
In Chapter 4, we discussed some of the more important organic molecules. But what makes these molecules so important? Glucose, starch, and fat are all energy-rich. However, the energy is packed in chemical bonds holding molecules together. To carry out the processes necessary for life, cells must find a way to release the energy in these bonds when they need it and store it away when they don’t. The study of how cells accomplish this is called bioenergetics. Generally, bioenergetics is the study of how energy from the sun is transformed into energy in living things.
During chemical reactions, such bonds are either broken or formed. This process involves energy, no matter which direction we go. Every chemical reaction involves a change in energy.
We’ve all heard the expression “nothing in life is free.” The same holds true in nature.
Energy cannot be created or destroyed. In other words, the sum of energy in the universe is constant.
This rule is called the First Law of Thermodynamics. As a result, the cell cannot take energy out of thin air. Rather, it must harvest it from somewhere.
The Second Law of Thermodynamics states that energy transfer leads to less organization. That means the universe tends toward disorder (entropy).
Exergonic reactions are those in which the products have less energy than the reactants. Simply put, energy is given off during the reaction.
Let’s look at an example. The course of a reaction can be represented by an energy diagram. Here’s an energy diagram for an exergonic reaction.

You’ll notice that energy is represented along the y-axis. Based on the diagram, we see that our reaction released energy. An example of an exergonic reaction is the oxidizing of molecules in mitochondria of cells, which then releases the energy stored in the chemical bonds.
Reactions that require an input of energy are called endergonic reactions. You’ll notice that the products have more energy than the reactants. An example is plants’ use of carbon dioxide and water to form sugars.

Even though exergonic reactions release energy, the reaction might not occur naturally without a little bit of energy to get things going. This is because the reactants must first turn into a high energy molecule, called the transition state, before turning into the products. The transition state is sort of a reactants-products hybrid state that is difficult to achieve. In order to reach this transition state, a certain amount of energy is required. This is called the activation energy.
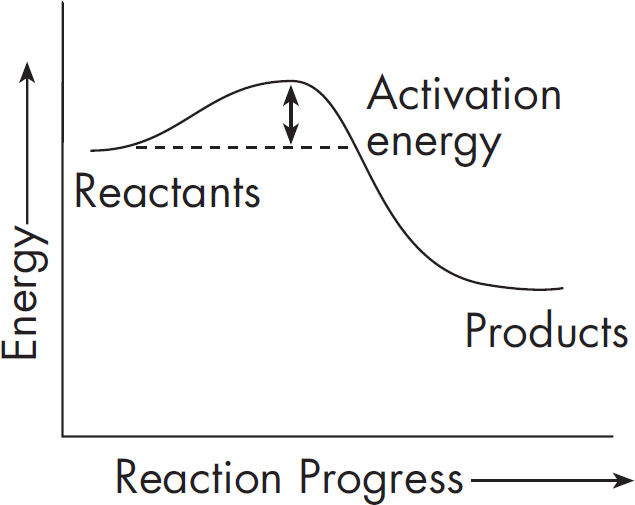
You’ll notice that we needed a little energy to get us going. That’s because chemical bonds must be broken before new bonds can form. This energy barrier—the hump in the graph—is called the activation energy. Once a set of reactants has reached its activation energy, the rest of the reaction is all downhill. Reaching the transition state is the tough part.
A catalyst is something that speeds something up. Enzymes are biological catalysts that speed up reactions. They accomplish this by lowering the activation energy and helping the transition state to form. Thus, the reaction can occur more quickly because the tricky transition state is not as much of a hurdle to overcome. Enzymes do NOT change the energy of the starting point or the ending point of the reaction. They only lower the activation energy.
Most of the crucial reactions that occur in the cell require enzymes. Yet enzymes themselves are highly specific—in fact, each enzyme catalyzes only one kind of reaction. This is known as enzyme specificity. Because of this, enzymes are usually named after the molecules they target. In enzymatic reactions, the targeted molecules are known as substrates. For example, maltose, a disaccharide, can be broken down into two glucose molecules. Our substrate, maltose, gives its name to the enzyme that catalyzes this reaction: maltase.
Many enzymes are named simply by replacing the suffix of the substrate with –ase. Using this nomenclature, maltose becomes maltase.
Enzymes have a unique way of helping reactions along. As we just saw, the reactants in an enzyme-assisted reaction are known as substrates. During a reaction, the enzyme’s job is to bring the transition state about by helping the substrate(s) get into position. It accomplishes this through a special region on the enzyme known as an active site.
The enzyme temporarily binds one or more of the substrates to its active site and forms an enzyme-substrate complex. Let’s take a look:

Once the reaction has occurred and the product is formed, the enzyme is released from the complex and restored to its original state. Now the enzyme is free to react again with another bunch of substrates.
By binding and releasing substrates over and over again, the enzyme speeds the reaction along, enabling the cell to release much-needed energy from various molecules. Here is a quick review on the function of enzymes.
Enzymes Do:
increase the rate of a reaction by lowering the reaction’s activation energy
form temporary enzyme-substrate complexes
remain unaffected by the reaction
Enzymes Don’t:
change the reaction
make reactions occur that would otherwise not occur at all
However, scientists have discovered that enzymes and substrates don’t fit together quite so seamlessly. It appears that the enzyme has to change its shape slightly to accommodate the shape of the substrates. This is called induced-fit. Sometimes certain factors are involved in activating the enzyme and making it capable of binding the substrate. Because the fit between the enzyme and the substrate must be perfect, enzymes operate only under a strict set of biological conditions.
Enzymes sometimes need a little help in catalyzing a reaction. Those factors are known as cofactors. Cofactors can be either organic molecules called coenzymes or inorganic molecules or ions. Inorganic cofactors are usually metal ions (Fe2+, Mg2+). Vitamins are examples of organic coenzymes. Your daily dose of vitamins is important for just this reason: vitamins are active and necessary participants in crucial chemical reactions.
Enzymatic reactions can be influenced by a number of factors, such as temperature and pH. The concentrations of enzyme and substrate will also determine the speed of the reaction.
The rate of a reaction increases with increasing temperature, up to a point, because an increase in the temperature of a reaction increases the chance of collisions among the molecules. But too much heat can damage an enzyme. If a reaction is conducted at an excessively high temperature (above 42°C), the enzyme loses its three-dimensional shape and becomes inactivated. Enzymes damaged by heat and deprived of their ability to catalyze reactions are said to be denatured.
Here’s one thing to remember: all enzymes operate at an ideal temperature. For most human enzymes, this temperature is body temperature, 37°C.
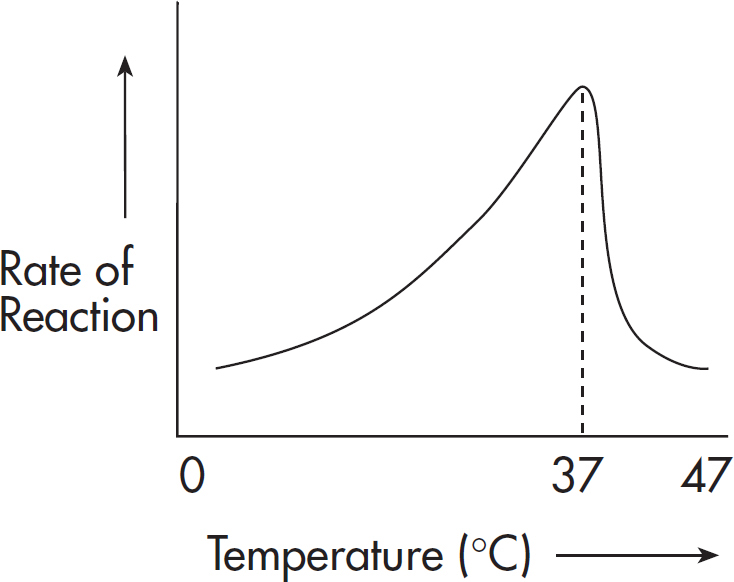
Not all organisms have a constant body temperature. For example, ectotherms depend on the environment to control their varying body temperatures. Q10 is a measure of temperature sensitivity of a physiological process or enzymatic reaction rate. The equation for Q10 is included on the AP Biology Equations and Formulas sheet.

The temperature unit must be in either Celsius or Kelvin, and the same unit must be used for T1 and T2. The two reaction rates (k1 and k2) must also have the same unit. Q10 has no unit; it is the factor by which the rate of a reaction increases due to a temperature increase. The more temperature-dependent a reaction is, the higher Q10 will be. Reactions with Q10 = 1 are temperature independent.
Enzymes also function best at a particular pH. At an incorrect pH, the hydrogen bonds can be disrupted and the structure of the enzyme can be altered. For most enzymes, the optimal pH is at or near a pH of 7.

Other enzymes operate at a low pH. For instance, pepsin, the digestive enzyme found in the stomach, is most effective at an extremely acidic pH of 2. The enzymes in the lysosome also function best in an acidic pH.
We know that enzymes control the rates of chemical reactions. But what regulates the activity of enzymes? It turns out that a cell can control enzymatic activity by regulating the conditions that influence the shape of the enzyme. Enzymes can be turned on/off by things that bind to them. Sometimes these things can bind at the active site, and sometimes they bind at other sites, called allosteric sites.
If the substance has a shape that fits the active site of an enzyme (i.e., similar to the substrate or the transition state), it can compete with the substrate and block the substrate from getting into the active site. This is called competitive inhibition. If there was enough substrate available, the substrate would out-compete the inhibitor and the reaction would occur. You can always identify a competitive inhibitor based on what happens when you flood the system with lots of substrate.
If the inhibitor binds to an allosteric site, it is an allosteric inhibitor and it is noncompetitive inhibition. A noncompetitive inhibitor generally distorts the enzyme shape so that it cannot function. The substrate can still bind at the active site, but the enzyme will not be able to catalyze the reaction.
As we just saw, almost everything an organism does requires energy. How, then, can the cell acquire the energy it needs without becoming a major mess? Fortunately, it’s through adenosine triphosphate (ATP).
ATP, as the name indicates, consists of a molecule of adenosine bonded to three phosphates. The great thing about ATP is that an enormous amount of energy is packed into those phosphate bonds.

When a cell needs energy, it takes one of these potential-packed molecules of ATP and splits off the third phosphate, forming adenosine diphosphate (ADP) and one loose phosphate (Pi), while releasing energy in the process.
ATP → ADP + Pi + energy
The energy released from this reaction can then be put to whatever use the cell pleases. Of course, this doesn’t mean that the cell is above the laws of thermodynamics. But within those constraints, ATP is the best source of energy the cell has available. It is relatively neat (only one bond needs to be broken to release that energy) and relatively easy to form. Organisms can use exergonic processes that increase energy, like breaking down ATP, to power endergonic reactions, like building organic macromolecules.
But where does all this ATP come from? It can be formed in several ways, but the bulk of it comes from a process called cellular respiration. Cellular respiration is a process of breaking down sugar and making ATP.
In autotrophs, the sugar is made during photosynthesis. In heterotrophs, glucose comes from the food we eat. We will start by going over photosynthesis, since in plants it is a precursor to cellular respiration.
Plants, algae, and cyanobacteria are producers. The earliest photosynthesis was conducted by prokaryotic cyanobacteria, and later eukaryotes developed photosynthetic abilities. All they do is bask in the sun, churning out the glucose necessary for life. In this section, we’ll focus on how plants conduct photosynthesis.
As we discussed earlier, photosynthesis is the process by which light energy is converted to chemical energy. Here’s an overview of photosynthesis.
6CO2 + 6H2O  C6H12O6 + 6O2
C6H12O6 + 6O2
You’ll notice from this equation that carbon dioxide and water are the raw materials used to manufacture simple sugars. But remember, there’s much more to photosynthesis than what’s shown in the simple reaction above.
There are two stages in photosynthesis: the light reactions (also called the light-dependent reactions) and the dark reactions (also called the light-independent reactions). The whole process begins when photons (energy units) of sunlight strike a leaf, activating chlorophyll and exciting electrons. The activated chlorophyll molecule then passes these excited electrons down to a series of electron carriers, ultimately producing ATP and NADPH. Both of these products, along with carbon dioxide, are then used in the dark reactions (light-independent) to make carbohydrates. Along the way, water is also split and oxygen gets released.
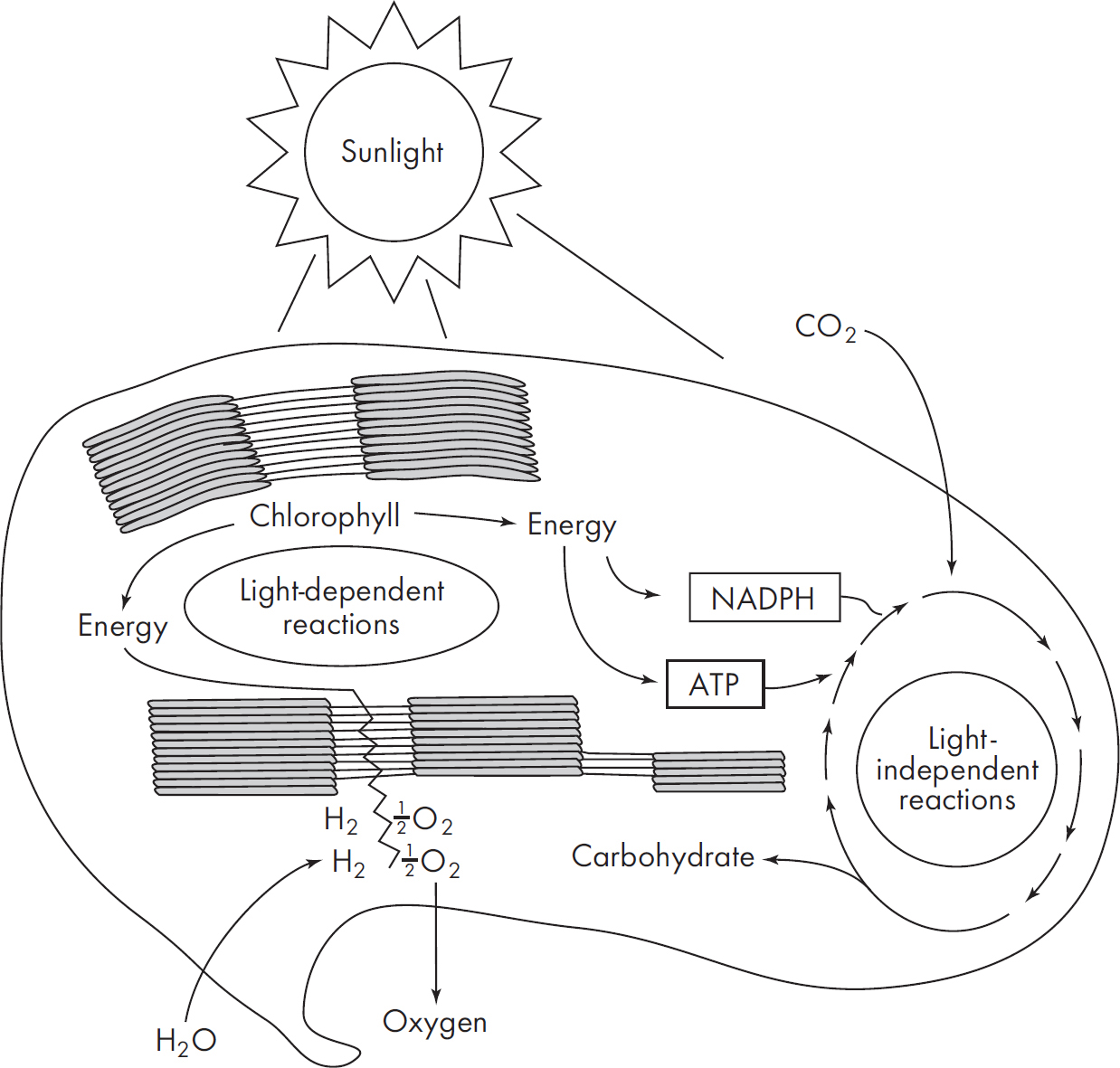
We’ll soon see that this beautifully orchestrated process occurs thanks to a whole host of special enzymes and pigments. But before we turn to the stages in photosynthesis, let’s talk about where photosynthesis occurs.
The leaves of plants contain lots of chloroplasts, which are the primary sites of photosynthesis.
Now let’s look at an individual chloroplast. If you split the membranes of a chloroplast, you’ll find a fluid-filled region called the stroma. Inside the stroma are structures that look like stacks of coins. These structures are the grana.
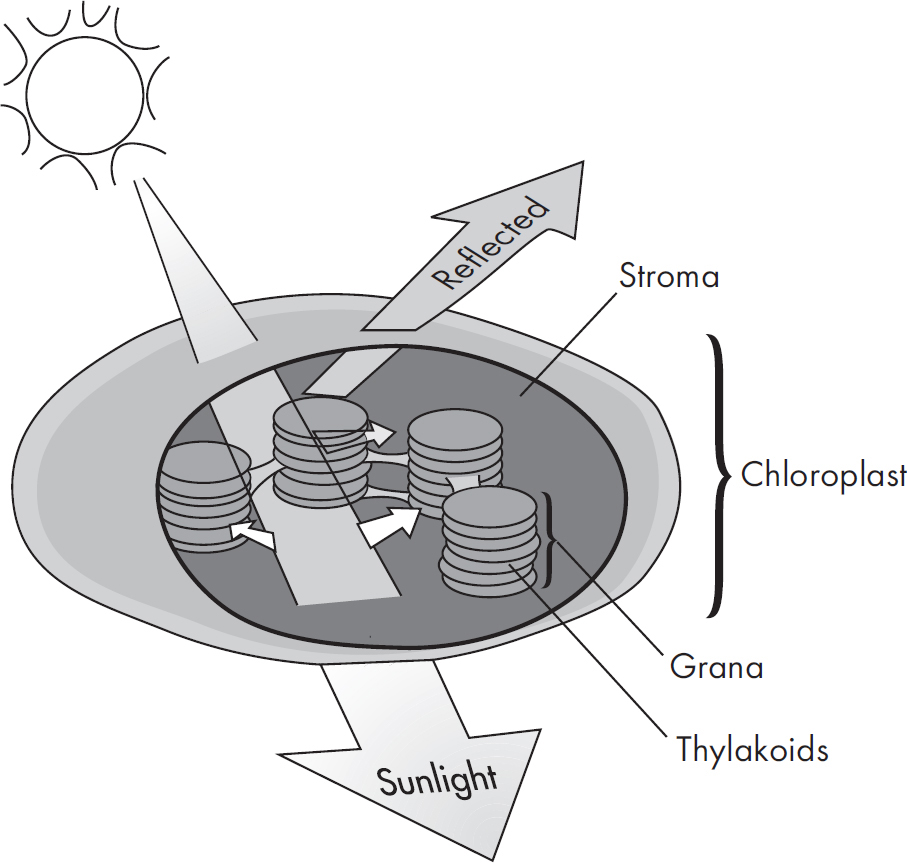
The many disk-like structures that make up grana are called thylakoids. They contain chlorophyll, a light-absorbing pigment that drives photosynthesis, as well as enzymes involved in the process. The very inside of a thylakoid is called the thylakoid lumen.
Many light-absorbing pigments participate in photosynthesis. Some of the more important ones are chlorophyll a, chlorophyll b, and carotenoids. These pigments are clustered in the thylakoid membrane into units called antenna complexes.
All of the pigments within a unit are able to “gather” light, but they’re not able to “excite” electrons. Only one special molecule—located in the reaction center—is capable of transforming light energy to chemical energy. In other words, the other pigments, called antenna pigments, “gather” light and “bounce” energy to the reaction center.

There are two types of reaction centers: photosystem I (PS I) and photosystem II (PS II). The principal difference between the two is that each reaction center has a specific type of chlorophyll—chlorophyll a—that absorbs a particular wavelength of light. For example, P680, the reaction center of photosystem II, has a maximum absorption at a wavelength of 680 nanometers. The reaction center for photosystem I—P700—best absorbs light at a wavelength of 700 nanometers.
When light energy is used to make ATP, it is called photophosphorylation. Autotrophs are using light (that’s photo) and ADP and phosphates (that’s phosphorylation) to produce ATP.
An absorption spectrum shows how well a certain pigment absorbs electromagnetic radiation. Light absorbed is plotted as a function of radiation wavelength. This spectrum is the opposite of an emission spectrum, which gives information on which wavelengths are emitted by a pigment. The absorption spectrum for chlorophyll a, chlorophyll b, and carotenoids is below. You can see that chlorophyll a and chlorophyll b absorb blue and red light quite well but do not absorb light in the green part of the spectrum. Light in the green range of wavelengths is reflected, and this is why chlorophyll and many plants are green. Carotenoids absorb light on the blue-green end of the spectrum, but not on the other end. This is why plants rich in carotenoids are yellow, orange, or red.

When a leaf captures sunlight, the energy is sent to P680, the reaction center for photosystem II. The activated electrons are trapped by P680 and passed to a molecule called the primary acceptor, and then they are passed down to carriers in the electron transport chain.
To replenish the electrons in the thylakoid, water is split into oxygen, hydrogen ions, and electrons. That process is called photolysis. The electrons from photolysis replace the missing electrons in photosystem II.
As the energized electrons from photosystem II travel down the electron transport chain, they pump hydrogen ions into the thylakoid lumen. A proton gradient is established. As the hydrogen ions move back into the stroma through ATP synthase, ATP is produced.

After the electrons leave photosystem II, they go to photosystem I. The electrons are passed through a second electron transport chain until they reach the final electron acceptor NADP+ to make NADPH.
The light reactions of photosynthesis occur in the thylakoid membranes.
Here is a summary of the most important points from the first part of photosynthesis:
P680 in photosystem II captures light and passes excited electrons down an electron transport chain to produce ATP.
P700 in photosystem I captures light and passes excited electrons down an electron transport chain to produce NADPH.
A molecule of water is split by sunlight, releasing electrons, hydrogen, and free O2.
The light-dependent reactions occur in the grana of chloroplasts, where the thylakoids are found. Remember: the light-absorbing pigments and enzymes for the light-dependent reactions are found within the thylakoids.
Most plants follow this electron flow, which is called linear, or noncyclic. However, some plants (such as C4 plants discussed below) perform cyclic electron flow instead. Cyclic photophosphorylation is similar to noncyclic, but generates only ATP, and no NADPH. It takes place only at photosystem I: once an electron is displaced from the photosystem, it is passed down electron acceptor molecules and returns to photosystem I, from where it was emitted.
Now let’s turn to the dark side. The dark reactions use the products of the light reactions—ATP and NADPH—to make sugar. We now have energy to make glucose, but what do plants use as their carbon source? CO2, of course. You’ve probably heard of the term carbon fixation. All this means is that CO2 from the air is converted into carbohydrates. This step occurs in the stroma of the leaf. The dark reactions are also called the Calvin-Benson Cycle (or just Calvin cycle).
Let’s summarize important facts about the dark reactions:
The Calvin cycle occurs in the stroma of chloroplasts.
ATP and NADPH from the light reactions are necessary for carbon fixation.
CO2 is fixed to form glucose.
The following table summarizes the stages of photosynthesis:

The light-dependent and light-independent reactions are inexorably linked; neither set of reactions alone can produce carbohydrates from CO2. The light-dependent reactions use water and light to produce ATP, NADPH, and O2; the light-independent reactions use CO2, ATP, and NADPH to produce ADP, NADPH+, and carbohydrates.
Stomata are pores on the leaf surface that allow CO2 to enter the leaf, and O2 and water to exit. Most plants close their stomata on hot, dry days, to prevent water loss by transpiration (the evaporative loss of water from leaves). While this conserves water, it also limits access to CO2 and thus reduces photosynthetic yield. With less CO2 available, O2 accumulates and plants start performing photorespiration instead of photosynthesis. Photorespiration is a wasteful process that uses ATP and O2, produces more CO2, and doesn’t produce any sugars.
Plants that live in hot climates have evolved two different ways around this: CAM plants deal with this problem by temporally separating carbon fixation and the Calvin cycle. They open their stomata at night and incorporate CO2 into organic acids. During the day, they close their stomata and release CO2 from the organic acids while the light reactions run. In contrast, C4 plants have slightly different leaf anatomy that allows them to perform CO2 fixation in a different part of the leaf from the rest of the Calvin cycle. This prevents photorespiration. C4 plants produce a four-carbon molecule as the first product of carbon fixation and perform cyclic electron flow in the light reactions (discussed above).
In the shorthand version, cellular respiration looks something like this.
C6H12O6+ 6O2 → 6CO2 + 6H2O + ATP
Notice that we’ve taken a sugar, perhaps a molecule of glucose, and combined it with oxygen to produce carbon dioxide, water, and energy in the form of our old friend, ATP. However, as you probably already know, the actual picture of what really happens is far more complicated.
Generally speaking, we can break cellular respiration down into two different approaches: aerobic respiration and anaerobic respiration. If ATP is made in the presence of oxygen, we call it aerobic respiration. If oxygen isn’t present, we call it anaerobic respiration. Let’s jump right in with aerobic respiration.
Aerobic respiration consists of four stages:
glycolysis
formation of acetyl-CoA
the Krebs (citric acid) cycle
oxidative phosphorylation (the electron transport chain + chemiosmosis)
In the first three stages, glucose is broken down and energy molecules are made. Some of these are ATP, and others are special electron carriers called NADH and FADH2. In the fourth stage, these electron carriers unload their electrons, and the energy is eventually used to make even more ATP.
The first stage begins with glycolysis, the splitting (-lysis) of glucose (glyco-). Glucose is a six-carbon molecule that is broken into two three-carbon molecules called pyruvic acid. This breakdown of glucose also results in the net production of two molecules of ATP.
Glucose + 2 ATP + 2NAD+ → 2 Pyruvic acid + 4 ATP + 2NADH
Although we’ve written glycolysis as if it were a single reaction, this process doesn’t occur in one step. In fact, it requires a sequence of enzyme-catalyzed reactions. Let’s look at a summary of these reactions:

If you take a good look at the reactions above, you’ll see two ATPs are needed to produce four ATP. You’ve probably heard the expression “You have to spend money to make money.” In biology, you have to invest ATP to make ATP: our investment of two ATP yielded four ATP, for a net gain of two. The ATP molecules generated in glycolysis are created from combining ADP and an inorganic phosphate with the help of an enzyme.
Glycolysis also creates two NADH, which result from the transfer of electrons to the carrier NAD+, which then becomes NADH. NAD+ and NADH are constantly being turned into each other as electrons are being carried and then unloaded.
There are four important tidbits to remember regarding glycolysis:
occurs in the cytoplasm
net of 2 ATPs produced
2 pyruvic acids formed
2 NADH produced
Pyruvic acid is transported to the mitochondrion. Each pyruvic acid (a three-carbon molecule) is converted to acetyl-coenzyme A (a two-carbon molecule, usually just called acetyl-CoA) and CO2 is released.
2 Pyruvic acid + 2 Coenzyme A + 2NAD+ → 2 Acetyl-CoA + 2CO2 + 2NADH
Are you keeping track of our carbons? We’ve now gone from two three-carbon molecules to two two-carbon molecules. The extra carbons leave the cell in the form of CO2. Once again, two molecules of NADH are also produced for each glucose you started with. This process of turning pyruvic acid into acetyl-CoA is catalyzed by an enzyme complex called the pyruvate dehydrogenase complex (PDC).
The next stage is the Krebs cycle, also known as the citric acid cycle. Each of the two acetyl-coenzyme A molecules will enter the Krebs cycle, one at a time, and all the carbons will ultimately be converted to CO2. This stage occurs in the matrix of the mitochondria.
The Krebs cycle begins with each molecule of acetyl-CoA produced from the second stage of aerobic respiration combining with oxaloacetate, a four-carbon molecule, to form a six-carbon molecule, citric acid or citrate (hence its name, the citric acid cycle).
Citrate gets turned into several other things, and because the cycle begins with a four-carbon molecule, oxaloacetate, it eventually gets turned back into oxaloacetate to maintain the cycle by joining with the next acetyl-CoA coming down the pipeline.

With each turn of the cycle, three types of energy are produced:
1 ATP
3 NADH
1 FADH2
To figure out the total number of products per molecule of glucose, we simply double the number of products—after all, we started off the Krebs cycle with two molecules of acetyl-CoA for each molecule of glucose!
Now we’re ready to tally up the number of ATP produced.
So far, we’ve made only four ATP—two ATP from glycolysis and two ATP from the Krebs cycle.
Although that seems like a lot of work for only four ATP, we have also produced hydrogen carriers in the form of 10 NADH and 2 FADH2. These molecules will, in turn, produce lots of ATP in the next stage of cellular respiration.
As electrons (and the hydrogen atoms to which they belong) are removed from a molecule of glucose, they carry with them much of the energy that was originally stored in their chemical bonds. These electrons—and their accompanying energy—are then transferred to readied hydrogen carrier molecules. In the case of cellular respiration, these charged carriers are NADH and FADH2.
Let’s see how many “loaded” electron carriers we’ve produced. We now have:
2 NADH molecules from glycolysis
2 NADH from the production of acetyl-CoA
6 NADH from the Krebs cycle
2 FADH2 from the Krebs cycle
That gives us a total of 12 electron or energy carriers altogether.
These electron carriers—NADH and FADH2—“shuttle” electrons to the electron transport chain, the resulting NAD+ and FADH can be recycled to be used as carriers again, and the hydrogen atoms are split into hydrogen ions and electrons.
H2 → 2H+ + 2e–
Then, two interesting things occur. First, the high-energy electrons from NADH and FADH2 are passed down a series of protein carrier molecules that are embedded in the cristae; thus, it is called the electron transport chain. Some of the carrier molecules in the electron transport chain are NADH dehydrogenase and cytochrome C.
Each carrier molecule hands down the electrons to the next molecule in the chain. The electrons travel down the electron transport chain until they reach the final electron acceptor, oxygen. Oxygen combines with these electrons (and some hydrogens) to form water. This explains the “aerobic” in aerobic respiration. If oxygen weren’t available to accept the electrons, they wouldn’t move down the chain at all, thereby shutting down the whole process of electron transport.
At the same time that electrons are being passed down the electron transport chain, another mechanism is at work. Remember those hydrogen ions (also called protons) that split off from the original hydrogen atom? The energy released from the electron transport chain is used to pump hydrogen ions across the inner mitochondrial membrane from the matrix into the intermembrane space. The pumping of hydrogen ions into the intermembrane space creates a pH gradient, or proton gradient. The hydrogen ions really want to diffuse back into the matrix. The potential energy established in this gradient is responsible for the production of ATP. This pumping of ions and diffusion of ions to create ATP is chemiosmosis.

These hydrogen ions can diffuse across the inner membrane only by passing through channels called ATP synthase. Meanwhile, ADP and Pi are on the other side of these protein channels. The flow of protons through these channels produces ATP by combining ADP and Pi on the matrix side of the channel. Overall, this process is called oxidative phosphorylation because when electrons are given up it is called “oxidation” and then ADP is “phosphorylated” to make ATP.
You’re also expected to know the following two things for the AP Biology Exam:
Every NADH from glycolysis yields 1.5 ATP and all other NADH molecules yield 2.5 ATP.
Every FADH2 yields 1.5 ATP.
You will also want to make sure you remember the major steps of cell respriation, and the outcome of each step:

The photosynthesis reactions share some similarities with cell respiration. In both cases, ATP production is driven by a proton gradient, and the proton gradient is created by an electron transport chain. In respiration, protons are pumped from the mitochondrial matrix to the intermembrane space, and they return to the matrix through an ATP synthase down their concentration gradient. In photosynthesis, protons are pumped from the stroma into the thylakoids compartment, and they return to the stroma through an ATP synthase down their concentration gradient.
The Krebs cycle and the Calvin cycle are both series of reactions that ultimately regenerate their starting product. Both cycles have an indirect need for a particular substance; although they do not use it directly, without it they cease to run. For the Krebs cycle, that substance is oxygen; for the Calvin cycle, that “substance” is light.
Furthermore, the goals of the two cycles are opposite; the Krebs cycle seeks to oxidize carbohydrates to CO2, while the Calvin cycle seeks to reduce CO2 to carbohydrates.
When oxygen is not available, the anaerobic version of respiration occurs. The electron transport chain stops working, and electron carriers have nowhere to drop their electrons. The mitochondrial production of acetyl-CoA and the Krebs cycle cease too.
Glycolysis, however, can continue to run. This means that glucose can be broken down to give net two ATP. Only two instead of 30!
Glycolysis also gives two pyruvates and two NADH. The pyruvate and NADH make a deal with each other, and pyruvate helps NADH get recycled back into NAD+ and takes its electrons. The pyruvate turns into either lactic acid (in muscles) or ethanol (in yeast). Since these two things are toxic at high concentrations, this process, called fermentation, is done only in emergencies. Aerobic respiration is a better option.

What types of organisms undergo fermentation?
Yeast cells and some bacteria make ethanol and carbon dioxide.
Other bacteria produce lactic acid.
Although human beings are aerobic organisms, they can actually carry out fermentation in their muscle cells. Have you ever had a leg cramp? If so, that cramp was possibly the consequence of anaerobic respiration.
When you exercise, your muscles require a lot of energy. To get this energy, they convert enormous amounts of glucose to ATP. But as you continue to exercise, your body doesn’t get enough oxygen to keep up with the demand in your muscles. This creates an oxygen debt. What do your muscle cells do? They switch over to anaerobic respiration. Pyruvic acid produced from glycolysis is converted to lactic acid. As a consequence, lactic acid causes your muscles to ache.
bioenergetics
First Law of Thermodynamics
Second Law of Thermodynamics
entropy
exergonic reaction
energy diagram
endergonic reaction
transition state
activation energy
enzymes
enzyme specificity
substrates
active site
enzyme-substrate complex
induced-fit
cofactors
coenzymes
denatured
Q10
allosteric sites
competitive inhibition
allosteric inhibitor
noncompetitive inhibition
cellular respiration
photosynthesis
light reactions
dark reactions
photons
stroma
grana
thylakoids
chlorophyll a and b
carotenoids
reaction center
antenna pigments
photosystem I (PS I)
photosystem II (PS II)
P680
P700
photophosphorylation
absorption spectrum
emission spectrum
photolysis
NADPH
carbon fixation
Calvin-Benson Cycle
photorespiration
CAM plants
C4 plants
aerobic respiration
anaerobic respiration
NADH
FADH2
glycolysis
pyruvic acid
acetyl-coenzyme A (acetyl-CoA)
pyruvate dehydrogenase complex
Krebs cycle (citric acid cycle)
matrix
oxaloacetate
citric acid
electron transport chain
cytochrome C
pH gradient (proton gradient)
chemiosmosis
ATP synthase
oxidative phosphorylation
lactic acid
ethanol (ethyl alcohol)
fermentation
Energy cannot be created or destroyed. There are endergonic reactions and exergonic reactions. The change in Gibbs free energy indicates whether the reaction will require energy or release energy.
The study of how cells accomplish biological processes is called bioenergetics.
Chemical reactions are catalyzed by enzymes.
Enzymes lower the activation energy of chemical reactions by stabilizing the transition state.
Enzymes do not change the energy difference between reactants and products, but by lowering the activation energy, they facilitate chemical reactions to occur.
Enzymes are proteins that are highly specific for their substrate (or reactant, which binds at the active site).
Enzymes have an optimal, narrow range of temperature and pH in which they have the highest rate of reaction. Outside this range, they may undergo denaturation and, therefore, no longer be active.
Enzyme activity may also be regulated or altered by allosteric/noncompetitive inhibitors, competitive inhibitors, and activators.
Enzymes may require coenzymes or cofactors to help catalyze reactions.
ATP is the universal energy molecule in cells. It is created via cellular respiration.
Photosynthesis is the process by which plants use energy from sunlight to make sugar.
In the light-dependent reactions, sunlight is absorbed by chlorophyll, electrons are passed down a chain, and the energy molecules ATP and NADPH are produced. Water is also split into hydrogen and oxygen.
In the light-independent reactions, sugar is made in the Calvin-Benson Cycle (Calvin cycle) by using energy from ATP and NADPH, and the carbon from CO2.
Cellular respiration consumes oxygen and produces a lot of ATP energy for the cell, using NADH and FADH2 as electron carriers. There are four stages to cellular respiration:
glycolysis
formation of acetyl-CoA via the PDC
Krebs cycle
oxidative phosphorylation
Anaerobic respiration occurs when cells lack oxygen to act as a final electron acceptor. In this process, the pyruvates generated by glycolysis are broken down by fermentation to produce lactic acid or ethanol and NAD+, which permits glycolysis to continue and provide the 2 ATP it makes.
Answers and explanations can be found in Chapter 15.
1. The mitochondrion is a critical organelle structure involved in cellular respiration. Below is a simple schematic of the structure of a mitochondrion. Which of the structural components labeled below in the mitochondrion is the primary location of the Krebs cycle?
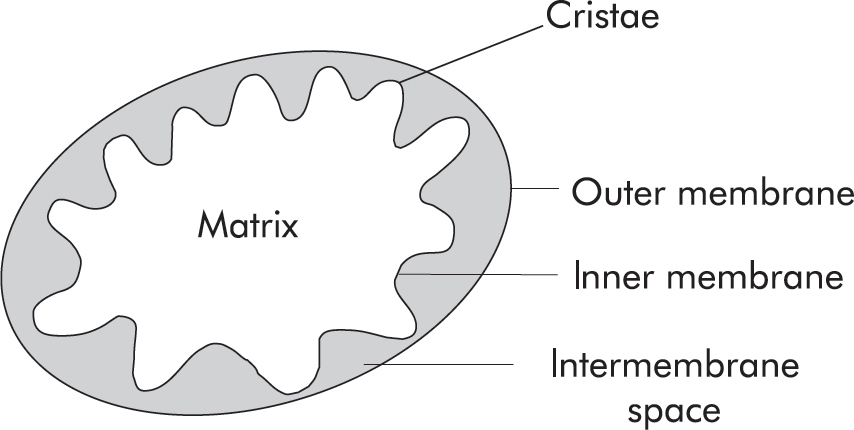
(A) Inner membrane
(B) Matrix
(C) Intermembrane space
(D) Outer membrane
2. Binding of Inhibitor Y as shown below inhibits a key catalytic enzyme by inducing a structural conformation change. Which of the following accurately describes the role of Inhibitor Y?

(A) Inhibitor Y competes with substrates for binding in the active site and functions as a competitive inhibitor.
(B) Inhibitor Y binds allosterically and functions as a competitive inhibitor.
(C) Inhibitor Y competes with substrates for binding in the active site and functions as a noncompetitive inhibitor.
(D) Inhibitor Y binds allosterically and functions as a noncompetitive inhibitor.
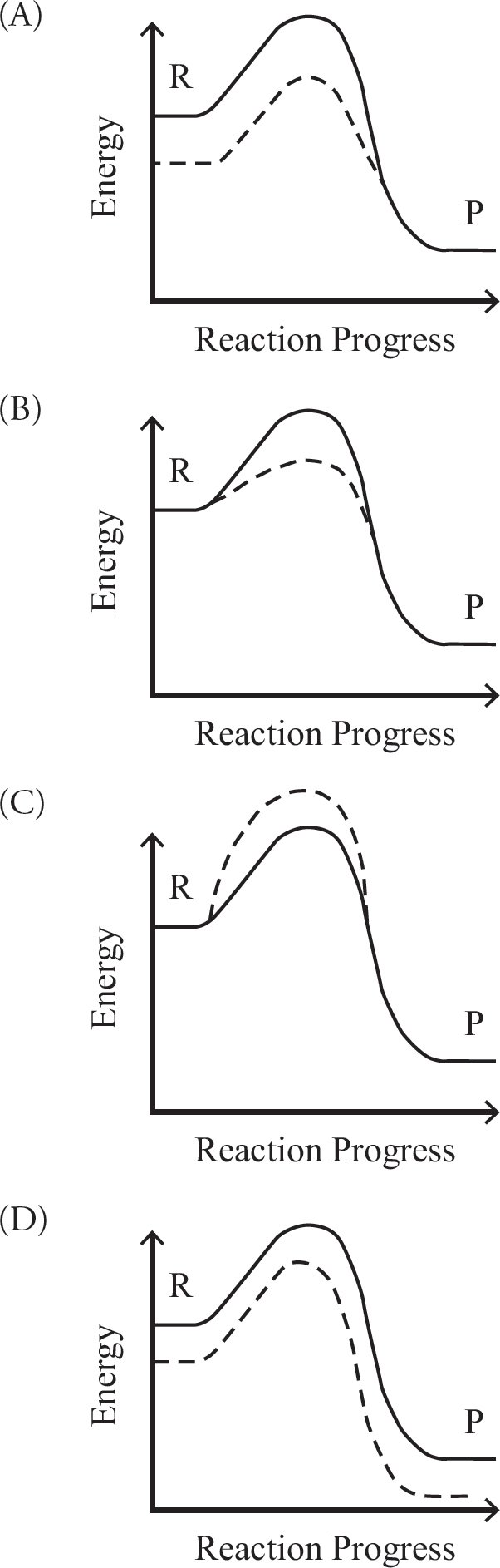
3. A single-step chemical reaction is catalyzed by the addition of an enzyme. Which of the reaction coordinate diagrams accurately shows the effect of the added enzyme (represented by the dashed line) to the reaction?
Questions 4–6 refer to the following diagram and paragraph.
Glycolysis (shown below) is a critical metabolic pathway that is utilized by nearly all forms of life. The process of glycolysis occurs in the cytoplasm of the cell and converts 1 molecule of glucose into 2 molecules of pyruvic acid.

4. How many net ATP would be generated directly from glycolysis from the breakdown of 2 glucose molecules?
(A) 2
(B) 4
(C) 8
(D) 12
5. Glycolysis does not require oxygen to occur in cells. However, under anaerobic conditions, glycolysis normally requires fermentation pathways to occur to continue to produce ATP. Which best describes why glycolysis is dependent on fermentation under anaerobic conditions?
(A) Glycolysis requires fermentation to produce more glucose as a substrate.
(B) Glycolysis requires fermentation to synthesize lactic acid and restore NADH to NAD+.
(C) Glycolysis requires fermentation to generate ATP molecules to complete the early steps of the pathway.
(D) Glycolysis requires fermentation to generate pyruvate for a later step in the pathway.
6. Which of the following most accurately describes the net reaction of glycolysis?
(A) It is an endergonic process because it results in a net increase in energy.
(B) It is an exergonic process because it results in a net increase in energy.
(C) It is an endergonic process because it results in a net decrease in energy.
(D) It is an exergonic process because it results in a net decrease in energy.
7. Taq polymerase, a DNA polymerase derived from thermophilic bacteria, is used in polymerase chain reactions (PCR) in the laboratory. During PCR, Taq catalyzes DNA polymerization, similar to how it would in bacteria. A normal PCR cycle is as follows:
|
1. Melting/Denaturing |
95°C |
|
2. Primer Annealing |
50°C |
|
3. Elongation of DNA (repeat 20–30 cycles) |
72°C |
Which of the following conditions likely describes the living environment of Taq bacteria?
(A) Freshwater with acidic pH
(B) Hydrothermal vents reaching temperatures between 70–75°C
(C) Hot springs of 40°C
(D) Tide pools with high salinity
8. The biggest difference between an enzyme-catalyzed reaction and an uncatalyzed reaction is that
(A) the free energy between the reactants and the products does not change
(B) the free energy difference between the reactants and the products does not change
(C) the catalyzed reaction would not occur without the enzyme
(D) a different amount of energy is required to reach the transition state of the reaction
9. Two groups of cells were grown under identical conditions. Mitochondria from each group were isolated and half of them were placed in a low pH (approximately pH 6.8) and the other half were placed in a neutral pH. Small molecules were allowed to diffuse across the outer membrane via facilitated diffusion. Both samples were exposed to oxygen bubbles through the growth media. What would you expect to see in terms of ATP production in the sample of cells placed in a low pH, with respect to the control population?
(A) ATP production decreases.
(B) ATP production increases.
(C) ATP production stays the same.
(D) ATP production ceases entirely.
10. The Second Law of Thermodynamics states that entropy, or disorder, is constantly increasing in the universe for spontaneous processes. Therefore, how is it possible that organisms exist in very ordered states?
(A) The Second Law of Thermodynamics does not apply to biological life.
(B) Energy can be created or destroyed.
(C) The catabolic reactions are always equal and opposite of the anabolic reactions.
(D) Biological life actually creates an increase in entropy through dissemination of heat and waste.
Respond to the following questions:
Which topics from this chapter do you feel you have mastered?
Which content topics from this chapter do you feel you need to study more before you can answer multiple-choice questions correctly?
Which content topics from this chapter do you feel you need to study more before you can effectively compose a free response?
Was there any content that you need to ask your teacher or another person about?