The diagnosis of Lyme disease is made based on a constellation of considerations, including the patient’s history of exposure to Ixodes ticks, time spent in Lyme endemic areas, clinical symptoms and signs, findings on the physical exam, and results from laboratory tests. Patients who present with the characteristic erythema migrans (EM) rash do not require testing; indeed, antibody tests for Lyme disease in the early weeks of infection are often negative.
The ideal diagnostic test for Lyme disease would clarify with 100 percent certainty whether the individual has been infected with B. burgdorferi and whether—after treatment—the infection has resolved. Such a test would aid diagnosis and help in treatment planning. Given that many of the symptoms associated with Lyme disease are common to other diseases as well, this test should also have no false positives; in other words, patients who do not have Lyme disease would not test positive on the Lyme disease test. While these goals for a test are simple to understand, they are impossible to achieve in actual practice. Even the best tests for other infections do not have 100 percent sensitivity and specificity. The aim therefore is for diagnostic tests that come closer to these ambitious ideals. With Lyme disease testing, there is much room for improvement.
This chapter reviews many of the tests—those that are now standard as well as new tests that are under development or newly released. This chapter also reviews some of the additional tests that doctors might order; these tests do not inform the physician about the presence or absence of infection, but they do provide information about bodily processes that may be consistent with a diagnosis of Lyme disease. For example, tests of the brain or peripheral nerves may provide objective evidence of neurologic dysfunction that may be seen among patients with current or prior B. burgdorferi infection.
The three types of blood tests most commonly used to check for Lyme disease are the indirect fluorescent antibody (IFA), the enzyme-linked immunosorbent assay (ELISA), and the Western blot. These blood tests do not detect the B. burgdorferi spirochete directly. They act indirectly instead. They detect antibodies present in the patient’s blood formed in response to antigens of the B. burgdorferi spirochete. Because the immune system has a long memory, once an antibody response develops, it tends to last for years, even in the absence of infection.
Because these tests measure antibodies and because the antibody response to B. burgdorferi requires time to develop (one to three weeks), most patients with a solitary Lyme disease rash will test negative. An additional problem with these antibody-based tests is that they do not necessarily tell us whether the infection is currently present. A positive antibody test simply indicates that a B. burgdorferi infection occurred at some point in the past. This infection may have occurred recently or in the distant past, months or even years previously. The infection may be active now or it may have been killed off years ago by the immune system and is no longer present. Because these antibody-based tests are not sufficiently sensitive at all stages of disease, a person may have Lyme disease but test negative. Alternatively, a healthy person may have fully positive antibody tests but not recall ever having been sick with Lyme disease.
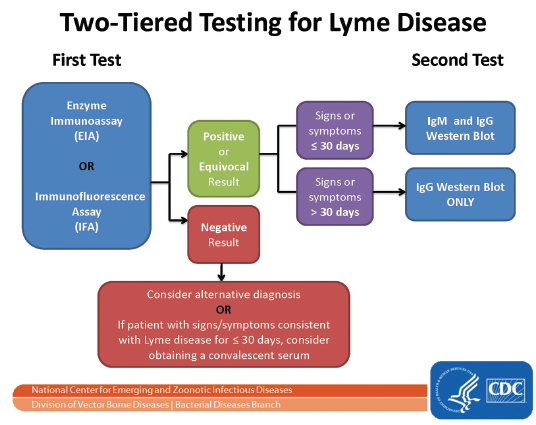
The Two-Tiered Approach for Antibody Testing
In an effort to enhance diagnostic standards, in the mid-1990s the CDC adopted the criteria from a national review committee that recommended a two-tiered strategy for laboratory testing of Lyme disease. According to the two-tiered approach, the first step involves ordering an ELISA or IFA. If the ELISA or IFA is negative, then no further testing is recommended. If the ELISA or IFA is equivocal or positive, then the next step is to order a Western blot. If the infection has been present for less than four weeks, then an equivocal or positive ELISA with a positive IgM and/or IgG Western blot is considered good evidence to confirm recent infection with B. burgdorferi. If the infection has been present longer than four weeks, then an equivocal or positive ELISA with a positive IgG Western blot is considered good evidence of prior or current infection with B. burgdorferi. According to the CDC guidelines, the IgM Western blot is not recommended for determining disease in persons with infection greater than four weeks in duration.
This two-tiered strategy is commonly used in the United States but is considered particularly problematic in Europe and other countries because there are several genospecies of B. burgdorferi in Europe that vary in their surface proteins and therefore may not be detected by the particular antigens chosen in the CDC’s Western blot scoring criteria.
An additional problem with this two-tiered standard is that the individual tests—both the ELISA/IFA and the Western blot—have problems both in sensitivity, the ability of the test to correctly identify those with the disease, and in specificity, the ability of the test to rule out those who do not have the disease. For example, a patient may have Lyme disease but test negative on the initial ELISA or the follow-up Western blot. Or a patient may have a positive test on an ELISA and/or IgM Western blot but never have had Lyme disease. In general, the two-tiered approach errs on the side of enhancing specificity at the expense of sensitivity, especially in early Lyme disease.
Various research studies and reports have assessed the two-tiered strategy (Dressler et al. 1993; Bacon et al. 2003; Aguero-Rosenfeld et al. 2005; Branda et al. 2010; Wormser et al. 2013; Molins et al. 2016), revealing different estimates of the sensitivity and specificity of the two-tiered strategy. However, some general conclusions about the sensitivity of the two-tiered approach include:
• All studies agree that in early Lyme disease, the sensitivity of the two-tiered approach is poor—only 29 to 45 percent of patients will test positive using this two-tiered approach.
• In acute disseminated neurologic Lyme disease, the sensitivity of the two-tiered approach is much improved but still problematic—about 72 to 87 percent of patients will test positive.
• In Lyme arthritis, the sensitivity is excellent—97 to 100 percent of patients will test positive.
FIGURE 4.1
The two-tiered testing strategy recommended by the CDC.
Courtesy of the CDC.
A central problem in the two-tiered method is that sensitivity varies depending both on how early in the course of infection one is tested and on the actual manifestation of Lyme disease. Equally problematic is that a patient may test positive using this two-tiered approach due to past exposure to Lyme disease but may not have a currently active B. burgdorferi infection. Another problem is that the IgM Western blot is recommended for use only during the first four weeks after infection; unless the tick bite was seen, it is often difficult to know when infection actually occurred and data analyses from other researchers have recommended that six weeks would be a more useful interval (Branda et al. 2010). Finally, our research at Columbia among patients with chronic symptoms after previously treated Lyme disease indicates that about 10 percent of those who have an IgG Western blot positive serum (i.e., five or more CDC significant bands) will have a negative ELISA.
Physicians relying solely on the initial ELISA test could miss patients who have good evidence of prior infection by IgG Western blot. Indeed, the CDC surveillance criteria accept a single positive IgG Western blot as lab evidence of prior infection. The two-tiered criteria, while enhancing specificity, may actually be misleading if the physician stops after a negative ELISA, as the IgG Western blot may provide additional information that is quite useful even if the initial ELISA is negative.
The Western blot, however, has its own problems, one of which is that some of the bands that are labeled as meaningful also are known to have cross-reactive antigens. This means, for example, that a patient’s serum might have multiple bands that are read as positive on Western blot that are actually not reflective of B. burgdorferi antibodies. This is because some of the B. burgdorferi proteins used in the Western blot contain regions (epitopes) that are similar to other proteins from other bacteria. In other words, test results may reveal positive bands for example on an IgG or IgM Western blot even though that person has never had Lyme disease. This is because the blood came from a person who was previously exposed to other non–Lyme disease bacteria that have similar protein profiles. This is discussed further under “cross-reactivity.”
These tests and their interpretation can therefore be confusing to both patients and doctors alike. These first-generation assays—developed in the 1980s and 1990s—are clearly inadequate and outdated. The newer-generation antibody-based assays (described later) have markedly reduced the problem of cross-reactivity by selecting protein fragments (epitopes) that are unique to the Borrelia species and by eliminating other fragments that elicit this cross-reactivity with other bacterial species.
While many aspects of Lyme disease are open to discussion and disagreement in academic circles, the problem of testing is widely accepted as an area in need of novel ideas and solutions. The fact that in early Lyme disease the false negative rate is as high as 60 to 70 percent means that the testing is of little use at one of the most important phases of infection—early Lyme disease—when it is most responsive to treatment. Antibody-based tests will not be able to solve this problem because antibodies take time to develop. The additional problem is that antibody levels do not necessarily correlate with treatment success in eradicating the B. burgdorferi spirochete. Because of these problems, many scientists from around the world are focusing their energies on developing more sensitive and accurate Lyme disease tests.
The following sections are meant to provide reasonable guidelines regarding how best to understand and use tests for Lyme disease. Some of the problems—and potential solutions—are delineated. Some of the standard approaches as well as more novel approaches and developments are also presented.
Indirect Fluorescent Antibody
This was the first Lyme antibody test and is less commonly used today. In this initial test, a dead B. burgdorferi spirochete is affixed to a glass slide, the patient’s serum is applied to the slide, and any antibodies (IgM or IgG) from the patient’s serum that bind to the spirochete on the slide are detected under a microscope after a fluorescent dye attached to a second antihuman antibody is applied. This test differs from the ELISA partly because it uses a fluorescent dye as opposed to an enzyme to signal spirochetal antigen detection. When observed under a microscope using ultraviolet light, serum antibodies bound to the spirochetes on the slide will light up a bright fluorescent color.
PROS
• The IFA provides semiquantitative information by reporting the highest dilution at which fluorescent spirochetes are still visible.
• The IFA allows for visualization of the binding of the antibodies to the spirochetes.
• The IFA requires a highly trained technician to interpret the results and is more subject to error than the ELISA or Western blot.
• The IFA is more labor intensive than the ELISA and thus more costly.
Enzyme-Linked Immunosorbent Assay
This is an automated, widely used quantitative initial test for Lyme disease that most often uses a “whole-cell sonicate” (i.e., B. burgdorferi spirochetes grown in culture are broken apart by sound waves); typically, this is the first test performed. A single number (or in some cases dilution titer) is reported that shows the relative level of antibodies against B. burgdorferi in the patient’s serum compared to the level usually found in the serum of individuals without Lyme disease. Some laboratories will not report the numerical value, instead reporting only whether the index or titer is positive, indeterminate, or negative. (The cutoff for a positive result requires that the patient’s antibody levels be three standard deviations higher than the average level in the non-Lyme group.) In the two-tier testing strategy, an indeterminate or positive ELISA then would lead to a Western blot.
PROS
• The ELISA is inexpensive.
• It is widely available, automated, and many samples can be run simultaneously.
• It is more quantitative than the IFA and Western blot.
• It produces a clear numeric value that reflects the magnitude of the antibody response (IgM and IgG) to the spirochetal proteins expressed under culture conditions.
CONS
• False positives and false negatives: The whole-cell sonicate ELISA sometimes produces false negative or false positive results. False negative rates on the ELISA have been reported as high as 67 percent in early Lyme disease and 21 percent in early neurologic Lyme disease. False positives also can occur, particularly in the context of certain diseases. One recent study (Schriefer 2015), for example, found that the ELISA had high false positive results among patients with syphilis (FP 85 percent), infectious mononucleosis (FP 53 percent), and multiple sclerosis (FP 18 percent); however, it performed well in this study with no false positives among patients with fibromyalgia and only 10 percent false positives among those with rheumatoid arthritis.
• Because many of the ELISA tests used today detect both IgM and IgG antibodies, there is a greater risk for false positive results because the IgM antibodies are known to be more “sticky” (and less specific) than the IgG antibodies. In other words, these antibodies may have been generated in response to another non-Lyme bacterial or viral infection but then bind to a Lyme disease spirochetal protein that is similar, thus leading to a false positive result. This phenomenon is known as “cross-reactivity.”
• Cross-reactivity: ELISAs that are based on whole-cell sonicate B. burgdorferi have an inherent problem of cross-reactivity. This is because the whole-cell spirochete contains components that are common among other bacterial species—i.e., not unique to the organism and thus “non-specific.” Some of the more nonspecific components of B. burgdorferi include the 21–23 kDa, 30 kDa, 41 kDa, 60 kDa, 66 kDa, and 73 kDa antigens that are known to have considerable cross-reactivity to other spirochetes (e.g., the oral treponemes common in the mouth may seed the blood in patients with periodontal disease), heat-shock proteins found on other microbes, and viruses (e.g, Epstein-Barr virus) or bacteria (e.g., Helicobacter pylori). False positives on the ELISA due to cross-reactivity may also occur among individuals with endocarditis or autoimmune disorders. Overall, the problem of cross-reactivity is recognized as a leading explanation for false positives on the ELISA (and Western blot).
• Differences between the proteins expressed in culture and the proteins expressed in the actual infected human: The traditional ELISA and Western blot assays are made most often with antigens obtained from Borrelia spirochetes grown in culture in a laboratory setting. However, what happens in culture is not the same as what happens in the human host. In culture some outer spirochetal surface proteins are expressed that are suppressed in the human host. Similarly, in the human host, some new surface proteins emerge that are not expressed in the culture setting. This means that a lab test that is developed based on what happens in culture may not be able to detect all of the outer surface proteins expressed in spirochetes in different stages of human B. burgdorferi infection. Some researchers speculate this may contribute to the variable sensitivity of the two-tiered assays in different stages of Lyme disease. Fortunately, newer assays with antigens expressed in the human (e.g., C6 and VlsE ELISAs) have been developed that begin to address this problem.
• Inconsistency between blood and cerebrospinal fluid: In our studies of Lyme disease, we have found occasional patient samples that test positive by Lyme ELISA in the cerebrospinal fluid but negative in the serum; this presents a problem when a physician relies solely on the ELISA in the serum to rule in or rule out neurologic Lyme disease.
° In a study of eighteen patients with cognitive problems after Lyme disease, three of the eighteen patients tested negative in the serum but positive in the cerebrospinal fluid for B. burgdorferi–specific antibodies (Logigian, Kaplan, and Steere 1999). This suggests that as many as one in six patients with evidence of central nervous system invasion by B. burgdorferi would be at risk for misdiagnosis if clinicians relied solely on blood test studies.
NEW DEVELOPMENTS
• Recombinant or Synthetic Peptide ELISAs. To reduce the number of false positives, more specific ELISAs (based on “recombinant” or “synthetic” proteins) have been developed that dramatically reduce the percentage of false positive results. One such test is the C6 Peptide ELISA. A similar test is the VlsE ELISA. Both provide tests for Lyme antibodies with improved sensitivity and specificity; each represents a much more specific test than the standard “whole-cell sonicate” ELISA.
° In one large U.S. study (Branda et al. 2010), the C6 ELISA was shown to have a sensitivity of 56 percent in early Lyme disease compared to only 42 percent for the two-tiered approach. In that study both approaches had excellent specificity—98.4 percent for the C6 and 99.5 percent for the two-tiered method. In another large-sample-size study (Wormser et al. 2013) compared to two-tiered testing, the C6 ELISA as a stand-alone test had a sensitivity in early Lyme disease of 66.5 percent versus 35.2 percent; in early neurologic Lyme disease of 88.6 percent versus 77.3 percent; and in Lyme arthritis of 98.3 percent versus 95.6 percent. In this study, the specificity was only slightly less for the C6 assay at 98.9 percent versus 99.5 percent for the two-tiered method. While more sensitive than the standard ELISA, particularly in early Lyme disease, the C6 ELISA is still not sensitive enough to be considered an adequate test for early infection.
° Some research studies on the C6 ELISA suggest that changes in the C6 ELISA value may reflect successful response to treatment. One study reported that six months or more after treatment there was a fourfold or greater decline in titer on the C6, corresponding with successful treatment in humans with culture-confirmed erythema migrans (Philipp et al. 2005). Other human studies, however, have not confirmed these findings. Additionally, in a primate animal study, C6 peptide ELISA antibody tests became negative over time in several rhesus macaques despite evidence of persistent B. burgdorferi infection (Embers et al. 2012).
• Combining Multiple Synthetic Peptides. To improve the sensitivity and specificity of the ELISA, other investigators are now creating ELISAs that combine multiple synthetic peptides, such as pepC10, OppA2, Decorin-binding protein A, and Decorin-binding protein B. The improved sensitivity is likely due to the use of multiple peptides that are expressed both at the very early localized phase and at the later stages of infection. The improved specificity may be due in part to the elimination of structures that are found in similar proteins expressed by other bacteria.
• Point-of-Care tests. More rapid and sensitive point-of-care ELISA tests are being developed that can provide results within minutes in the health care provider’s office.
• Adopting a two-tier ELISA. To reduce problems associated with interpretation of the Western blot that can be technically complex and subjective, the Centers for Disease Control and Prevention may move to replace or complement their laboratory recommendation for diagnostic testing in clinical practice from the ELISA–Western blot combination of the two-tiered system with a two ELISA combination.
° Recent analyses indicate that using a whole-cell sonicate ELISA as the first-tier test with the C6 ELISA as the second-tier test is either equivalent to or significantly better than any comparison algorithms that included immunoblots (Molins et al. 2016). This provides the advantages of simplicity and removes the subjective aspects of visually read immunoblots.
Western Blot
The Western blot (a type of immunoblot) is also widely used as a test for Lyme disease antibodies, usually during the two-tiered testing as a follow-up test if the ELISA is equivocal or positive. While the ELISA is a quantitative test of the magnitude of the IgG/IgM antibody response, the Western blot provides qualitative data regarding which antibodies are actually present in the patient’s serum that recognize proteins of B. burgdorferi. Interpretation of the Western blot is often conducted by a skilled laboratory expert who visually assesses the intensity of each “band.” More recently, automated methods have been developed to measure each band’s intensity and report whether it is present or absent based on a cutoff intensity. According to the CDC, a Western blot is considered positive if there is reactivity at two out of three bands of the IgM class (23, 39, or 41 kDa) or five out of ten bands of the IgG class (18, 23, 28, 30, 39, 41, 45, 58, 66, or 93 kDa). (The apparent molecular mass of OspC is dependent on the strain of B. burgdorferi being used and in the early Western blots was identified as 21 or 24 kDa, instead of the 23 kDa weight more commonly reported today.)
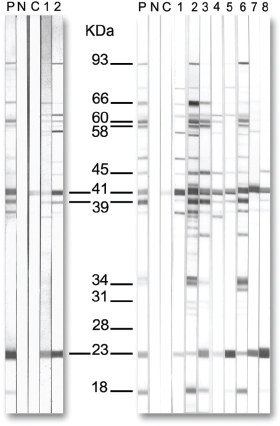
FIGURE 4.2
Western blot showing the location of significant bands that support the presence of B. burgdorferi antibodies.
Courtesy of the CDC.
The Western blot IgM and IgG provide different information. Both IgM and IgG antibodies are produced during active infection, but the less specific IgM antibodies wane once the more specific IgG antibodies are generated. While it is generally known that the IgM antibody can be a marker of new or recent infection, it is not well known that the production of IgM antibodies does not necessarily stop, even after the infection has been treated and the organism eradicated. Both antibodies can stay elevated for long periods, although the IgG stays elevated for much longer. In the case of Lyme disease, IgM and even IgG do decline to undetectable levels in some patients, but in many patients they remain elevated (in comparison to baseline) for many years. This is likely a function of the duration of active infection, strain of organism, and genetic predisposition of the human. In almost everyone, these antibodies keep declining over the years at various rates after peaking at some point during or shortly after active infection. A persistent IgM antibody response therefore is of uncertain significance. The CDC and the IDSA state that the IgM Western blot result has meaning only during the first four weeks after infection. Subsequent to that interval, the IgG Western blot should be the focus of attention because by that time a healthy immune system in the human should be able to generate the more specific IgG response.
The IgG Western blot is considered highly specific when interpreted using the CDC criteria of five out of ten bands. In other words, if an IgG Western blot is positive, one can be almost certain that the person has had prior exposure to B. burgdorferi. This is obviously advantageous because knowledge of prior infection can help clarify why an individual might have persistent symptoms. On the other hand, a positive Western blot alone does not mean a person has active infection because the IgG antibody response can stay positive for many years, long after the infection has been eradicated.
PROS
• The Western blot is widely available.
• It provides a fingerprint of the Lyme-specific antibody profile in the blood.
• A positive result (five out of ten bands) is highly specific for Lyme disease—in other words, a positive test is strong evidence that a patient had or has infection with the agent of Lyme disease.
° A lab test that reveals four out of five IgG bands, while not positive by CDC standards, would be considered very highly suggestive of prior exposure to B. burgdorferi. In the setting of a carefully evaluated patient with good evidence of tick exposure and clinical features consistent with Lyme disease, such a test result would provide valuable supportive evidence of prior infection (although not confirmatory).
• The sensitivity of the IgG Western blot is considered to be excellent for Lyme arthritis. For acute neurologic Lyme disease, the sensitivity in various studies is less, falling between 72 and 87 percent (Dressler et al. 1993; Bacon et al. 2003).
CONS
• Western blots are much more expensive than ELISAs.
• Unlike ELISAs, which are quantitative assays, the density of bands on a Western blot is usually visually interpreted by a technician, so there is variability in results depending on the skills of the “reader.” In other words, the test is more subjective. This problem is being addressed by new striped immunoblots that use a scanning densitometer (rather than a technician) to measure band intensity; this improves consistency and objectivity.
• There have been questions about the bands selected for inclusion in the list of Lyme-significant bands. For example, the CDC’s list of significant bands excludes certain bands, such as the 31 kDa and 34 kDa, even though Lyme vaccines were developed or considered that target these specific proteins. If these markers are important enough to be the focus of vaccine development, clearly they should also be considered in Western blot interpretation. Why were these bands excluded?
° Some speculate that the 31 kDa band was excluded in the mid-1990s because a vaccine was under development for the U.S. market that triggered the healthy patient’s immune system to produce antibodies against the 31 kDa band. Vaccinated patients would therefore be more at risk of a misleading result on the Western blot if the 31 kDa band were included in the CDC list.
° Another reason given for the exclusion of the 31 and 34 kDa bands is simpler. The CDC adopted the Dressler and colleagues criteria (1993) in which the ten most frequently observed IgG bands among patients with new-onset Lyme disease were chosen; although the 31 and 34 kDa bands were detectable among some patients with later stage disease (e.g., arthritis or neurologic Lyme), the frequency of antibody responses to these peptides did not make it to the top ten. In support of this conclusion, we examined the frequency of bands on IgG Western blots in a large sample of patients with chronic persistent symptoms after well-defined Lyme disease and found that the OspA and OspB antigens were the eleventh and twelfth most frequently elicited antibody responses.
° Worth noting in regard to OspA and OspB antigens is that they are downregulated during tick feeding and not initially expressed in the human until later in the course of infection. Positive antibodies to OspA and OspB are therefore more relevant for the detection of later stage than earlier stage infection.
• As noted in the ELISA section earlier, cross-reactive antibodies originating from triggers unrelated to B. burgdorferi can occur. While it was originally thought that the Western blot would solve this limitation of the ELISA by selecting protein bands that were more specific to B. burgdorferi, it is now known that some of these proteins also have regions that are not specific and thus contribute to the problem of cross-reactivity. This is confusing to both patients and clinicians. The 21–23, 30, 41, 58, and 66 kDa antigens (included in the ten bands specified by the CDC) can each attract cross-reactive antibodies triggered by infections unrelated to B. burgdorferi. The common oral treponemes in the mouth, for example, can trigger the generation of antibodies that cross-react at the 41 kDa band (Magnarelli 1990) while the heat-shock proteins found on other microbes may lead to misleading reactivity on the 58 and 66 kDa bands (Hansen et al. 1988; Arnaboldi and Dattwyler 2015; Luft et al. 1991). Reactivity on a Western blot to other antigens previously thought to be “Lyme specific” may also reflect false positivity; these include the 31 kDa antigen (due to less specific protein migration to that 31 kDa location) and the 93 kDa antigen (Nowalk et al. 2006; Chandra et al. 2011). The problem of cross-reactivity common to these antibody-based tests is now being corrected by new tests that use peptide regions unique to the B. burgdorferi spirochete; the cross-reactive regions on the immunoblot bands that are common to other bacteria have been identified and eliminated from these new assays.
° As an example of the problem of cross-reactivity, one study showed that 40 percent of healthy individuals with no history of Lyme disease had positive reactivity to the Borrelia flagellin (41 kDa) (Liang et al. 1999). In other words, reactivity to the 41 kDa band is common in the general public and should not be considered by itself as a sign of infection with B. burgdorferi.
• Limitations of the IgM Western blot: While a positive IgG Western blot is relatively easy to interpret, the considerable limitations of the IgM Western blot lead to problems.
° Unclear time course: A positive IgM Western blot after the first one to two months can be confusing. It has been shown in Lyme disease that the IgM Western blot can stay positive for many months, long after the infection has been treated and the patient has recovered.
° Cross-reactivity: The IgM Western blot is recognized as less specific than the IgG Western blot. That means that a positive Lyme IgM antibody test result may actually have been elicited by infection with a microbe other than B. burgdorferi. In other words, the Lyme IgM Western blot is more likely than the IgG Western blot to have problems with false positives as a result of cross-reactivity. This cross-reactivity creates quite a confusing situation for patients and doctors. Does an intermittently positive IgM Western blot reflect new B. burgdorferi infection, a failure to turn off a previously activated IgM response even after the infection is gone, or a false positive due to cross-reactivity with other common microbes in the human body? Patients may be told that they need more antibiotics because the IgM Western blot is positive—such a recommendation may be correct or it may be incorrect. The IgM Western blot is unfortunately not specific enough to allow us to make that determination.
° CDC criteria versus “in-house” criteria: To enhance the sensitivity of diagnostic tests, some Lyme specialty labs have devised an alternative (“in-house”) set of criteria for determining whether a Western blot is positive; not surprisingly, when criteria are modified to enhance sensitivity, there is often also an increase in false positives. To assist clinicians, these labs report their interpretation of the Western blot results using the CDC criteria as well as the lab’s in-house criteria.
• Limitations of in-house criteria. The problem with interpretation of the IgM Western blot when using these in-house criteria was demonstrated in a study (Fallon et al. 2014) in which we reported that 37 percent of healthy individuals whose serum was tested at a Lyme specialty lab were found to have a positive IgM antibody response for Lyme disease, even though they did not feel sick and even though they had no knowledge of ever having had Lyme disease. Of course, while we cannot rule out that some of these healthy controls may have been previously bitten by a tick and gotten infected without a memorable illness, we consider it highly unlikely that this explanation would apply to most of these healthy individuals. This study demonstrated that these tests—when relied upon too strongly by clinicians—can be misleading, particularly when in-house criteria are used for the IgM Western blot.
NEW DEVELOPMENTS
• Switching from IgM to IgG may be impaired: Recent research in mice indicates that B. burgdorferi infection damages the architecture of the lymph nodes in such a way as to diminish maturation from the production of IgM antibodies to IgG antibodies generated by long-lived plasma cells (Elsner et al. 2015). It is unclear whether this finding in mice has relevance to humans, as mice are the primary reservoir carriers of B. burgdorferi infection in nature. This is, however, an important area of current research as it could provide an additional explanation as to why people can get Lyme disease more than once, why in some patients IgM antibodies are more common than IgG antibodies, and why infection may persist in some hosts.
• Use of recombinant or synthetic proteins on immunoblots: To improve the accuracy of the Western blots, some laboratories are using recombinant or synthetic proteins rather than the protein mixture currently used to target the antibodies in the serum. These purified proteins ensure that the “positive” band is truly a Lyme-significant band as opposed to an antigenic marker that is not specific for Borrelia but has migrated to that location on the blot. The 31 kDa band, for example, is the site of one such migrating antigen that can lead to misleading conclusions. Some labs now provide Western blots that have a combination of antigens on the blot—one composed of synthetic antigens, recombinant antigens, and whole-cell sonicate antigens. These should improve the specificity of both the IgM and IgG Western blots.
• Multiplex bead arrays: Recent research has focused on identifying the best antigen combination or best cytokine/chemokine combination by screening a wide array of novel and established biomarkers to see which worked best to improve diagnosis and detect early infection.
° Rather than relying solely on the ten antigens recommended on the CDC’s IgG Western blot panel, one such approach screened multiple antigens to identify the ones most sensitive and specific (Lahey et al. 2015). In this study, the investigators screened sixty-two candidate antigens (including seven B. burgdorferi surface proteins and fifty-five synthetic peptides derived from B. burgdorferi–specific antigenic epitopes); of these, ten were selected for a multiplex bead array using Luminex technology. This detection strategy avoids the need for immunoblotting altogether by using specific peptides in a multiplex microsphere assay. The results from a sample of early Lyme disease cases demonstrated an improved sensitivity of 55 percent for detection rate of B. burgdorferi antibodies (using the multiplex array) compared to only 40 percent using the CDC two-tiered methodology; both approaches had excellent specificity (100 percent). The sensitivity of this multiplex assay however is similar to the sensitivity reported for the Lyme C6 ELISA assay in early Lyme disease. Both represent improvements for early detection compared to the two-tiered method, but one that falls short of the 90 percent or greater detection desired for an assay in early Lyme disease.
° Another approach used a multiplex bead array to look not at antigens but at the immune signature associated with early Lyme disease (Soloski et al. 2014). In this study, fifty-eight immune mediators (cytokines and chemokines) and seven acute phase markers (e.g., C-reactive protein and serum amyloid) were measured. The analysis revealed an immune biosignature that differentiated the early patients with Lyme disease from the controls. Importantly, after antibiotic treatment of the EM rash, the levels of the three most highly expressed chemokines (CXCL9, CXCL10, and CCL19) returned to normal, suggesting that this immune signature may have a role as a diagnostic marker of early Lyme and/or a biomarker of treatment response. Could this research lead to the much-needed test for active infection? Possibly. Future studies need to examine how specific this immune signature is when compared to other inflammatory illnesses and whether this biosignature is also sensitive for active infection in later stages of acute Lyme disease.
Culture is a test in which blood or tissue is placed in a special medium to see if B. burgdorferi will grow. Culture is the “gold standard” for diagnosis of any infection, but in the case of Lyme disease, culture is rarely used except in the research setting.
PROS
• If the organism can be cultured, you know that the blood, spinal fluid, or tissue contains healthy Borrelia spirochetes. This is the definitive proof of active infection.
• In the research setting, B. burgdorferi spirochetes have been grown from approximately 50 percent of skin biopsy samples of patients presenting with the Lyme disease rash. This is helpful in clinical research because one can then be sure that the rash is indeed an EM rash. By conducting biopsies on all rashes on individuals after tick bite or exposure to a Lyme endemic area (regardless of whether it looked like a Lyme rash), this approach has demonstrated that the EM rash of Lyme disease may not always look like a Lyme rash (e.g., it could look like a spider bite).
CONS
• Culturing B. burgdorferi is time consuming because the organism is slow growing.
• Not all B. burgdorferi will grow readily in standard culture.
• Test results may not come back for eight to sixteen weeks—far too long to be helpful to the doctor trying to decide what to do now for the patient currently in his or her office.
• Culture of Borrelia is not offered by the majority of commercial labs.
• Culture is less useful once the infection has disseminated outside the bloodstream and beyond the stage of the EM rash. Even at the EM stage, when the quantity of Borrelia spirochetes is greatest in the blood, only about 40 to 50 percent of patients will test positive using the best culture techniques currently available (e.g., high-volume plasma culture).
• False positive results can occur even at the best labs due to inadvertent contamination of culture specimens.
NEW DEVELOPMENTS
• Modified Culture Medium. There are increased efforts to improve the sensitivity of culture by modifying the culture medium. One lab in the United States has reported a remarkably improved sensitivity (greater than 90 percent after sixteen weeks) (Sapi et al. 2013). These results are extraordinary given prior studies that reported positive cultures using high volumes only 45 percent of the time after eight to twelve weeks (Wormser et al. 2001). The CDC has raised concerns about this new culture assay, particularly raising the question of implausibility and suspected contamination (Johnson, Pilgard, and Russell 2014; Nelson et al. 2014). For these reasons, the authors of this critique recommended additional validation studies of this new culture assay by an independent lab or academic medical center.
Polymerase Chain Reaction
The polymerase chain reaction (PCR) detects genetic material of the B. burgdorferi spirochete itself. This is considered by many to be a direct test of active infection.
PROS
• A positive PCR result is strongly suggestive of current or recent infection as the Borrelia genetic material does not linger in the body for long after the infection is gone.
• Because the PCR detects DNA, positive results can be seen immediately when infection first appears and does not require the delay of two or more weeks typical of antibody-based tests.
• PCR is a sensitive assay in skin biopsy samples from EM patients (approximately 64 percent) and in synovial fluid specimens (approximately 83 percent) from patients with Lyme arthritis.
• PCR studies of blood from patients with early Lyme disease typically detect DNA of B. burgdorferi in only 18 to 26 percent of blood samples.
• PCR of the cerebrospinal fluid is insensitive in detecting active infection among patients with neurologic Lyme disease, with sensitivities as low as 38 percent in early neurologic Lyme and 25 percent in late neurologic Lyme disease.
• False negatives can occur when the genetic load in the blood specimen is just below the detection level of the assay.
• False negatives in the blood can also occur because spirochetes have already disseminated outside the bloodstream and have lodged in tissues; the test result is correct but the conclusion that the human is not infected with Borrelia spirochetes would be incorrect.
NEW DEVELOPMENTS
• Large volume sampling. Recent studies have revealed an increased sensitivity by sampling larger blood volumes and by using different PCR techniques such as qPCR or nested PCR.
• Discovery-based techniques. One of the more promising research areas has been the introduction of “discovery” diagnostic methods to enhance microbe detection.
Currently, doctors order a diagnostic assay such as a PCR based on a hypothesis that a specific organism, such as B. burgdorferi, may be present. This is a limited approach to microbial detection because it requires knowing in advance what microbe might be causing the infection.
In research settings, we can now probe a sample of fluid and “discover” whatever microbes may be located within that sample. This is very helpful in biowarfare situations. In some hospital centers, it is now being used for patients who come in with “fevers of unknown origin.” To be able to rapidly detect and label a foreign microbe is a major advance in medical science.
• This test is done through a variety of techniques, one of which is a combination of isothermal amplification followed by broad-range PCR and electrospray ionization mass spectrometry (PCR/ESI-MS). This approach has been used to identify novel pathogens inside ticks as well as in blood samples of patients with recent Lyme disease (Eshoo et al. 2012, Marques et al. 2014). This technique has many advantages, including the ability to rapidly identify and genotype pathogens and to identify new genetic variants.
Other Blood Tests
• In previous decades, various investigators have explored other laboratory methods for detecting Lyme disease, such as the immune complex dissociation assay and the borreliacidal assay. Most of these tests are no longer commercially available due to problems with sensitivity, specificity, or marketability.
NEW DEVELOPMENTS
• Lymphocyte or macrophage stimulation assays: Much like the successful cell stimulation tests for tuberculosis, one of the newer approaches takes advantage of the immune cell’s memory. When the infection with B. burgdorferi is current or recent, the patient’s blood in the lab will show a much stronger release of a specific cytokine (immune mediator) when exposed to stimulation by Borrelia protein than when the infection has resolved. This is because the immune cells (for example, T-cells or macrophages) will have been “primed” by recent exposure to the Borrelia spirochete itself. These tests would be considered indirect markers of infection.
° The application of this approach to early Lyme disease using monocytes or macrophages is partly based on the novel immunological discovery that the very early immune response is not just a shotgun response with no specificity but an immunologic attack that does retain some specificity for a period of time (Saeed et al. 2014).
° Another report applied this approach to the stimulation of T-cells using peptide antigens derived from B. burgdorferi to stimulate the release of interferon-gamma (IFN-γ) (Callister et al. 2016). Among twenty-nine patients with early Lyme disease, 69 percent tested positive; among these patients who initially tested positive on the T-cell stimulation assay, 80 percent were subsequently negative on this test when reevaluated two months post-treatment. This approach to testing bypasses the problems associated with the time delay for antibody generation and represents a significant departure from the antibody tests that remain positive for years, long after the infection is gone.
° Most of these assays are still in the testing and validation phase and hence will not be discussed in further detail here. They do, however, provide the promise of a test that serves as a surrogate marker of active infection with the Borrelia spirochete at any stage of disease. As an alternative to serologic testing, this approach to diagnostic testing brings us closer to the clinically important goal: a test that is positive early in infection and one that becomes negative after the infection has resolved.
• Next-generation “omics” and discovery-based science: Rather than developing assays with a “best guess” of which proteins or markers are most important to use, discovery-based science starts with a blank slate. The method involves uncovering and identifying all of the molecules in a fluid (e.g., blood or spinal fluid) that distinguish individuals with Lyme disease from those who do not have Lyme disease. Or one could compare the blood or CSF components from patients with early Lyme disease to those components found in later stage Lyme disease. In this way, one is uncovering or discovering the markers that are there as opposed to searching for what one hypothesizes will be there. This breakthrough in science is made possible by recent advances in biotechnology. The components that are scrutinized might start at the most basic level or higher up the information chain; for example, studies probing a sample of human blood may seek to discover all the genetic material (DNA), genetic transcripts (mRNA), or the products of the gene transcriptions (proteins or other metabolic products). Metabalome-derived assays are an example of the application and power of this discovery approach.
° Recent work (Molins et al. 2015) has focused not just on the proteins and peptides but also on other features of the “metabolome” in the blood to distinguish early Lyme disease from healthy controls. This approach examines the pattern of molecules produced in the patients’ serum in response to B. burgdorferi infection. This research group identified a biosignature of early Lyme disease containing forty-four molecular features. The sensitivity of this assay was 88 percent in early Lyme disease with a specificity of 95 percent. These results are vastly superior to the current sensitivity of the two-tiered approach (ELISA and Western blot) while retaining high specificity. Given that many clinicians are most interested in highly sensitive tests so that they don’t miss the opportunity to treat a case of Lyme disease early in the course of infection, this high degree of sensitivity represents a marked advance in the diagnosis of early Lyme disease. This assay combined mass spectrometry and liquid chromatography to identify metabolic patterns in the blood that differentiate early Lyme from other clinical or healthy states.
Nanotechnology and Urine-Based Assays
• An early version of urine-based tests that purported to detect Borrelia antigen was shown to be unreliable in one report (Klempner et al. 2001b).
• A more recent version has been reported using the extraordinary sensitivity of nanotube technology (Magni et al. 2015) to detect the OspA antigen, which is common to the Borrelia genospecies. OspA is a surprising choice because it is not thought to be expressed in early disease. However, another recent diagnostic study reported detection of OspA in the blood of three patients with acute Lyme disease (Cheung 2015). By directly detecting the spirochetal protein rather than the antibody response, these tests come closer to reaching the holy grail of clinically useful diagnostics—a test of active infection. If future studies are able to confirm that this is a reliable and sensitive marker of active infection at all stages of disease, many of the treatment decisions faced by clinicians would be made easier. While we cannot be certain the persistent OspA antigen represents active infection, the detection of the protein does suggest that the Borrelia spirochete has been present in the recent past. The initial nanotechnology study reported excellent sensitivity and specificity. Because this is a relatively new assay and because OspA is an unexpected marker for early Lyme disease, this test requires independent validation for confirmation.
Lumbar Puncture
Patients with symptoms suggestive of central nervous system involvement that may be due to Lyme disease should have a lumbar puncture, also known as a spinal tap. The cerebrospinal fluid (CSF) obtained in this test is sent for routine studies, including cell count as well as protein and glucose levels. In addition, the CSF is sent for studies of B. burgdorferi–specific antibodies and for Borrelia DNA by PCR. When spinal fluid is collected, it is equally important to collect serum from the patient so that the CSF-serum pair can be sent together to the lab for testing. This allows for a comparison of the relative levels of Lyme-specific antibodies in the CSF compared to the serum; with this information, the lab can then calculate the “intrathecal index.” This index refers to the ratio of B. burgdorferi antibodies in the CSF compared to the serum. A positive index indicates that more antibodies are being produced in the CSF than in the blood, strongly suggesting that the Borrelia spirochete has invaded the central nervous system. Please note that the serum must be drawn on the same day as the lumbar puncture for this study to be effective. If the physician suspects the symptoms could also be caused by multiple sclerosis, he or she is likely to order more tests on the CSF, such as an assay for oligoclonal bands and an IgG index and synthesis rate.
PROS
• The spinal fluid is the closest fluid to the brain and thus provides information related to a variety of brain infections and diseases that may not be detectable from the blood alone. It is also relatively protected from the blood by the blood–brain barrier.
• A positive intrathecal index can confirm invasion of the central nervous system by B. burgdorferi.
CONS
• In the setting of active Lyme disease in the central nervous system, a false negative result for B. burgdorferi antibodies in the CSF among U.S. patients may occur as often as 20 percent of the time (Coyle 1995). This false negative means that the B. burgdorferi antibodies are not always detected by routine tests and that the white blood cell count and protein level of the CSF may be normal even in the setting of neurologic Lyme disease; the 20 percent false negative was detected by “immune complex dissociation assays.”
• Like the serum antibody tests, the intrathecal index may remain positive long after the initial infection has been treated and cleared as the antibody response can continue due to immunologic memory.
NEW DEVELOPMENTS
• Chemokine CXCL-13. The chemokine CXCL13 was reported in early studies to be a highly sensitive (94 percent) and specific biomarker of early neurologic Lyme disease (Schmidt et al. 2011). This B-cell attractant, however, has also been found to be elevated in patients with neurosyphilis, B-cell lymphoma, and in patients with N-methyl-d-aspartate (NMDA)-receptor encephalitis. Therefore, while it may be a sensitive marker of active infection in Lyme neuroborreliosis, it is not 100 percent specific when compared to other diseases associated with CNS infection or B-cell mediated pathologies.
• Recombinant Antigen Index. A study (Wutte et al. 2014) demonstrated that the diagnosis of neurologic Lyme disease varies considerably depending on the test method used. Sensitivity of detection of neurologic Lyme disease was 24 percent higher when the intrathecal index used a recombinant antigen ELISA (OspC and VlsE) to identify B. burgdorferi–specific antibody production compared to when another B. burgdorferi–specific antigen (flagellin) capture ELISA was used. When either the immunoblot alone or the CXCL13 assay were used to detect neurologic Lyme disease, the recombinant assay with OspC and VlsE continued to detect about 10 to 14 percent more cases. While this study is encouraging in demonstrating considerable improvement in diagnostic testing of the CSF, it also reminds us that a negative result using one testing method may be a false negative and that a clinician’s decisions regarding treatment should not be based solely on the results of the CSF assay; further test improvements are needed to enhance the clinician’s confidence regarding these tests.
• CSF Proteome. To determine whether the cerebrospinal fluid of patients with post-treatment neurologic Lyme disease contains a protein profile unique to patients with post-treatment Lyme disease when compared to patients with chronic fatigue syndrome and to healthy controls, a proteomic study (Schutzer et al. 2011) using samples from the Columbia Lyme encephalopathy clinical trial was conducted in collaboration with Pacific Northwest National Laboratories. The method enabled identification of all of the proteins contained in the spinal fluid from people in each of these three groups; this is known as the “proteome.” The study demonstrated that post-treatment Lyme disease has 692 unique proteins in the cerebrospinal fluid and therefore can be differentiated from chronic fatigue syndrome, even though the clinical symptoms of each are quite similar. While not yet a diagnostic assay, studies such as these provide additional clues for diagnostic test development.
Structural MRI
Magnetic resonance imaging (MRI) is a medical imaging technique that uses magnetic fields to show the anatomy of the brain without using radiation. MRI is able to detect abnormalities that may be seen in some patients with neurologic Lyme, such as white matter hyperintensities. White matter hyperintensities can occur as a result of Lyme disease, but can also occur for a wide variety of other reasons. It is important to note that these findings are not unique to Lyme disease. White matter hyperintensities tend to increase with age even in adults who do not have Lyme disease. Individuals with a history of stroke, multiple sclerosis, or smoking may also have large or numerous white matter hyperintensities. For these reasons, MRI is informative but it cannot be used to make a diagnosis of Lyme disease.
PRO
• MRI does not use radiation and is noninvasive.
CONS
• Lyme disease cannot be diagnosed on the basis of MRI findings, which are nonspecific.
• MRI cannot be used on patients with pacemakers or certain metallic implants.
• Some patients report feeling claustrophobic during the MRI scan, which takes approximately forty-five minutes. MRI scans are also somewhat noisy, making it hard for individuals with heightened sensitivity to sound (hyperacusis) to tolerate.
Functional MRI
Functional MRI (fMRI) is an approach to magnetic resonance imaging that allows researchers to answer certain questions about brain function. It is not commonly used in the clinical setting in the assessment of patients. One example of its potential research use among patients with Lyme disease is to ask whether the brain function of patients with Lyme disease has been altered compared to those who are healthy. If we hypothesize, for example, that patients with post-treatment Lyme disease syndrome (PTLDS) might suffer from “central sensitization,” we would expect that their central pain networks might be hyperactivated compared to healthy controls. In other words, a moderate level of pain would result in greater activation of the brain’s pain networks in a patient with a history of Lyme disease than would occur in an individual without a history of Lyme disease. This “brain pain” can be studied using fMRI approaches; “brain pain” might help explain the chronic diffuse pain that many patients with PTLDS experience. At Columbia, we are conducting an fMRI study to address this question.
Magnetic Resonance Spectroscopy
MR spectroscopy is not commonly used to assess patients with Lyme disease in routine clinical practice, but it is used in the research setting to teach us about the relative concentrations of different chemicals in the brain. For example, a study of brain chemistry among patients with fibromyalgia (Natelson et al. 2015) demonstrated using MR spectroscopy that the level of lactic acid in the cerebrospinal fluid was initially elevated but declined after successful pharmacologic treatment with a medication (“milnacipran”); this medication modulates important brain neurotransmitters involved in pain, fatigue, and depression. Whether patients with persistent fatigue or pain after Lyme disease also have elevated levels of lactic acid in the cerebrospinal fluid is a question that the Columbia Lyme Center is currently investigating using this noninvasive brain imaging approach.
Single-Photon Emission Computerized Tomography Imaging
Unlike structural MRI, which provides a picture of the brain’s anatomy, single-photon emission computerized tomography (SPECT) enables an examination of how the brain is actually functioning. In Lyme disease, the most common finding is “heterogeneous hypoperfusion” diffusely throughout the brain. This finding might lead one to conclude that there is a problem in the blood vessels because less blood is reaching various parts of the brain than normal. However, vascular narrowing or blockage is not the only reason for diminished blood flow. Decreased blood flow can also occur due to decreased demand by the brain tissue. In other words, if the brain tissue is not metabolically active or if it is impaired in its functioning, the demand for blood flow may be diminished. In fact, our studies at Columbia have documented that patients with Lyme disease have deficits in both metabolic demand and blood flow; the most striking finding, however, is that the brain tissue’s reduced metabolism creates the appearance of insufficient cerebral blood flow (Fallon et al. 2009).
Approximately 50 percent of patients with persistent symptoms after Lyme disease may have multiple areas of hypoperfusion on SPECT imaging (Fallon et al. 1997). Unfortunately, this same pattern of heterogeneous hypoperfusion is also seen in Lupus, chronic cocaine users, and certain vasculitic inflammatory disorders. Although SPECT has limitations and the blood flow perfusion pattern does not provide a diagnosis, it may be a helpful tool to clarify whether or not the blood flow in the brain appears normal or abnormal in a patient with central nervous system symptoms.
In the research setting, repeated SPECT scans over time after treatment with intravenous ceftriaxone has been shown to result in improved perfusion among patients with Lyme encephalopathy (Logigian, Kaplan, and Steere 1999). In the clinical setting of an individual patient, however, it is not clear that monitoring changes in blood flow on SPECT imaging is helpful. We have seen patients whose clinical symptoms improved after treatment for Lyme disease but whose brain SPECT scan did not change; this led to considerable discouragement and in some cases despair. Our impression is therefore that improvement in blood flow on the brain SPECT scan may lag far behind the improvement in clinical symptoms. While helpful as a research tool, it is not yet clear that SPECT scans among individual patients with Lyme disease adds valuable information in the assessment of treatment response in the clinical setting.
PROS
• It is relatively inexpensive compared to positron emission tomography (PET) scans and widely available.
• It can show brain function.
• It is particularly useful if a seizure is suspected as that area of the brain may reveal increased perfusion.
• It may be useful in the assessment of young adults: an abnormal brain SPECT demonstrating diffuse moderate to severe heterogeneous hypoperfusion in a young adult with Lyme disease would support brain involvement if the individual is not abusing drugs, has never had a brain injury, and does not suffer from another disease known to cause perfusion or metabolic brain problems, such as another autoimmune or infectious disease.
• Lyme disease cannot be diagnosed on the basis of SPECT scans alone.
• SPECT scans may only be useful in patients with moderate to severe central nervous system disease, as healthy people may have mild to moderately decreased perfusion, especially associated with increased age.
• In many medical centers, the clinical reading of the brain SPECT is quite subjective—based on the clinical experience of the nuclear medicine physician and based on the assumption of normal flow in certain parts of the brain (such as the cerebellum or thalamus). In other words, unless the reading of the scan has been automated and compared to a large database of age- and gender-adjusted healthy controls, the subjective reading by an individual physician is vulnerable to considerable unreliability.
• Like PET imaging, SPECT imaging uses a small amount of a radioactive tracer; although the exposure is quite low and single or multiple scans are considered safe, repeated use over time would increase the risk of ionizing radiation toxicity and possibly place the individual at an increased risk for the development of cancer.
Positron Emission Tomography Imaging
PET imaging also tells us about how the brain is functioning. The most common radioactive tracer is 18F-fluorodeoxygluclose (18F-FDG) which assesses metabolism of brain cells. PET scan studies are common in research but also have a role in the clinical setting. The assumption behind all functional brain imaging, such as PET and SPECT, is that there is a close relationship among the brain’s nerve activity, glucose metabolism, and blood flow. The advantage of PET compared to SPECT is that the images have enhanced resolution allowing for improved quantification of the metabolism and blood flow in different brain areas. When different radioligands are used, PET requires a highly trained multidisciplinary staff with expertise in physics, chemistry, computers, medicine, and technology. Certain radioligands are more stable over time (such as radioactively labeled glucose—FDG), and these are commonly used in most hospitals or outpatient clinics. In general, PET scans tend to be more expensive and less widely available than SPECT.
Before the scan, the patient is infused with a small amount of a radiopharmaceutical, or “tracer,” that allows one to measure the blood flow or metabolism on the scan. PET scans can be used to differentiate between the problem of inflammation or blockage in small blood vessels in the brain (as might be seen in inflammatory Lyme encephalopathy) versus a problem with the nerve itself. It is unclear at this point whether PET has any advantage over SPECT for evaluation of an individual patient with possible Lyme disease, but with further research PET scans may emerge as a helpful adjunctive clinical tool in Lyme diagnostics.
PROS
• PET has enhanced resolution compared to SPECT scans.
• It is able to quantify both blood flow and brain metabolism, providing information that helps to tease out whether the brain problem is one of metabolism or blood flow.
CONS
• It is expensive and less widely available than MRI scans.
• It cannot be used to diagnose Lyme disease because findings are nonspecific.
• It requires highly trained, multidisciplinary staff.
Neuropsychological Testing
Neuropsychological testing assesses various aspects of brain function and is usually administered by a specially trained mental health professional or by a technician supervised by a neuropsychologist. Typically a comprehensive selection of tests is administered, including a measure of general intellectual functioning, verbal and visual memory and learning, attention/concentration, verbal fluency, processing speed, fine and gross motor functioning, and executive functioning. Testing may occur over one to two days and may span three to six hours or even longer. Shorter forty-five-minute to one-hour long computer-based neurocognitive batteries do exist; these are useful for screening but should not be considered a replacement for thorough neurocognitive assessment. Measures of depression, anxiety, and personality may also be administered because certain psychological states might affect cognitive performance. Lyme disease has been documented to cause impairments in memory, word finding, fine motor control, language conceptual ability, and motor functioning. The most consistently identified deficits in adults with Lyme disease have been problems with verbal memory, verbal fluency, and mental processing speed. Patients often refer to their cognitive limitations as “brain fog.” Studies by various researches (Keilp et al. 2006; Kaplan and Jones-Woodward 1997; Elkins et al. 1999) support the conclusion that cognitive impairments are not secondary to a psychological response to chronic illness or due to a mood disorder.
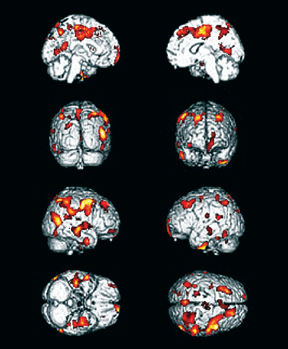
FIGURE 4.3
These are composite images representing areas of low blood flow in the brain of patients with memory impairment after Lyme disease (post-treatment Lyme encephalopathy). The low-flow regions are highlighted in yellow and red. To obtain these images, PET-H2O15 scans from thirty-seven patients with post-treatment Lyme disease syndrome were compared to the PET scans from eighteen age-, gender-, and education-matched healthy controls (Fallon et al. 2009).
• Neuropsychological testing offers an objective measure of a patient’s cognitive functioning at a specific point in time. Patients sometimes experience cognitive problems but perform well on objective testing. This may reflect a high level of intelligence on the part of the patient such that mild reductions in cognition function are not readily recognized as impairment. Alternatively, this may suggest that problems may have more to do with a distorted or negatively-biased perception of one’s functioning than an actual deficit. This may be seen for example among patients with depression or anxiety. It is well known among neuropsychogists that patients’ reports of cognitive difficulties, such as memory problems, do not necessarily correlate with objective data, so that without formal testing doctors may not be entirely sure that self-reported cognitive problems truly reflect a patient’s cognitive capacity. Neuropsychological test results, when interpreted by an experienced neuropsychologist, may help to differentiate between neurological and psychiatric causes of cognitive problems.
• By providing a quantitative result, neuropsychological testing also allows for the monitoring of changes in response to treatment. This can be quite helpful as patients often ask, “Have I gotten any better over the last six months?”
• A better understanding of their own cognitive deficits may allow patients to better cope with them by advanced planning and special accommodations—for example, a patient with auditory attention problems might learn better from visually presented information.
• Comprehensive neurocognitive testing can be costly. This is because the assessment requires not only hours to administer, but also hours after the testing to score the results, analyze the significance, and write the report. A neuropsychologist or psychiatrist may administer an abbreviated battery of tests as a screen to see if further testing is needed. Computer-assisted neurocognitive batteries are available online, but the cognitive domains that can be tested using this approach are not as comprehensive or informative as the in-person testing; this approach, however, is considerably less expensive.
• These tests are not able to diagnose Lyme disease specifically, although they are very effective at picking up brain dysfunction. These tests may be used as additional evidence in support of a suspected diagnosis of Lyme disease.
Electromyography/Nerve Conduction Studies
Electromyography (EMG) and nerve conduction studies (NCS) assess the function of muscles and nerves, respectively. The EMG allows the neurologist to distinguish between muscle and nerve disease and to identify precisely the muscle involved. Nerve conduction studies inform the neurologist about the integrity of sensory and motor nerves. An electrical stimulation is applied to the skin overlying a peripheral nerve to enable a recording of the speed of conduction and the amplitude of the “downstream” action potential, or the electrical impulse traveling through a nerve. Nerve conduction studies assist in the diagnosis of nerve disorders, such as demyelinating neuropathy (slow conduction velocity), axonal neuropathy (reduced amplitude of compound muscle action potential), and nerve root compressions.
PRO
• EMG and NCS can detect muscle or nerve damage.
CON
• Lyme disease cannot be diagnosed on the basis of EMG or NCS alone.
Skin Biopsy to Study Nerve Fibers
Lyme disease can cause a neuropathy, or nerve damage, that results in symptoms of burning, pain, tingling, or numbness, typically in the extremities. These sensory abnormalities may be due to damage to tiny pain and temperature nerve fibers in the skin (i.e., small nerve fibers). To detect small fiber damage, skin biopsies are performed. Many diseases may cause small fiber sensory neuropathy including diabetes, lupus sarcoidosis, Sjogren syndrome, celiac disease, hypothyroidism, and Lyme disease. The skin biopsy procedure is typically performed with a 3-mm disposable circular punch needle with a sterile technique using local anesthesia.
PROS
• Skin biopsy can reliably detect sensory nerve damage.
• The procedure is minimally invasive.
CON
• Lyme disease cannot be diagnosed on the basis of skin biopsy alone.
Electrocardiogram or Echocardiogram
Electrocardiogram (ECG/EKG) and echocardiograms are widely performed to assess the structure and function of the heart. Though the abnormalities detected by these tests, such as partial or complete heart block or carditis, are not specific to Lyme disease alone, they are useful in detecting potential damage that may be caused by the infection.
PROS
• ECG and echo are minimally invasive.
• They can detect abnormalities in the heart’s rhythm and function that may be caused by Lyme disease.
CON
• Lyme disease cannot be diagnosed on the basis of these tests alone.
Autonomic Nervous System Testing
Because Lyme disease can affect the nervous system, tests that probe the autonomic nervous system can sometimes be quite helpful. Heart rate variability testing involves monitoring an ECG during cyclic deep breathing to assess the variability between beats. Tilt table testing involves measuring the heart rate and blood pressure response to a change in position from lying down to standing up (i.e., table tilts vertical). A systolic blood pressure drop of 20 to 30 mm Hg is considered abnormal and a diastolic drop of 10 mm Hg is considered significant. Disturbances of the ability to sweat—a marker of peripheral autonomic nervous system dysfunction—may be one of the earliest signs of a small fiber neuropathy. Sweat gland function can be assessed noninvasively. One such approach applies a low-voltage potential of varying current to the hands and feet; the electrochemical skin conductance is then measured to quantify the sweat gland functioning.
PRO
• Autonomic testing is minimally invasive and inexpensive.
CON
• Lyme disease cannot be diagnosed on the basis of these tests alone.
Most of the neurologic and imaging tools are ancillary tests used to document different effects of infection with B. burgdorferi. Only the Borrelia-specific lab tests—ELISA, immunoblot, IFA, PCR, or culture—can provide the biological evidence needed to confirm current or past infection with B. burgdorferi specifically. The antibody-based tests are being improved by using synthetic peptides that contain only the regions that are highly specific to B. burgdorferi. The emerging “biosignature” approaches that use inflammatory, proteomic, nucleic acid, cellular, or metabolomic biomarker profiles show promise, as do the T-cell stimulation assays. The ancillary tests that are not specific to Lyme disease may reinforce or refute the clinical suspicion of disseminated Lyme disease and may therefore help guide treatment. Because disseminated Lyme disease and PTLDS are complex multisystem diseases, the clinician may make use of many different tests, as if putting a puzzle together, to create a coherent composite picture to help guide diagnosis and treatment.