DENDRITES ARE BRANCHED EXTENSIONS in neurons that receive signals from other neurons at synapses. They bud on the neuronal cell body in the opposite direction from the axon. Dendrites synthesize multiple incoming messages. Signals from dendrites determine an axon signal, which, in turn, triggers neurotransmitters at the terminal synapse. The resulting message is received by dendrites in the next cell of the neural circuit.
Dendrites also respond to the changing needs of neuronal networks by rapidly growing new branches. These branching arbors consist of many small subcompartments with diverse chemical and electrical properties. It is the elaborate back-and-forth communication among these varied compartments that produces the dendritic signal to the axon.
Until recently, dendrites have been considered passive calculators of multiple inputs. But now it’s been shown that thousands of dendritic compartments talk with each other to determine what signal will be sent along the axon to trigger the next neuron in a circuit. Conversations have even been found inside individual dendrites, among spines and other subcompartments, where each compartment produces its own electrical signals that interact and influence the final decision for the entire dendritic complex. Spines are described in detail below; they are protrusions from the dendrite that most of the time receive the signals from other neurons. Computation of inputs can occur with one spine, multiple nearby spines, several distant spines, or across the entire dendritic arbor. Research has only scratched the surface as to how decisions are made among these many dendrite subcompartments.
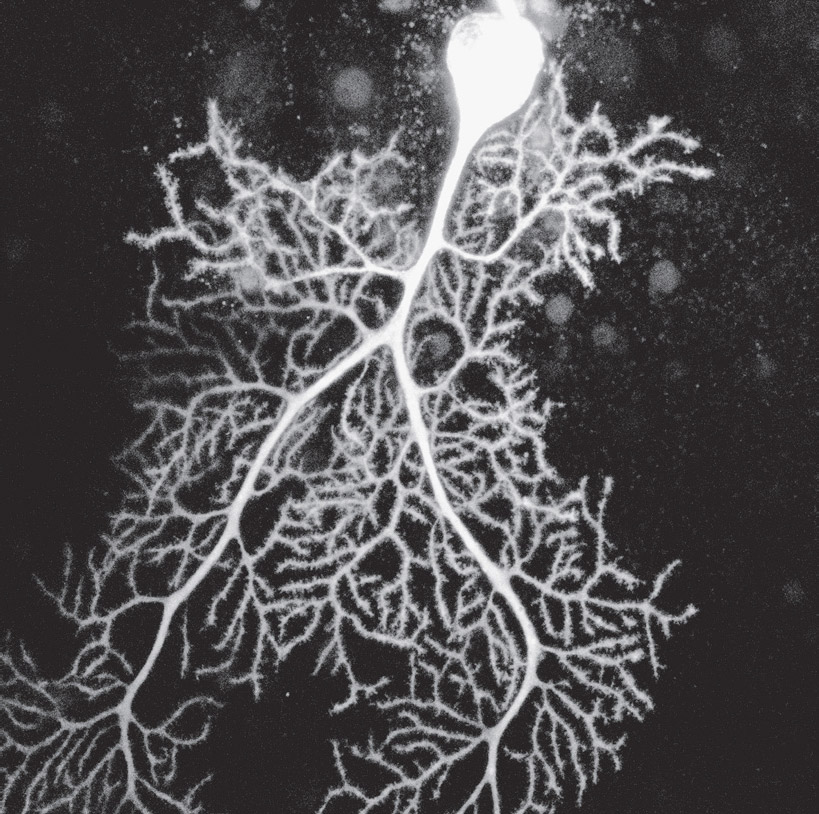
An elaborate dendrite arbor on a neuron in the cerebellum. Confocal light micrograph. (Dana Simmons/Science Source)
THE THREE REGIONS OF THE DENDRITE
On their large branches, dendrites generate a great number of small protrusions. Some protrusions become mushroom-shaped structures—called spines—that attract axons from other neurons to establish contact. One neuron can have up to a hundred thousand spinal connections with other neurons. Spines have a narrow neck, which is a channel extending at right angles from the dendrite shaft. The neck leads to the bulbous head, which has multiple receptors in its membranes. Heads also have elaborate signal-receiving complexes.
These three different regions of the dendrite—large dendrite shafts, thin spine necks sprouting from the shaft, and spine heads on top of necks—can have different molecular and electrical compositions. Also, multiple spines can combine to form compartments, each with diverse ion channels and electrical properties.
Variations of size and shape for spine necks and heads enable diverse chemical and electrical environments in dendrite subcompartments. The neck is quite tiny—a tenth the size of a small bacterium. Neck shapes have been called “tall,” “short,” “thick,” or “thin.” Spine heads have been called “thin,” “stubby,” “mushroom-like,” and “branched.” But there is a continuum of shapes for both. Shapes are altered by environment, seasonal change, age, estrogen, and stress. The mushroom-shaped head appears to be the most stable. Particular shapes of these isolated compartments allow segregation and storage of different types of signals in spine heads.
Types of cargo, such as various proteins and other molecules, brought into large dendrite shafts on microtubule tracks are sorted for various destinations, as they are in axons. Even though the cell nucleus is thousands of times larger than a spine, signals from a single spine can rapidly stimulate production of special proteins in the cell nucleus.
These proteins are transported to the particular spine from the cell body on microtubule tracks with destination tags. As the proteins travel along the dendrite shaft, they are grabbed by the appropriate spine. These specially recruited proteins with particular properties can sometimes benefit nearby spines as well.
Even more rapid production of proteins used in spines can occur from locally placed ribosomes in the shaft. These proteins are then actively transported into spines. Various electrical properties of individual spines affect how easily they will receive such molecules. For example, short, wide necks are optimal for accepting protein transported from the shaft.
NEUROPLASTIC INFLUENCES
Alterations of neuronal connections based on learning—collectively called neuroplasticity—influence dendritic spines in multiple ways. This includes alterations of spinal shapes and spatial arrangements of multiple nearby spines. Various types of neuroplasticity trigger particular motors and material transport to change microtubule and actin scaffolding in these spines.
Neuroplasticity can alter one spine, multiple spines, the entire dendrite, the entire neuron, or multiple neurons in wide circuits across the brain. Most often, spinal changes occur rapidly, with alterations of actin structures, which are then later built more permanently using specially manufactured proteins for a lasting neuroplasticity effect.
Enriched environments provided for animals in experiments, such as increased opportunity for a mouse to exercise, have shown how neuroplasticity works. In these cases, the number of spines rapidly increases. These increases can be stimulated by manufacturing more receptors. As these receptors are transported along the dendrite shaft, new spines sprout and grow actin scaffolds to enable the receptors to enter.
Actin scaffolds also recycle these receptor molecules after use. Some of these spines can become quite large and also incorporate microtubule structures. Neuroplasticity can alter the spine neck so that increased electrical resistance affords protection of specific information in the spine head from interacting with signals in nearby spines. Particular proteins are also produced that maintain these spine modifications as long-lasting neuroplasticity effects.
There are other complex effects of neuroplasticity that are not well understood. Spine necks can shelter heads with diverse shapes that restrict flow of electric current in and out. The longer and wider the neck, the greater the electrical separation from other spines. In some cases, neuroplasticity can alter the neck, making it forty times larger. In addition, it can also amplify the strength of the signal by fortyfold. When a signal becomes stronger, the neck’s resistance to electrical changes becomes greater. Neuroplasticity can also decrease the resistance, producing other changes in the spine head.
The elaborate protein complexes that receive signals in each spine are also altered during the neuroplastic process. Receiving complexes take up to 10 percent of a spine’s total area and are varied in each brain region and each type of neuron. Receiving complexes are built with a thousand interlocking proteins surrounded by a dense matrix, which connects to the membrane and holds everything in place. To build these protein complexes, proteins must be transported into the spine—first on microtubule tracks and then via actin scaffolds.
Recent research shows that spines might also manufacture some of their own proteins for these elaborate structures. Advanced high-powered imaging devices have enabled researchers to discover small ribosomes in the spines, giving them the possibility of manufacturing their own proteins. Alterations of these receiving platforms by neuroplasticity are just now being deciphered, but several involve substituting alternate proteins in and out of these protein complexes.
PRODUCING THE SIGNAL THAT FINALLY LEAVES THE AXON
To produce the signal that finally leaves the neuron, at least two major interacting factors are at play, each with many variations—dendrites sending their own electrical signals toward the axon, and the axon initial segment sending electrical current backward to interact with dendritic signals. Only recently have these retrograde signals backward from the initial segment been shown to be important for the outcome.
In addition to constantly sprouting multiple tiny spines in all regions, dendrites rapidly alter their shape by producing new large branches. Signals from the dendrite to the axon arise from various combinations of spines and larger dendrite branches, each with multiple shapes and geometrical formations that change rapidly. Spines can grow suddenly or vanish, with 20 percent appearing or disappearing in hours. A strong signal to one spine can immediately cause others to sprout around it, with each connecting to a different incoming axon.
Large shafts and small spines are built in different ways. To build new large dendritic branches, microtubules grow into dendritic shafts, bringing motors and cargo. Microtubules produce the primary structure of the large shafts, but in much smaller spines, it’s mostly actin scaffolding. Actin-associated motors regulate all action in the spines. Actin is very active in spines—building and rebuilding structures, including rings that hold the neck’s shape, similar to rings in the axon. An anchor at the base of the spine neck attaches to the shaft to keep out random molecules.
COMPONENTS THAT INFLUENCE DENDRITIC COMPUTATION
Somehow, dendrites trigger specific messages after receiving thousands of simultaneous signals. Many variables have been found that affect this computation process—spine shapes, geometrical arrangements of spine groups, and active and passive electrical properties. Recently, a wide range of electrical signals circulated among dendrites has been found to be important for the outcome. Also, retrograde signals from the initial axon segment mingle with various dendritic signals to alter the final output.
To further complicate the process, synapses on spines can be twenty-six different sizes—the largest sixty times the size of the smallest—and each one can affect the outcome in different ways. Also, each of the thousand different types of neurons can have variable dendritic characteristics.
The most recent research shows that computations can include a single spine, multiple spines, particular local geometrical configurations of spines, or even widely separated spines. One type of computation is the simple addition of multiple signals that all strike a particular dendrite region, but these have to be well synchronized. Two signals can also add to each other in a simple way by hitting different regions at the same moment, including on multiple large branches. Addition of the effects of various signals can also occur, with signals striking thousands of locations on the tree at the same time.
Computations by addition can take into account where signals hit. For instance, strikes closer to the cell body can magnify others. Another factor determining the computation is that strong signals can alter the entire dendrite arbor at once. But at the same time, even one small dendritic signal can have a dramatic effect on the final axon spike.
As well as addition, competition can occur among incoming signals. Several spines can be struck in one region of the dendrite and compete with another large signal in a different region. Particular signals can block others. More complex interactions can include addition of several inputs and inhibition of others—early signals from a distant branch and a large hit near the cell body.
Ion Channels
In dendrites, ion channels produce various electrical currents that also affect the outcome. Ion channels are large proteins sitting across membranes, where molecules with particular electrical properties are allowed to pass. Each type of channel has multiple subtypes with diverse spatial arrangements that respond in various ways to reverse axon signals. For example, more than a hundred unique potassium channels have been identified in neurons, each with varied functions.
Dendrites in memory centers can have multiple potassium channel types at the same time, each based on how distant they are from the cell body. Sodium and calcium channels also have multiple variations, with particular sodium channels present in some brain regions and not others. Moreover, particular neurotransmitters associated with learning can alter ion channels and the computations based on them.
Dendritic Spikes
Historically, without detailed knowledge of spines, scientists assumed the computation process to be a passive linear summation of inputs. Later, varied signals from spines, called dendrite spikes, were found, with research appearing to be contradictory. It was eventually demonstrated that the findings were not inconsistent. Rather, a wide range of dendrite spike signals occur in different circumstances, based on communication among individual spines, groups of spines, regions of branches, and the entire dendrite system. These spikes interact and produce different computations.
Studies of neuroplasticity in animals are still contradictory. Some conclusions emphasize the prominence of dendrite spikes. Others emphasize interactions of backward axon signals, but only if they are timed pulses. Particular temporal patterns of spikes appear to be necessary for some learning and not others. Some computations require dozens of hits at the same millisecond in small geometrical regions. These inputs must be in sync in exact locations. Also, other cellular modulating pathways can alter this situation, requiring fewer strikes.
Details about the influence of backward signals from the initial axon segment on the final result were also slow to emerge. Various brain regions, such as those for different types of memory, have distinct interactions among dendrite spikes and retrograde axon electrical signals. Some dendritic signals add to the axon signal and others subtract. When axon signals are strong, dendritic signals might have greater influence on the axon signal. Also, signals can start from one dendrite and then be altered by other spines when mingling with the axon signal.
Various types of signal integration can operate in specific brain regions. Linear addition of dendrite spikes often occurs with vision, sound, and touch neurons. But touch related to social behavior is more complex when neuroplasticity alterations from learning are occurring. With motor learning of tasks, such as bicycling, inputs from far apart on the dendrite arbor join to produce combined dendrite spikes. Another variation is in memory regions, where the columnar architecture allows one strong dendritic signal to have a strong effect on others.
Considerable research will be needed to figure out exactly how output is determined in dendrites and how they correlate with mental events. This is complicated in that most mental events involve activity in multiple brain circuits across the entire brain, simultaneously. Each region has large numbers of dendrites operating independently and in sync. One moment a particular spine is triggered, and a millisecond later, it’s a totally different one on the same dendrite. Much of the brain’s mystery resides in this process. It is surprising that we understand as much as we do!