MICROBIAL CONVERSATIONS WITH ORGANELLES
ORGANISMS HAVE ORGANS—structures that perform specific functions in the body. In the same way, cells have organelles: mitochondria, nucleus, protein factories, membrane factories, and multiple large vesicles with diverse roles to play.
When microbes invade a cell, physical and chemical barriers keep them near particular organelles as the cell tries to kill them. But microbes are able to manipulate these organelles with their own signals. Several species of bacteria and protozoa are able to live their entire lives inside certain cellular organelles that are large sacs whose purpose is to corral and destroy the microbes. Other microbes are able to briefly travel inside these sacs and then attack the cell in various ways.
Microbes produce a wide range of assaults directed at particular organelles. They send “molecular missiles” via secretion systems to the cell nucleus or mitochondria from either inside or outside the cell. Microbes can also manipulate tags normally placed by human cells for transporting material to particular organelles. The microbes alter these tags to enable the transport of microbial toxins.
Microbes are able to target organelles by altering genes in the cell’s nucleus. These DNA alterations can interfere with an organelle’s metabolic pathways. Targeting a gene often means placing or removing tags on human DNA or the protective proteins around DNA. Tags either stimulate genes that help microbes or block those that don’t. Microbes can accomplish this by altering enzymes handling tags. Another genetic technique of targeting organelles is to alter editing of messenger RNAs as proteins are produced. These new edits instead manufacture abnormal proteins for a specific organelle.
CONTROLLING CELLULAR VESICLES
Chapter sixteen mentioned how microbes can create a home in large sacs, even those filled with enzymes designed to kill them. These large sacs are part of a cell-wide system that provides multiple important functions, such as storing water and fat and removing misfolded proteins. Vesicles can serve as destruction factories that take apart molecules and recycle them.
Microbial signals manipulate the sac, but also inadvertently alert the host cell of the microbe’s whereabouts. The bacteria that cause tuberculosis is one species that hides in large sacs. When even a small amount of their DNA leaks out, it triggers immune signals from the cell. To further identify the microbe, cellular-attack molecules produce holes in the vesicle, releasing particles that trigger more specific cellular attacks. The microbe returns the volley with defense signals, sometimes by hijacking the vesicle’s innate secretion system.
Microbes use signals to help strengthen their vesicle home against assaults. Signals trigger scaffolding around the vesicle for protection. They attract food into the vesicle by modifying the membrane to allow small molecules to enter. Microbes are able to produce channel proteins in the membrane to receive larger molecules. In essence, microbial signals control the basic supply chain of the cell. They place tags on sacs filled with food and reroute them to their vesicle home. Microbial signals are also able to alter the outer membrane of the vesicle home to make it less visible to the host cell’s sensors. Another technique alters the vesicle membranes so it cannot fuse with the vesicles of destruction.
Protozoa parasites that cause such diseases as malaria and toxoplasma are particularly adept at building permanent homes in vesicles. They control the pH nearby to avoid acid attacks from the cell. These microbes can even build their own vesicle homes by grabbing a cell membrane with special appendages as they enter the cell. New materials are stimulated to create a uniquely shaped home. Parasites commandeer the cell’s scaffolding to build a double membrane around the vesicle for better protection.
To inhibit immune activity, protozoa decorate the outside of the vesicle with their own proteins. They send signals to the nucleus to alter genetic networks. This action stops receptors from tracking the vesicle home. With multiple human cell attacks, these protozoa counter each one and maintain their lifestyle inside the vesicle.
TARGETING THE CELLULAR NUCLEUS
The nucleus houses a cell’s genetic machinery and is protected from microbes and their signals by multiple barriers. The outer membrane has pores with elaborate protection to only allow transit of specific molecules in and out. Only molecules with vital importance are permitted to transit the pore, such as signals coming into the nucleus and messenger RNAs going out to protein factories near the pore.
Placing tags on DNA and the proteins that protect DNA, microbes wage war for control of the nucleus. Tags alter what genes can be used and, therefore, what proteins are able to be produced. More than fifty different types of tags are currently known that either allow a particular strand of DNA to be used or inhibited. Also, each tag can be placed on multiple different sites to produce even more effects. Somehow, microbial signals manipulate enzymes that work with tags, allowing placement and removal at specific locations. Microbes are particularly adept at hijacking a cell’s tagging systems related to production of sensors that detect microbes in the cell. By denying the cell its sensors, the cell is unaware of the presence of the microbes.
One way bacteria alter the normal tagging process in the nucleus is by producing two competing versions of the enzyme handling tags. This maneuver decreases operation of genes that are critical for cellular defenses. For example, a microbial signal sent by a secretion system changes the enzyme-placing tags, causing severe diarrhea. Another signal injected by a secretory system inhibits immune signals for inflammation.
A wide range of other microbial signals produces various results in the nucleus. One such signal opens the highly defended nuclear pore to allow the entry of other molecules. Microbial signals can affect the three-dimensional shape of the large DNA molecule. By altering the scaffolding that holds the DNA strand in a particular shape, genes interact in new ways. Bacterial signals can also alter proteins that protect the DNA by replacing them with the microbes’ own proteins. Another microbial signal alters protein production machinery, allowing the microbe to multiply more rapidly.
ORGANELLE FACTORIES THAT PRODUCE PROTEINS AND MEMBRANES
Microbes are able to influence two vital cell compartments, one where proteins are manufactured and the other where membranes are assembled. These two organelles have hard-to-remember names—endoplasmic reticulum, or ER, for proteins, and the Golgi apparatus for membrane assembly. A transit system between these two compartments also transports material to all other organelles and is called the secretory pathway.
The protein factory (the ER) is a maze of membranes physically connected to the outside of the nucleus. It sits near the nuclear pore, where messenger RNAs are sent from the nucleus to the ER to produce proteins. The other side of the ER is open to the rest of the cell. This arrangement allows a flow of material and signals between the nucleus and the rest of the cell through the cavernous membranes of the ER. It also allows the ER to filter what goes into the nucleus.
The protein factory is constantly building and rebuilding various membrane shapes, with particular sequences of molecules embedded in the membranes. These form subcompartments that serve as individual factories for particular molecules. Large molecules embedded in membranes include ribosomes that manufacture proteins, enzymes that modify proteins, and chaperone molecules that fold proteins. When proteins are completed, they are sent to the outer section of the ER, where sugars and fats are added as coatings for the protein, altering the molecule’s properties. Microbes influence the ER in a variety of ways to produce their own molecules.
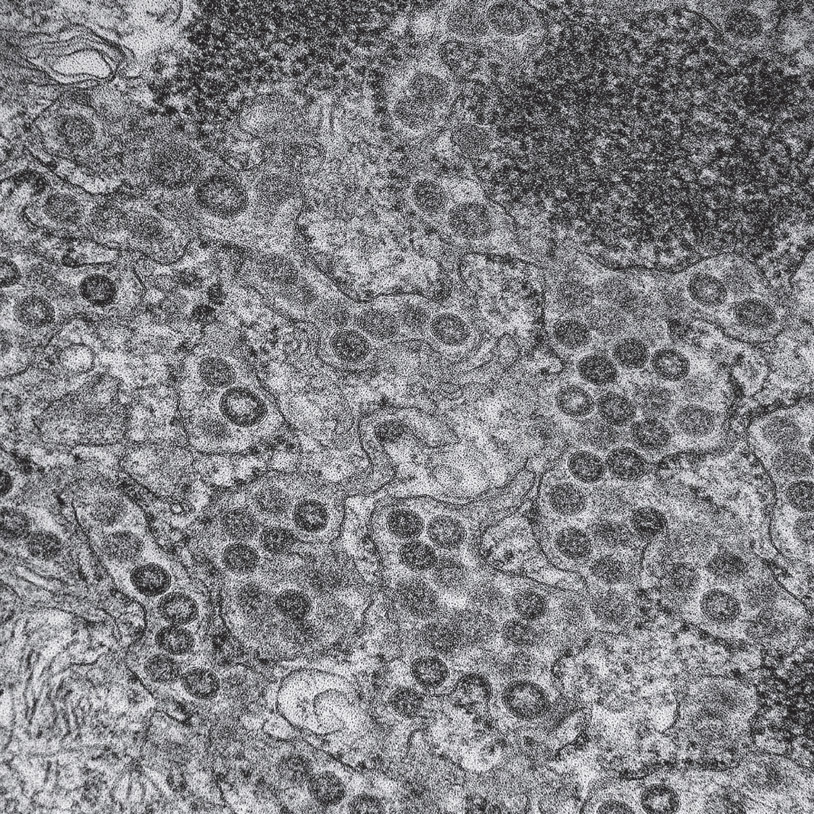
MERs viruses using the endoplasmic reticulum to produce their proteins. Electron micrograph. (NIAID/Wikimedia Commons)
The other associated organelle—the Golgi apparatus—is also a maze of membranes that organizes and transports lipids for use throughout the cell. It produces and transports all membranes for cell components, such as for mitochondria, ER, vesicles, and the outer-cell membrane. Lipid molecules are placed in sacs, tagged for exact destinations, and sent along transit pathways. Microbes target the Golgi when they need lipids and membranes.
INVADING PROTEIN FACTORIES
Protein factories are a logical place for invading viruses to produce their offspring. Upon entering the cell, viruses are transported to the ER, where they spend much of their lives. The ER’s vast channels covered by multiple membranes also serve as safe places to avoid detection. When a virus first arrives at the ER, human enzymes disassemble the virus. Viral proteins that are carried along with the disassembled virus are then placed on specific membranes of the ER.
Viruses are able to build their own special subcompartments of the ER for reproduction by using their own key proteins embedded in ER membranes. Several viruses stimulate unique membrane shapes for this purpose by altering scaffolding molecules. In these subcompartments, viruses utilize both human and viral proteins at the same time, when, for example, a human protein is used to cut the microbial protein into several pieces, which are then used for different purposes.
Viruses can produce a large protein that is broken into as many as ten smaller functional proteins by human enzymes. Also, specific microbial proteins can attract fatty molecules from the Golgi to the compartment, to build exactly shaped membranes covering the virus. They can also help build the sugar-coated proteins that stick out of the viral surface. In addition, shapes of viral proteins can be altered, enabling them to hide as they enter the nucleus.
Microbes can influence transport vesicles that travel in the secretory pathway between the two factories and all other cell components. This can allow a vesicle home to avoid being merged with the cell’s destruction factories. Multiple microbial signals can alter switches in the secretory pathway to reroute materials. Also, viruses can use the secretory pathway to travel throughout the cell. Bacterial signals to the Golgi can release fatty molecules that are then sent in the pathway directly to a vesicle home. Another vesicle in the pathway carries particular proteins from the ER to the vesicle home to help microbes reproduce.
MANIPULATING MITOCHONDRIA
Mitochondria were once independent microbes that, perhaps a billion years ago, set up shop in larger cells with a nucleus. A deal was set for mitochondria to live a protected life inside the larger cell in exchange for producing energy for the cell. Mitochondria travel throughout the cell, providing energy wherever it is needed, in conjunction with frequent communication with the ER. Conversations determine fulfillment of specific energy needs in precise locations. They also regulate other cell functions such as planned cell suicide. Signaling between mitochondria and the ER, as well as other organelles, is discussed in chapter twenty-four.
Manipulating mitochondria, which also participate in protein folding, is a vital part of the lifestyle of both bacteria and viruses. Bacteria can send their own unfolded proteins to mitochondria, where they are taken in and folded by mitochondrial enzymes. These folded microbial proteins then alter mitochondrial function. Other mitochondrial enzymes then cut their newly folded protein molecules into pieces and send each fragment to various subregions in the labyrinth of mitochondrial membranes. Situated in particular subcompartment factories in the mitochondria, the pieces are then able to alter cellular functions to benefit the microbe.
Microbial signals can change the ability of mitochondria to produce energy and carry out other vital cellular functions. Bacterial and viral signals can cut holes in mitochondria membranes, killing them. Other bacterial signals alter calcium metabolism to blunt the cell’s responses to viruses. Microbial signals to mitochondria can trigger the cell’s programmed death pathway. The suicide pathway is normally used when there isn’t enough energy for the cell to survive or the cell is severely infected with microbes. Microbial toxins can initiate cellular suicide when there is enough energy. Or microbes can stop cell suicide when it should be triggered to keep a sick cell alive, allowing microbes to continue using the cell’s machinery for as long as possible.
VESICLES THAT CARRY INFORMATION MOLECULES
Vesicles filled with information molecules are a major way that tissue cells, capillaries, and cancer cells send signals to other cells. Organelles also produce various vesicles to carry signals and regulate these vesicles with energy particles. Such sacs can be formed inside the cell or by using material from the cell’s outer membrane. Vesicles from organelles have a wide range of sizes and carry diverse molecules.
There are increasing examples of microbes using information sacs as signals. For instance, a strain of deadly fungus is able to attack ordinarily healthy humans because of communication with their own information sacs. A common strain of this fungus only infects those with poor immunity. This new strain is able to attack otherwise healthy people using increased coordination of fungal activity throughout the body with signals in these information sacs.
Vesicles that house particular molecules are in some ways similar to viruses with covering membranes. Because both are produced with the same cellular mechanisms related to varied production of membranes for the cell, viruses can co-opt pathways that produce sacs for travel inside and outside the cell. Viruses are not only able to escape from the cell in vesicles; they can also alter messages sent in vesicles or even send their own messages. Vesicles with proteins, RNAs, or even an entire virus are sent to nearby cells, altering the receiving cell’s behavior, including immune responses. Viruses causing long-term infections are able to alter the content of vesicles to promote the infection.
Vesicles can carry viral information, or the virus itself, during active infection or even when the virus is quietly living in the cell. Vesicles allow transfer of the infection from cell to cell, without using direct cell contact or nanotubes between cells. Vesicles containing viruses traveling in the blood and lymph are mostly received by lining cells in distant regions and organs. The vesicles’ role for viruses is similar to that of exosome sacs used for cancer metastasis, in that they alter distant regions to prepare for a spread of infection. This information primes the immune system in the new region to accept the virus. Messages also promote leaks in lining cells and particular matrixes in the new site.
TARGETING SCAFFOLDING PROTEIN MOLECULES
A typical cell produces and organizes constantly, changing LEGO-like scaffolding molecules. With a network of regulation, scaffolding molecules are able to build and rebuild all cellular structures powered by energy particles. Three basic types of scaffolding molecules and their regulation are discussed in chapter twenty-six. The largest scaffold molecules—called microtubules—provide transportation highways throughout cells. Microbial manipulation of this transport process was already mentioned in relation to the herpes virus commandeering a motor for rapid transit along the axon into the brain. The smallest and most widely used scaffold molecule that is commonly manipulated by microbial signals, especially viruses, is a protein called actin.
Actin contributes to multiple cellular functions, including muscle contraction, vesicle and organelle movement, and cell signaling. In addition, actin scaffolds provide not only the shape of all cell compartments but also the structures within organelles, such as placement of DNA in the nucleus. Using attached energy particles, actin works with protein motors to move muscles. Actin forms a protective mesh just under the outer cellular membrane, which helps bring in specific molecules and avoid others. It links receptor molecules on the outer membrane surface to signaling pathways inside the cell. Actin provides multiple shape changes in special appendages used for cell movement. The cell’s ability to gobble up bacteria and debris is also based on changing actin structures.
Viruses manipulate actin to spread infections. Newly discovered nanotubes between cells are made from parallel actin filaments surrounded by a membrane. Viruses travel in these nanotubes, which can be as long as ten cell lengths, spreading infection to other cells. Wider tubes between cells for transport of larger molecules can be built with actin plus microtubules.
Viruses stimulate a wide range of actin manipulations. To alter construction, the microbes place tags, such as phosphates, on the enzymes that produce scaffolds. For example, viruses stimulate actin-based changes in cell shapes to enable virus entry. Cells become more rounded or produce more invaginations, protrusions, or spikes on the cell surface. Motor proteins working with actin can be inhibited by other viral tags. Stimulated by HIV, actin alterations can increase cell division and movement. Viral signals can alter the mesh under cell membranes, making it more rigid, for example, or allowing more contact with other cells. These alterations can lead to cancer. In addition, viruses are able to use their own proteins to manipulate actin within a cell even when the virus is still outside the cell.
A viral sugar-coated protein from outside the cell reaches through the membrane and attaches to scaffold below the membrane. This stimulates proteins beneath the membrane to remodel the actin matrix, allowing entry of the virus. The cell’s motors then pull the virus into the cell along actin filaments. Grabbing the virus, actin filaments can even pull the virus sideways on the outside of the membrane to find a better entry point with altered actin mesh below.
After entry, actin filaments and motors help the virus in multiple ways. They facilitate travel to the ER. Actin helps several viruses enter the nucleus through the nuclear pore. When exiting the cell, viruses manipulate actin to hold the virus while the membrane is wrapped around it for travel outside the cell, without disrupting the outer cellular membrane. Another interaction occurs when actin alterations allow vesicles with viruses inside to fuse with the cell membrane. Cell proteins direct the virus to locations in the membrane that are normally hidden, allowing easier fusion of the vesicle and membrane.
The next section continues the discussion about organelles and shows how these major cellular compartments signal to each other, just like cells do.