruses fit into evolution’s tree of life. Viruses are not cells, and, therefore, some scientists do not consider them to be “alive”—a term that can be difficult to define. Regardless, the makeup of viruses is complex and intriguing. In the past several years, large viruses have been discovered that are closer to the size of small bacteria, which further complicates such research.
Even more recently, larger viruses that exceed the size of small bacteria have been discovered. These have enough DNA to produce fourteen hundred proteins. Such discoveries are consistent with the theory that some viruses were once bacteria that gave up genes for a lifestyle that’s now dependent on other cells. Large viruses are also surrounded by their own sets of viruses, just as bacteria have particular viral species that are focused on them.
Viruses consist of strands of DNA or RNA, proteins, and a protective capsule. It is remarkable how much they can accomplish with as few as seven genes and a handful of proteins. Even viruses with this small number of molecules are able to enter cells and hijack genetic machinery to make copies of themselves while evading multiple attacks from the immune system. HIV, with nine genes, and Ebola, with only seven genes, respond in elaborate ways, as bacteria do, to defend against human cell attacks.
The herpes virus is a much larger virus, with more than seventy genes. With this larger number, it is able to enter and manipulate human skin cells and neurons. It commandeers motors for axon transport systems and travels up and down the axon from the skin all the way into the brain. Herpes tricks the heavily guarded nucleus of the neuron. There, the virus alters its own behavior and hibernates for years. When it reactivates, it travels back down the axon, jumps to the skin, and waits for skin-to-skin contact to infect someone else.
Viruses are able to avoid the extensive human cellular machinery designed to find and destroy them. Multiple types of receptors pick up molecules from viruses in the human cell nucleus and outside it. These receptors trigger aggressive immune attacks. But viruses can latch onto these receptors and modify them. Viruses can also interfere with the production of receptors with tags of their own. They are able to alter the very pathways designed to destroy them. Once inside the nucleus, viruses then trigger actions in the rest of the cell.
Viruses also use other techniques to fight receptors, enabling viruses to hide in their host cells. They mask their DNA and RNA by adding phosphate molecular tags. They take receptor molecules and move them to compartments where they don’t work. They are somehow able to trigger the growth of distinct new compartments surrounded by membranes to hide receptors. This is, in some ways, similar to bacteria that take over cell compartments to live in. Receptors are also moved to other larger organelles, such as mitochondria and protein factories. Another tactic is using enzymes to modify the shape of the receptors or to simply destroy them.

Two herpes viruses interacting with human cell. Electron micrograph. (Energy.gov/Wikimedia Commons)
PARTICIPANTS IN PEACEFUL AND DESTRUCTIVE RELATIONSHIPS
Viruses have both peaceful and destructive relationships with bacteria and human cells. Like bacteria, viruses alter their relationships in various situations. When bacteria travel to new spaces, their companion viruses travel with them, repelling both bacterial and viral enemies. To allow their bacterial comrades to fight off enemies, friendly viruses provide them with toxins to use as weapons.
But with stress, such as little food, friendly viruses can turn on their own bacterial comrades. Some viruses only attack individual bacteria that weren’t the conduit for their own personal birth. If there are no other viruses nearby, viruses can choose to kill a cell slowly so they can use the cell to make as many new viruses as possible. But if competing viruses are present, they kill the cell faster so it can’t be utilized by others.
When bacteria are attacked by antibiotics, viruses provide them with genes taken from other microbes to produce bacterial resistance. What is surprising is that viruses don’t only transfer these genes; they also steal other genes that work against antibiotics but aren’t being used at that moment, to be employed in future warfare. This process produces multidrug-resistant bacteria that are so dangerous to humans.
On the other hand, it’s been shown that some viruses bolster bacteria that are beneficial to the gut. Viruses that surround bacteria have been observed defending those microbes chosen by human gut lining cells as friends. When dangerous bacteria approach, these friendly viruses attack and kill the intruders, reducing their numbers by up to ten thousand times. Also, in studies in which bacteria are completely eliminated from a site in the gut, viruses remember the particular enemies when the bacteria are replenished.
Entirely new viral behavior, not yet understood, has been observed near mucus. It has been thought that the class of viruses hovering over bacteria does not directly interact with human cells. This variety of viruses is the most abundant on earth. Now, billions of these viruses have been observed being transported into the gut lining cells under mucus. They all appear to be traveling in the direction from the gut lumen toward gut tissue. It is not known if this produces another positive virus effect in humans.
VITAL VIRAL GENES
Viral genes have been vital for humans in multiple ways. Eight percent of all human DNA comes from viruses that have placed their DNA into human genomes. This DNA has been placed over millions of years into human ancestors and passed along. Most are fragments, not full viral genes. But some are actively producing RNAs and proteins that are used by human cells.
Embryonic stem cells have more products related to these viral genes than any other types of cells—2 percent of all RNA produced in stem cells. One of these viral-based RNAs is vital for stem-cell function and correlates with the power of stem cells to produce multiple types of cells.
Another vital gene from viruses that sits in human DNA provides a protein produced only by placental cells that connect to the uterus. This single layer of cells allows the fetus to receive nutrients from the mother via the placenta. Without this viral protein, fetuses would die.
Originally, the protein allowed viruses to fuse with membranes for entry into cells. When the viral gene first appeared in primate ancestors, this function was altered in evolution to become the connection to the placenta membrane. Multiple versions of this protein have appeared in various animal species from diverse viruses—at least six distinct times in evolution. For example, pigs and horses don’t have the type of viral DNA in humans and, therefore, don’t have the same type of placenta.
HIV WITH NINE GENES
With only nine genes and nineteen proteins, HIV is able to invade and disable human T-helper cells—the very immune cells that activate an army of other immune cells to kill such viral foreigners. HIV’s proteins help the virus evade multiple immune cells, enabling it to enter the T-helper cell unharmed.
These proteins also hide viral RNA by masking RNA activity and tricking sensors that protect the cell nucleus. With its proteins, the virus then produces double-stranded DNA from the codes on viral RNA—opposite to the way human cells make RNA from DNA. This new viral DNA then needs to be transported into the nucleus and placed into human genes.
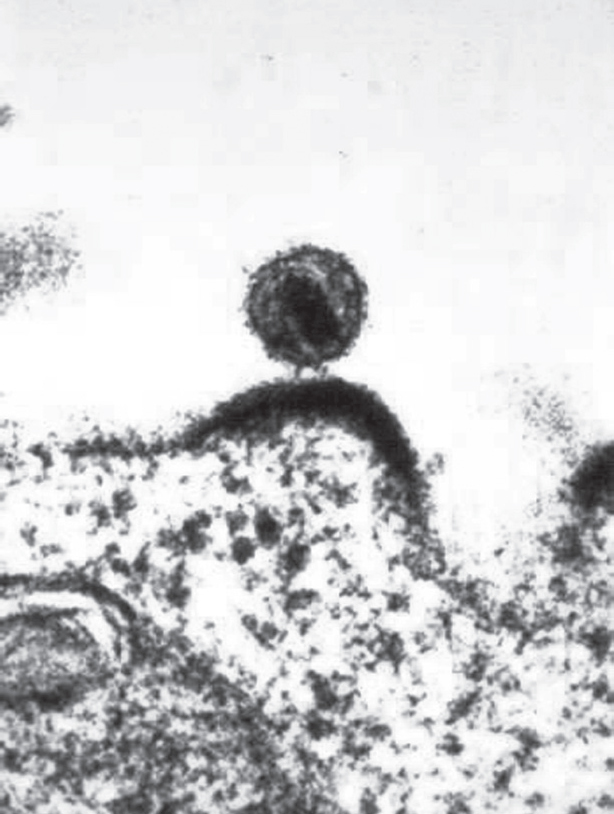
An HIV virus budding from a lymphocyte. Electron micrograph. (NIAID/Science Source)
Once HIV DNA is in human genes, there are various outcomes. Viral DNA can be quiet and hide for considerable periods of time, later repopulating HIV in the cell if needed. It can produce more viruses to leave the cell. These viruses can transfer to nearby cells via nanotubes or in communication sacs. Also, HIV can kill the cell or even kill nearby cells without entering them. To kill the nearby cell, first HIV stimulates the two cells to fuse together. Then signals from the host T cell trigger suicide in the second connecting cell. With this direct contact between the two cells, HIV can also enter the second cell without killing it.
Entering and Reproduction
Nothing in HIV’s behavior is simple. To enter the human cell, special proteins on the surface of the virus interact with receptors on an immune cell. The viral protein alters its own shape twice and then folds in half, forming coils that twist together. Coils join the virus and the cell membrane together. Sugar-coated proteins, called glycoproteins, on the viral surface fuse with the cell membrane, which then releases the viral protein cover into the cell with the virus inside. This same mechanism can be used later to leave the cell and travel elsewhere. When the virus contents are injected into the cell, four enzymes are included. Viruses then travel on transport microtubule highways in the cell to the nucleus.
To reproduce in the cell, HIV produces its own messenger RNA from the strand of DNA that the virus had placed in human genes. Ordinarily, human messenger RNA is edited with multiple pieces spliced together before proteins are made. HIV stops the splicing process by actions of its own proteins. This allows the entire HIV RNA genome to be made as one continuous messenger RNA strand. This full RNA copy is used to assemble new viruses in the cell’s membrane.
Assembly and release of new viruses are also complex. HIV proteins travel to the T-helper cell organelle that makes proteins. There, one protein produced from the HIV DNA is cut in half by the host enzyme to produce two glycoproteins for the viral cover. These two proteins are then transported to the membrane site where the virus is being assembled. To bud from the cell, two other viral proteins are used.
HIV produces a protease, an enzyme that breaks down proteins and peptides, and uses it to cut the other HIV protein into pieces at the membrane where the virus is being assembled. The pieces form a matrix to hold the cover in place. (Medications that inhibit these particular protease enzymes are a major form of HIV treatment.) HIV then stimulates the cell membrane to produce a sac that bulges out from the cell, which HIV uses as its cover as it leaves.
Cloaking Techniques
Perhaps HIV’s most remarkable behavior is evasion. HIV’s ability to avoid triggering special sensors in the T cell makes it successful. Ordinarily, cells find any DNA or RNA that is not in the right place. When the RNA of the virus first produces DNA outside the nucleus, cell sensors are alerted about unusual DNA in the wrong place in the cell. HIV uses several molecules to cloak the operation and to transport the hidden DNA into the nucleus.
A multistep mechanism takes the virus coat off in stages, while hiding the newly produced DNA from sensors. A human enzyme that would normally cut up HIV DNA is manipulated instead to help the virus evade sensors. Other molecules in communication with the nucleus protect the HIV DNA in a hiding place. Then the HIV DNA is sent from this safe location into the nucleus. Because the nucleus is where DNA is supposed to be, this DNA no longer triggers sensors.
Other cloaking techniques use two cofactors provided by the T-helper cell that are attracted to the virus as soon as the HIV cover enters the cell. The first is an enzyme that alters pieces of the cover. The second is a human nuclear protein that helps HIV enter the nucleus by hijacking a motor that normally transports cargo in the other direction.
This second factor also tears off molecules that protect the route into the nucleus—enlarging the nuclear pore and enabling HIV entry. Surprisingly, at the same time, these two factors also work with HIV to stop immune signals directed at them. During this activity, another attack is launched by the cell in which it reduces the amount of nucleotides available for HIV to make more DNA. HIV responds to this by altering its own enzymes to function with the reduced amount.
HIV becomes invisible to the cells’ attacks by using tags in multiple ways. The virus can alter its own tags to evade immune signals. Enzymes in the cell that stop production of viral DNA can be tagged and, thus, inhibited. In addition, a molecule in a cell’s membrane can be tagged to alert other cells about the HIV infection inside this cell. This molecule is also supposed to grab HIV, stopping its exit through the membrane. HIV is able to counter this operation by tagging the molecule with its own tag, making it useless.
EBOLA WITH ONLY SEVEN GENES
The deadly Ebola virus is equally clever with only seven genes. The virus is composed of a layer of proteins attached to RNA and a protective matrix. It surrounds itself with a cellular membrane stolen from an infected cell. The virus uses other proteins near the surface membrane that are sugarcoated to enable them to stick out of the membrane and attach to various molecules on the exterior of multiple types of human cells.
The virus also uses varied cofactors for each type of human cell in which they are able to gain entry. One cofactor in the cell membrane removes virus caps protecting the sugar-coated proteins, which alters these proteins to enhance attachment to the cell. Another cofactor molecule helps take vesicles carrying the virus into the cell. These cofactors also alter pH in the unusual membrane-covered cellular compartment to enable entry. The cell then cooperates further by altering Ebola’s glycoproteins to make loops with hairpin turns, which provide a strong attachment to the cell.
Just like HIV, Ebola uses various methods to escape detection. It builds a decoy to fool the immune system, for instance. Ebola makes two different versions of its glycoproteins. One covers the virus, while the other serves as a decoy that the immune system attacks. Ebola also has a special mechanism in which the enzyme-manufacturing proteins sway back and forth—first making one version of the sugar-coated proteins and then the other. Most of the proteins produced are decoy versions that provide safety for those actually used by the virus as it escapes from the cell.

Ebola virus. Electron micrograph. (CDC/Science Source)
To reproduce new viruses, Ebola builds a distinctive raft structure that floats in the cell’s membrane. When Ebola enters the body, it first attacks immune scavenger cells and cells that present material to T cells. Both of these cells ordinarily pick up microbes or their products to trigger defenses, but Ebola is able to damage these immune cells early in the process. As the cells die, release of immune signals further weakens all other immune cells, as well as cells in various tissue. Ebola then uses a diverse range of methods to begin attacking various organs. Unlike HIV, which only invades T-helper cells, Ebola enters most human cells except for lymphocytes and somehow is able to use multiple techniques with varied cofactors for each type of human cell.
Cooperation from the Enemy Cell
For cell entry, Ebola and human cell membranes fuse with a unique process. Ebola’s traveling vehicle is so large that a cell cannot use the ordinary process of taking in sacs. To accommodate this viral vehicle, the cell produces an unusually extra-large sac by using its outer-cell membrane, which takes in the entire virus at once.
This type of large vesicle is normally only triggered by a cellular pathway for cell suicide. But Ebola triggers an alternate cellular pathway that keeps the cell alive as it produces the large sacs. The large vesicle with Ebola inside is then brought to the lysosome, the organelle that ordinarily would kill the virus and recycle its molecules. With specially lowered pH produced by Ebola’s actions, the lysosome instead assists Ebola by removing the virus’s coat without injuring it. The organelle also opens the vesicle to release Ebola into the cell.
Without ever entering the nucleus, Ebola makes its own copy of RNA and then produces its proteins by hijacking the ribosomes, the host cell’s protein-producing organelles. Later, the virus uses three of its proteins in a complex arrangement to produce copies of the original RNA molecule that will travel with newly minted Ebola viruses.
Ebola RNAs produce eight distinct proteins with the help of the human cell’s genetic machinery. Each of these Ebola proteins provides multiple functions. They protect copies of RNA. They also build a reproduction complex in the cell membrane that assembles new viruses via a newly manufactured protective matrix.
When building new viruses, human cells cooperate with Ebola’s proteins in a variety of ways. To produce the final coat for a new virus, human enzymes cut the Ebola proteins into two pieces, which then self-assemble into a pattern on the cell’s outer membrane. Using Ebola’s proteins, the virus builds special fatty rafts to float in a cell’s membrane. The rafts also help rebuild the viruses as they leave the cell. Ebola proteins are assembled nearby, and using human proteins, they connect to a viral matrix layer. Then the entire structure attaches to the raft in the cell’s membrane. The raft produces the large bulge of membrane necessary to cover Ebola as it escapes from the cell. The entire virus and membrane covering it are then launched.
Evasion
While inside the cell, Ebola uses various evasion techniques. One viral protein that covers Ebola RNA helps evasion in multiple ways. Cell sensors recognize viral RNA and trigger attacks. Ebola’s protein places phosphate tags to alter production of the cell’s attack molecules. The same protein disrupts the cell’s attempt to place tags on the virus. At the same time, the protein also prevents viral RNA attachment to cell recognition receptors. It is hard to understand how one protein can do all of this.
Another Ebola protein fights immune signals. It blocks messages to the nucleus triggering genes that produce immune signals. It stops cell responses that are triggered by these signals. It binds to and alters the particular transport complex that moves these signals into place without hurting other cellular transport. Somehow, this single Ebola protein is able to use three techniques to block three different processes related to the same human immune signals.
When cells notice Ebola RNA, they shut down production of all protein in the cell to stop the virus from reproducing. An Ebola protein blocks this pathway and instead stimulates increased protein production for new viruses. Normally, small RNAs are produced by the cell to interfere with foreign RNAs. This cellular defensive measure cuts small pieces of the Ebola RNA and uses particular molecular patterns to kill the virus. Yet another Ebola protein interferes with the machinery for this entire process.
THE NOVEL CORONAVIRUS—VERY CAPABLE WITH FIFTEEN GENES
The novel coronavirus (technically called SARS-CoV-2), the virus that causes the illness COVID-19, has great capabilities, with fifteen genes that produce at least twenty-nine proteins. One very large protein is actually sixteen different ones that are cut and released by other proteins. Several of these proteins produce a bubble filled with fluid where the virus builds a factory to produce more viruses. Inside the bubble, two proteins produce new RNA, and another brings material to build the RNA; a medicine for SARS can attack this step. Three other proteins unwind the RNA to make it useful, correct errors, and cut up leftover RNA to keep the host cell from reacting to it.
Other proteins alter the human cell’s environment to help the virus survive in the cell and escape. Still others block immune signals to other cells, influence the flow of molecules in and out of the cell nucleus, and turn human genes on and off. Others poke holes in the cell’s membrane, making escape easier, and disable a protein on the surface of human cells trying to grab the escaping virus. One stimulates inflammation and another induces human cells to commit suicide.
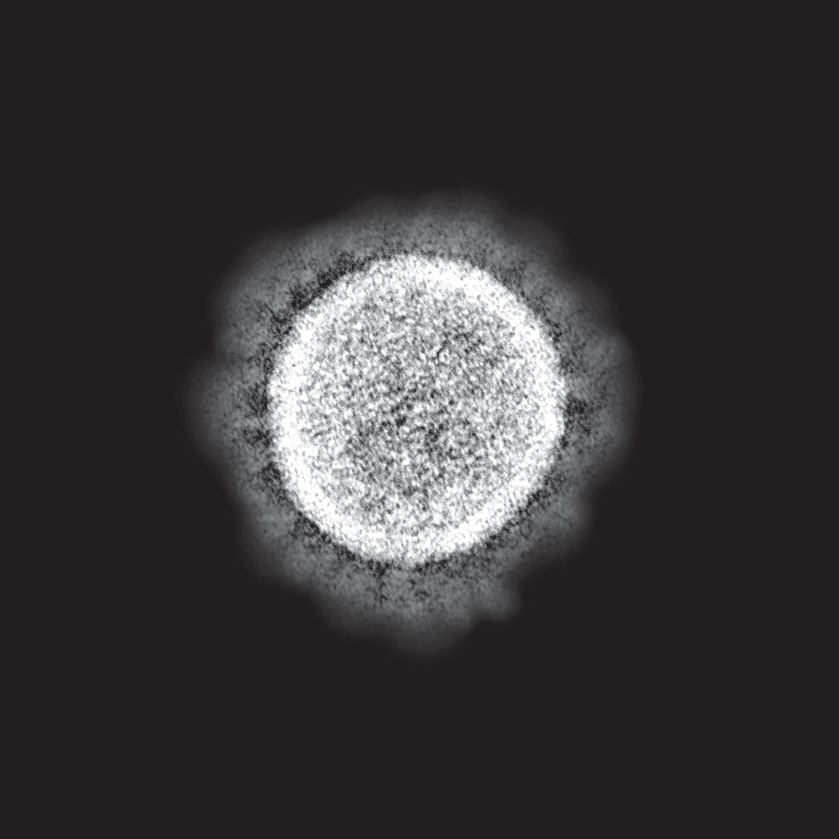
The novel coronavirus—technical name SARS-CoV-2. Electron micrograph. (NIAID/NIH/Science Source)
Four of SARS-CoV-2’s proteins build the structure that has been seen so frequently in the media. All four help with assembly and release from the host cell. One protects the RNA deep inside. Another produces the famous spikes that make it appear as a crown, also known as a corona. These spikes attach to the human cell receptor ACE2 molecule in the airways and other places. This molecule is also an important enzyme blocked by a class of blood pressure medications (ACE inhibitors), likely causing an association of increased risk of COVID-19 symptoms in those who have high blood pressure. Attaching to this receptor allows the virus to enter into cells and creates a strong attachment that causes the symptoms to linger. Of all the proteins, this one might be the one that causes the COVID-19 virus to be so dangerous to humans, since it attaches so firmly to human cells.