Embryo Rescue and Cytogenetic Manipulation
Dorin Gupta*; Rebecca Ford†; Prabhakaran Sambasivam†; Sajitha Biju* * School of Food and Agriculture, Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Dookie, VIC, Australia
† Environmental Futures Research Institute, School of Natural Sciences, Griffith University, Nathan, QLD, Australia
Abstract
Lentils, despite their tremendous importance as a human food, an animal feed, and in rotational cropping systems, have remained under-exploited and poorly researched. Although some traditional cytogenetic techniques have aided in our understanding of the lentil genome, the organization and structure of the genome remains largely undiscovered. Understanding the genetic potential of both cultivated and wild lentils is essential for mining useful genetic variations, as well as for adding considerable resources to lentil breeding programs. This chapter addresses the knowledge on available lentil genomics derived through traditional cytogenetics and the potential to improve our understanding through applications of modern cytogenetic manipulations, including the use of fluorescence in situ hybridization (FISH), genome in situ hybridization (GISH), and distant hybridization, coupled with embryo ovule rescue and haploid breeding to widen the genetic resources of lentil.
Keywords
Molecular cytogenetics; Mutation; Embryo-ovule rescue; Haploid breeding; Aneuploids; Double-haploids; Chromosome banding; In-situ hybridization; Linkage mapping
5.1 Introduction
Lentil (Lens culinaris ssp. culinaris Medikus, 2n = 2X = 14) belongs to tribe Vicieae, family Fabaceae (Leguminoseae), and is a diploid self-pollinating legume. The crop provides both environmental and ecological benefits within an agricultural system, including the contribution of nitrogen fixation and breaking cereal crop pathogen and insect pest cycles. The lentil grain contains more than twice the amount of dietary protein (25%), essential amino acids, minerals, and carbohydrates, and is an integral part of the diet of poor people, for whom accessibility of animal protein is limited (Srivastava and Vasishtha, 2012). Among the world’s oldest crops, lentils remain an integral part of agricultural production systems. L. culinaris originated in the Near East around the Fertile Crescent and was later domesticated in southern Turkey with further spread to the Nile, Greece, Europe, and Asia. L. culinaris ssp. orientalis (Boiss.) Ponert is the presumed wild progenitor of the cultivated lentil and is found from western Greece to eastern Uzbekistan and the northern Crimean Peninsula to southern Jordan (Renfrew, 1969; Ladizinsky, 1979; Cubero, 1984). The occurrence of wild lentils around the Mediterranean basin suggests its popularity during the early agricultural settlement followed by its domestication, local adaptation, and further spread around the world, leading to emergence of geographically diverse landraces (Erskine, 1997). However, over time, South Asia became the world’s largest lentil growing area (approximately 48%), with little variation for the lentils of a specific ecotype (pilocea) growing in this region, which remained a major limitation for lentil breeding progress (Erskine et al., 1998).
Lentils are a cool season food legume cultivated in the Indian subcontinent, Canada, Australia, southern Europe, eastern and northern Africa, and the dry areas of the Middle East. Although ancient, the crop has not been exploited to its full potential as reflected by yield plateaus, and the breakdown of disease and insect resistance. Huge selection pressure for developing new varieties and narrow genetic diversity within the cultigen (Ford et al., 1997) are mainly responsible for the crop’s vulnerability to co-evolved pathogens (Podder et al., 2013). In a preferred cereal-based cropping system, lentils have been a crop of marginal lands, which might have contributed to loss of genes for higher productivity and hence further narrowing of the genetic base (Bejiga and Degago, 2000). To improve crop performance, variation should exist in the cultivated gene pool from which desirable genetic selections may be made. When this is not the case, in order to widen this gene pool, distant cultivated and wild relatives are relatively untapped sources of variations for high yields and resistance to stresses which can either be introduced into new regions, hybridized with existing varieties, or utilized in mutation breeding (Erskine et al., 1998). Although wild relative genomes may be used to improve and widen the genetic base of the cultivated gene pool, genetic and cytogenetic manipulations may be required. For this, considerations of genome size, composition, and compatibility/incompatibility leading to fertility barriers must be made.
5.2 Taxonomy of the Lens Genus
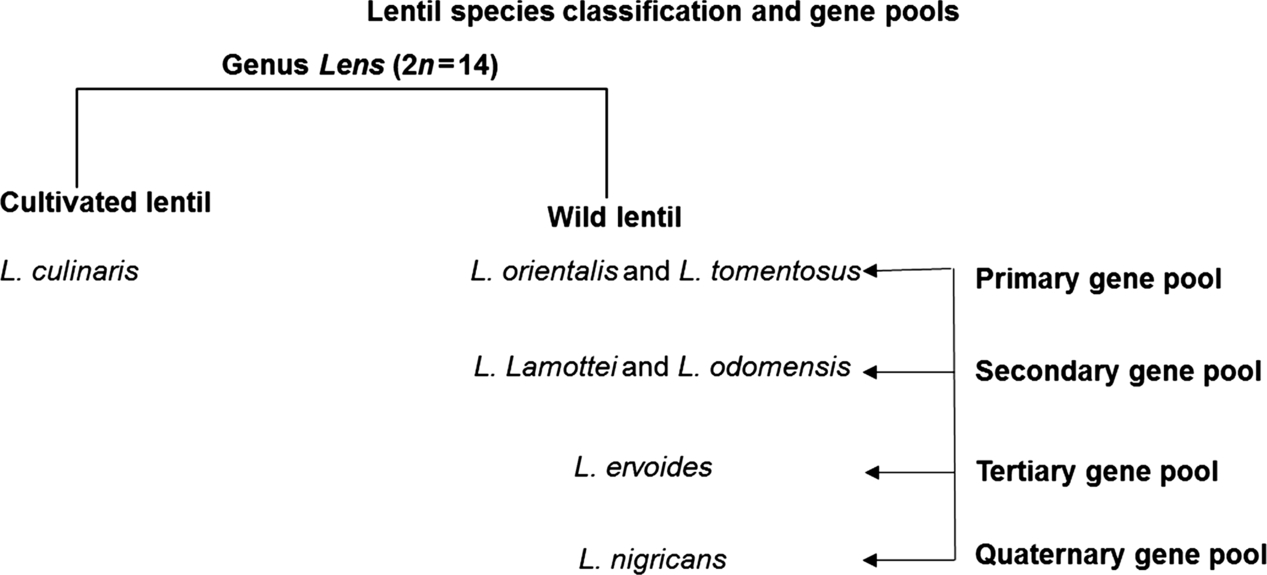
Lentil has a fairly large genome of about 4 Gbp with seven chromosome pairs (Arumuganathan and Earle, 1991). Cultivated lentils are broadly categorized into two classes—small-seeded (microsperma) and large-seeded (macrosperma) based on morpho-physiological traits. Wong et al. (2015) recently classified seven lentil species into four gene pools, in which L. culinaris/L. orientalis/L. tomentosus form primary, L. lamottei/L. odomensis form secondary, L. ervoides forms tertiary and L. nigricans forms quaternary gene pools (Fig. 5.1). Among the seven species, the genus Lens encompasses only one cultivated lentil species (L. culinaris), and the remaining six are wild species. Furthermore, the wild species L. orientalis is presumed to be the wild progenitor of cultivated lentils (Zohary, 1972). Wild species from primary and secondary gene pools are easily crossable with cultivated lentil, unlike with the wild species from the remaining two gene pools (Gupta and Sharma, 2007; Singh et al., 2013).
Traditional and molecular cytogenetic techniques have been employed to identify introgressed chromosomal segments among broad crosses (Goshen et al., 1982; Ladizinsky and Abbo, 1993). These approaches have also been used to aid in fertility restoration for intra/interspecific hybrids (Ahmad et al., 2005; Gupta and Sharma, 2007; Singh et al., 2013). Cytogenetic manipulations include biochemical and molecular biology approaches such as in chromosome banding, fluorescence in situ hybridization (FISH), and genome in situ hybridization (GISH) (Gall and Pardue, 1969; Rayburn and Gill, 1985; Pinkel et al., 1986; Le et al., 1989). These cytomolecular techniques have greatly facilitated the precise study of chromosomes (heterochromatin, centromeres, and telomeres) and their putative functions. In addition, molecular cytogenetics have played a vital role in genome analysis and sequencing of important crops to utilize distant relative genetic resources and combat the risk of genetic erosion by improving the chances of wide hybridization to introgress genes of interest into cultigens such as rice (Cheng et al., 2002), wheat (An et al., 2015; Zhang et al., 2017), brassica (Fredua-Agyeman et al., 2014), and banana (D’Hont et al., 2000).
Cytogenetic analysis of lentil chromosomes enabled an understanding of lentil genome evolution, particularly, species chromosome number and structure. Recently, a clear differentiation and evolution of chromosomes within Lens speciation was reported (Galasso, 2003; Fernández et al., 2005; Gaffarzadeh-Namazi et al., 2007). The future application of these techniques will be to facilitate the transfer of durable gene(s) of interest for biotic and abiotic stresses, and/or agronomical traits of high yield with reduced linkage drag from distant/wild species to provide new sources of variation. Furthermore, embryo-ovule rescue has been used to improve introgression of wild species chromatin into cultivated ones from noncrossable gene pools. The wide hybrids have been studied cytogenetically to ascertain their role in lentil breeding programs. Advanced cyto-molecular/genomic techniques have provided a clearer picture of introgressed chromosomes and/or chromosome arms, and aided in the association of physical and genetic maps and mapping of chromosome-specific markers. The creation of aneuploids is another method to introduce novel genetic variation in order to create new genetic combinations for desirable traits. These can be generated through mutagenesis in higher plants (Nagar, 2013) including lentils (Kumar and Gupta, 1998; Gupta et al., 1999), chickpeas (Pundir et al., 1983), and green grams (Sharma, 1990).
5.3 Cytogenetics of the Lens Genus
Traditional cytogenetic techniques were employed in the 20th century to understand the lentil genome. Bhattacharjee (1953) studied lentil meiotic chromosomes in detail using oxyquinoline. However, by that time, it was already known that there were six species within genus Lens with a basic chromosome number (n), which varied from five to eight. He reported the diploid chromosome number of lentil 2n = 14, which was in agreement with the earlier reports of Heitz (1926), Bleier (1928), and Miranda (1931), and for karyotype analysis with Bhattacharjee (1951). He concluded that the number of nucleoli corresponded with the number of secondary constrictions, which were a maximum of four in the somatic chromosomes. However, the meiotic study also revealed considerable irregularities in the behavior of the chromosomes and irregular disjunctions leading to pollen grain development with chromosome numbers greater or less than seven. Based on these findings, he suggested that the plant under study was not a true diploid (Bhattacharjee, 1953).
For almost 30 subsequent years, classical cytogenetic manipulations were the key drivers to further clarifying the number and status of wild Lens species, the wild progenitor of cultivated lentils, and the chromosomal structures and number of the various Lens species. Ladizinsky (1979) greatly expanded the knowledge in these areas using morphological and cytogenetic studies. He suggested that Lens comprised five annual species, four being wild (L. montbretii, L. ervoides, L. nigricans, and L. orientalis) with only L. culinaris being the cultivated species. He further suggested that L. nigricans, L. orientalis, and L. culinaris had the same chromosome number (2n = 14). He further confirmed the findings of Barulina (1930) and Williams et al. (1974), suggesting that the origin of cultivated lentil (L. culinaris) was L. orientalis. He also confirmed the similarity of the L. culinaris karyotype to fourteen L. culinaris lines studied by Sharma (1962). However, Williams et al. (1974) suggested a different karyotype for L. culinaris, and Ladizinsky subsequently reported that L. orientalis genotypes were cytogenetically uniform but differed from L. culinaris by a single chromosome interchange (Ladizinsky, 1979). Meanwhile, L. nigricans and L. culinaris cytogenetically differed by three chromosome interchanges (the presence of a large number of univalents, rod bivalents, trivalents, quadrivalents, and pentavalents) (Ladizinsky, 1979). However, their hybrids were fertile, indicating a monophyletic origin (Ladizinsky, 1979). Subsequently, Ladizinsky et al. (1984) proposed two species of Lens (L. culinaris and L. nigricans). Among these two species, L. culinaris included cultivated lentil (L. culinaris ssp. culinaris) and L. culinaris ssp. orientalis (wild progenitor of cultivated lentil) and L. culinaris ssp. odomensis.
In another study, the F1 progeny of a L. culinaris x L. nigricans cross showed irregular chromosome pairing due to the differences of three interchanges among the parents (Goshen et al., 1982). Only 19% of the F2 plants were meiotically stable and possessed chromosomal arrangements similar to that of cultivated lentil (Goshen et al., 1982). Slinkard (1985) studied karyotypes of all lentil species and suggested that seven pairs of chromosomes have median or near-median centromeres with a secondary constriction near the centromere of one or two pairs of chromosomes. Based on these findings and chromosome paring of F1, he confirmed Ladizinsky’s findings that L. culinaris and L. nigricans form two crossable groups with subspecies of L. culinaris spp. culinaris, L. culinaris spp. orientalis, L. culinaris spp. odomensis, L. nigricans spp. nigricans, and L. nigricans spp. ervoides. Slinkard’s (1985) classical cytogenetic and crossing results suggested the genus Lens should be considered one large gene pool from a breeding viewpoint, but as two separate gene pools for evolutionary consideration.
5.3.1 Cytogenetic Manipulations and Ploidy Status
The 1980s saw some remarkable work in classical cytogenetics and exploration of the wild species. Among various cytogenetic manipulations, polyploidy revolutionized monocots and dicots, and was considered an important crop improvement tool. The application of colchicine was a common method to artificially induce polyploidy, through chromosome doubling, also known as colchiploidy. Malaviya and Shukla (1983) undertook cytogenetic analysis of lentil cultivars to understand the presence and extent of ploidy level. They revealed three triploid and two tetraploid plants among 100 plants of variety WYR134 and one triploid plant in ILL467. Cytogenetic studies of these polyploids exhibited irregular meiotic events such as abnormal chromosomal synapsis, non-oriented chromosomes at metaphase 1, the presence of laggards, unequal chromosome numbers at the poles, and a high percentage of pollen sterility. They also supported the theory of a stabilized diploid level in Lens. One year later, Malaviya and Shukla (1984) reported cytological features of six colchiploid plants. They concluded that the developed colchiploids were not stable and observed tetraploid and mixoploids (pollen mother cells with 29 and 27 chromosomes) as well as irregular chromosomal behavior during different meiotic stages, which could further explain why natural polyploids do not exist in lentils. Understanding and utilizing constancy of nuclear DNA content of a species through cytogenetic manipulation helps to understand the evolutionary relationships (Rees, 1984). Considerable nuclear DNA content variation among interspecific individuals has been reported by various researchers for different crops including several legumes (Schweizer and Davis, 1972; Furuta, 1975; Laurie and Bennett, 1985). Ramesh (1994) looked for variation in chromatin content, nuclear DNA amount, and distribution of DNA among different chromosomes and also performed somatic karyotyping of ten cultivated lentil genotypes. They concluded that intervarietal variation was significant for chromatin and nuclear DNA content. Metacentric chromosomes were the highlight of the symmetric lentil karyotypes, with only one chromosome pair detected with secondary constrictions near to the centromere in only two of ten varieties. The 2C DNA content was positively correlated with total chromosome volume and nuclear area, and DNA density was negatively correlated with nuclear area. Ramesh (1994) consequently suggested that the changes in DNA content with genotypic evolution have affected all chromosomes at different magnitudes. In summary, using traditional cytogenetic approaches, many contradictory reports arose on the exact lentil karyotype (Bhattacharjee, 1953; Ladizinsky, 1979; Sharma, 1962; Williams et al., 1974; Ladizinsky et al., 1984; Goshen et al., 1982; Slinkard, 1985; Malaviya and Shukla, 1983, 1984). More advanced cytogenetic technologies were subsequently employed to more accurately identify the Lens chromosomes and establish robust karyotypes as follows.
5.3.2 Chromosome Banding Techniques
Kannan and Zilfalil (2009) reviewed and summarized various cytogenetic techniques. Among the banding techniques, Q-banding was the first (Caspersson et al., 1970) which involved the use of fluorochrome (quinacrine mustard or quinacrine dihydrochloride) to stain chromosomes followed by their examination under fluorescence microscopy. The G-banding technique (Rowley, 1973) became popular as this had a better resolution than Q-banding, where chromosomes were stained with Giemsa solution. Giemsa C-banding technique was also developed (Pardue and Gall, 1970), which darkly stains the constitutive heterochromatin regions of chromosomes, and helps to identify polymorphic regions of chromosomes and multiple centromeres of a chromosome. As the name implies, another technique, nucleolar organizing region (NOR)-banding or N-banding (Matsui and Sasaki, 1973) stains chromosomal NORs. This can localize centromeric heterochromatin and identify the NORs of SAT chromosomes in eukaryotic genomes that give rise to the nucleolus (and thus ribosomes), and identifying their exact location on a chromosome is of great significance for precise genome analysis. Mehra et al. (1986) used N-banding for karyotype analysis of lentils and to locate satellite (SAT) chromosomes. Near metacentric chromosomes were reported to be large, whereas, the small chromosomes were submetacentric. Further, they inferred that the SAT chromosome had comparatively small, short arms between the primary and secondary constrictions. These findings suggested the SAT chromosome as an acrocentric or sub-telocentric chromosome. Mehra et al. (1986) explained that the existence of the large satellite was responsible for the appearance of the SAT chromosome as a sub/medium-metacentric chromosome detected in earlier studies (Sinha and Acharia, 1972; Ladizinsky, 1979). The power of this new cytogenetic technique clarified Sinha and Acharia’s (1972) findings of not being able to locate the lentil SAT chromosomes using simple cytogenetic techniques and conventional staining; hence, the two constrictions might have appeared as one complete section. However, the karyotypic analysis using the N-banding technique (Mehra et al., 1986) was in agreement with that reported by Sinha and Acharia (1972), that lentils possessed large metacentric chromosomes, and that the SAT and small chromosomes were submetacentric. Meanwhile, the Mehra et al. (1986) finding differed from that of Ladizinsky (1979) who suggested that lentils possess a SAT chromosome as medium metacentric and a small chromosome as acrocentric.
Gaffarzadeh-Namazi et al. (2007) used C-banding to overcome gaps in the karyotypic knowledge of lentil chromosomes and identified individual lentil chromosomes which had four pairs of metacentric and three pairs of sub-metacentric chromosomes. They also assessed C-banding polymorphism among lentil lines and reported each chromosome to have a unique C-banding pattern. Also, they reported that the long arm of Chromosome 4 has a secondary constriction near the centromeric region, which was in agreement with earlier studies (Slinkard, 1985; Raziuddin et al., 1990; Ahmad et al., 1992). Gaffarzadeh-Namazi et al. (2007) also agreed with Kumar and Gupta (1997) and Galasso et al. (2001) in identifying the SAT chromosome as the fourth largest chromosome among the seven. This differed from Ahmad et al. (1992), who reported that the SAT chromosome was the second longest.
5.3.3 Cytogenetic and Mutation Studies
Mutation to increase genetic variation can be induced artificially to develop new varieties in less time than spontaneous mutation (Micke et al., 1990). Various chromosomal aberrations arise due to mutagen treatment including chromosomal interchanges/reciprocal translocations (exchange of non-homologous chromosome segments). Ionizing radiation has been used in various plant species to produce such interchanges (Gill et al., 1980). These interchanges can be employed as cytogenetic markers which play an important role in creating new variations and basic material for cytological studies (Burnham, 1932). From a plant cytogeneticist’s viewpoint, interchanges with known chromosome involvement are valuable, particularly when a tester set can be made from many interchanges (Gupta and Gupta, 1991; Gupta, 1995) for use in gene mapping (Mahama et al., 1999). Burnham (1962) and Gupta (1995) suggested analysis of somatic chromosomes, analysis of pachytene configurations, semi-sterility of genetic markers, meiotic analysis of the F1s developed through crossing of known trisomics and interchanges, and the crosses between unknown interchanges as methods to identify these potential interchanges. Understanding pairing configurations during meiosis provides insight into chromosome structure rearrangement, which is a significant contribution to understanding the plant’s evolution (King et al., 1993). Also, there are some successful examples of important trait transfers in crops like wheat and barley using reciprocal translocations (Driscoll and Sears, 1965).
Kumar and Gupta (1998) were the first to report the isolation of a complex translocation monosomic lentil plant from the F2 generation of a cross of two translocation homozygotes with different chromosomes. They found interchange heterozygotes carrying two trisomics (2n = 2x + 1 = 15) from the progeny of the monosomic. Simple microscopic meiotic studies of these plants revealed the presence of multivalents, which reflected the presence of multiple interchanges, possibly in five of the seven non-homologous chromosomes of the monosomic. This may help to explain why trisomic plants do not exist, since one of the studied plants had abnormal morphological traits (compact branching, narrow leaves, and dwarf nature) due to an extra chromosome. Gupta et al. (1999) described the significance of a standard interchange tester set for a plant species to identify individual chromosomes involved in interchanges, or aneuploids by studying crosses with the testers. Among different methods to identify individual chromosomes, they used meiotic analysis of the diallel cross (interchange homozygotes) hybrids, and chromosome morphology alterations to identify individual chromosomes. An interchange tester set can be utilized for chromosome mapping, and more specifically, for the identification of particular chromosomes, which might be involved in an interchange. To create this, Gupta et al. (1999) irradiated the seed of cultivar PL-639 with different doses of gamma rays and produced many chromosome interchanges. From studies of meiotic configurations at metaphase I and somatic chromosomes, they identified 14 interchange homozygotes through altered chromosome morphology, including seven interchange homozygotes assembled in the tester set. Nine of these 14 interchange homozygotes had easily identifiable interchanged chromosomes due to changed total lengths and the ratios of long and short arms of each chromosome. In a recent study, Goyal and Verma (2015) irradiated lentil seeds with different doses of gamma rays, and with different concentrations of ethyl methane sulphonate (EMS). They selected two mutant plants, one of which was a translocation heterozygote (gamma rays treated), and the other, an inversion heterozygote (EMS treated). At various meiotic stages, the plants showed different types of chromosomal configurations in most of the pollen mother cells such as the ring/chain formation of four and six chromosomes of the translocation heterozygote. Meanwhile, the inversion heterozygote bridged and contained fragments which were attributed to the crossover number and position in the inverted segment. In general, though, the inversion heterozygote had poor pollen fertility compared to the translocation heterozygote (Goyal and Verma, 2015).
In summary, there are few reports in which cytogenetics has played a role to understand changes in the lentil genome through tracking of induced mutations. This approach has not been used anywhere near the extent it has in other major crops. This leaves a large scope for future studies to understand these variations more precisely and to assess their potential for use in lentil mutation breeding programs.
5.3.4 Molecular Cytogenetics
Molecular cytogenetic approaches involving FISH and GISH of highly repetitive DNA sequences, restriction site analyses and nucleotide variations have proven to be a valuable tool in the more precise physical visualization of chromosomes. These are also used in the identification of structure, function, organization, and localization of chromosomes, genes and DNA sequences. These methods involve hybridization of DNA probes to denatured metaphase spreads, followed by direct or immunological staining. The probes used in FISH can be region-specific, including ribosomal DNA (rDNA), arm-specific, and chromosome or genome-specific. By employing this method, one can identify with accuracy the chromosomes involved in the organization of the nucleolus and the exact location of genes. They can also be used to detect numerical changes in chromosomes and to compare the genomes of interspecific or intergeneric hybrids (Lombello and Pinto-Maglio, 2004). FISH and its modifications, such as multiple-target fluorescence in situ hybridization (multicolor FISH) (Mukai, 1996), extended DNA fiber-FISH (Ersfeld, 2004), bacterial artificial chromosome (BAC)-FISH (Hanson et al., 1995), and super-stretched pachytene-FISH (Koo and Jiang, 2009), have been employed to reveal the minute details of chromosome structure and subsequently permit sophisticated analyses of chromosomal behavior (Sharma et al., 2016).
GISH is a technique used to identify alien chromosomes as well as chromatin and chromosomal rearrangements formed due to mosaic chromosomes (Ramzan et al., 2017). GISH distinguishes genomic relationships within polyploids. Also analysis of genome composition, visualization, and chromosome discrimination from different genomes in allopolyploids are carried out by GISH (Singh, 2003). GISH techniques follow the same procedure as FISH, but genomic and blocking DNA are utilized in GISH. These two techniques have been employed in crop improvement programs, molecular systematics, and in the conservation and utilization of plant genetic resources (Hizume et al., 2002; Chaudhary et al., 2011). They have been employed to unravel individual chromosomes in depth specifically in legumes like chickpea (Khattak et al., 2007), soybean (Findley et al., 2010, 2017), medicago (Pires et al., 2012), garden peas (Greilhuber and Ebert, 1994), faba beans (Caperta et al., 2008), sweet pea (Seijo and Fernández, 2003) and peanut (Robledo et al., 2009). However, these molecular chromosomal visualization techniques have not been so extensively employed in lentil. Most of the studies in lentils have focused on physical and genetic comparative mapping (Bhattacharjee, 1951; Sharma, 1962; Naithani and Sarbhoy, 1973; Gupta and Singh, 1981; Nandanwar and Narkhede, 1991; Tullu et al., 2011; Jha et al., 2015).
Abbo et al. (1994) used FISH to localize the nucleolus organizer region (NOR) in somatic chromosomes of L. culinaris and detected the hybridization sites of an rDNA probe coding for the 18S, 5.8S, and 26S genes. One conserved pair of hybridization sites of a ribosomal probe were detected among L. culinaris and L. orientalis, which corresponded to the secondary constriction site. Galasso et al. (2001) subsequently used FISH to characterize the chromosomal location of two repeated DNA sequences (pLc30: 466 bp long with 64% AT residues, and pLc7: 408 bp long with 61% AT residues). They reported two families with sequence differences, where the pLc30 family had four internal repeats and the pLc7 family had short direct sub-repeats. When they looked into the presence of these families in various Lens species and other genera, pLc30 was found only in lentil species; however, pLc7 was present in other genera. The FISH results further showed that pLc30 sequence hybridized on six of seven chromosomes, whereas, pLc7 hybridized with only one chromosome pair. A more robust identification of the seven chromosome pairs was proposed based on nine different hybridization sites of the pLc30 family. This led to assembly of a physical map based on FISH results with pLc7, 5S rRNA, 18S–5.8S–25S rRNA genes, and a telomeric sequence. They demonstrated that in situ hybridization with these two probes (pLc30 and pLc7) allowed the discrimination of all seven chromosome pairs and enabled the construction of a L. culinaris standard FISH karyotype (Galasso et al., 2001).
Meanwhile, Kumar and Ramesh (2001) provided evidence for two pairs of chromosomes carrying 18S–5.8S–25S rRNA loci by FISH using the interchange heterozygote (where a satellited chromosome is involved in the interchange) and decondensed prophase chromosomes. Balyan et al. (2002) also used Feulgen staining as well as fluorescent in situ hybridization for chromosomal analysis of five different species of Lens and observed that the position of the secondary constriction in cultivated and some wild species, namely L. culinaris, L. orientalis, L. odomensis, and L. ervoides, was close to the centromere, that is, interstitial. However, in L. nigricans, the position of secondary constriction was near the telomere of an acrocentric chromosome pair. Thus, the five species had similar karyotypes with minor modifications, which may be due to the chromosomal interchanges of chromosomal fragments of similar size during the evolution of the Lens genus.
Balyan et al. (2002) summarized the work of various researchers from the 1950s to the early 1990s (Bhattacharjee, 1951; Sharma, 1962; Sinha and Acharia, 1972; Naithani and Sarbhoy, 1973; Keshwani, 1973; Gupta and Singh, 1981; Sharma and Gupta, 1982; Lavania and Lavania, 1983; Nandanwar and Narkhede, 1991), who reported zero to three SAT chromosomes in cultivated lentils. However, in their own study, they concluded that the 18S–5.8S–25S rRNA genes and 18S–5.8S–25S rDNA (the intergenic spacer regions) are tandem repeats specifically present at the NORs and possibly localized at other chromosomal sites without connection with NORs. This was suggested by earlier studies (Mukai et al., 1991; Leitch and Heslop-Harrison, 1992; Jiang and Gill, 1994; Pedersen and Linde-Laursen, 1994). Balyan et al. (2002) suggested that since the 5S rRNA genes are present at one or more loci per chromosome set and occur in tandem repeats, these together with 18S–5.8S–25S may be detectable by FISH. Balyan et al. (2002) subsequently successfully mapped 18S–5.8S–25S rRNA at a single locus on a single chromosome pair and 5S rRNA at two loci with varying sizes of two different repeat units on two different chromosome pairs for each of the five species. They further suggested the use of the rRNA loci as landmarks for chromosome identification, which were found at different positions on chromosomes in different species. Their studies concluded that the karyotypes of all five species which was considered to be the same, were in fact different on the idiograms and the rRNA loci physical map.
Galasso (2003) conducted multiple-target fluorescence in situ hybridization studies (multicolor FISH) for the simultaneous detection of multiple targets to study the repeated DNA sequences in Lens species. For this, two highly repetitive sequences (pLc30 and pLc7) isolated from the cultivated lentil and the multigene families for the 18S–5.8S–25S (pTa71) and 5S rRNA (pTa794) from wheat were used simultaneously as probes. Each species showed a typical FISH karyotype, and few differences were observed among accessions belonging to the same species, except for the accessions of L. odomensis. The FISH karyotype most similar to the cultivated lentil was that of L. orientalis, whereas L. nigricans and L. tomentosus showed the most divergent FISH patterns compared with all taxa based on number and location of pLc30 and 18S–5.8S–25S rDNA sites. Sonnante et al. (2003) then isolated the internal transcribed spacer (ITS) region of the nuclear ribosomal DNA of Lens sp. They reported sequence divergence of 14 base substitutions between L. nigricans and L. lamottei, but close phylogeny among cultivated lentil to L. orientalis (wild progenitor) at this region. They proposed L. lamottei and L. tomentosus as separate species based on unusual autapomorphies.
Other than the FISH technique, restriction endonuclease digestion of DNA and the squash technique have also been employed to understand evolutionary relationships in Lens. Mayer and Soltis (1994) studied chloroplast DNA (cpDNA) variation using 399 restriction-sites to determine relationships among 114 cultivated and 11 wild accessions. They also used this approach to ascertain the wild progenitor of cultivated lentil and to construct a cpDNA phylogeny. Most of the accessions had the same cpDNA except three from the cultivated genome. Among the wild accessions, they identified restriction-site mutations and length mutations and confirmed L. orientalis as the wild progenitor. Based on limited cpDNA diversity, they were also able to conclude the possible loss of cytoplasmic variability during domestication. Based on ten autapomorphic restriction-site mutations, they also determined that L. nigricans was the most divergent taxon of the genus.
Recently, Pal et al. (2016) used the squash technique to study the somatic chromosome and used sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for seed protein profiling of five lentil varieties. Karyotype analysis highlighted small differences for each variety producing individual karyotypes, and protein profiling revealed an expected low diversity.
5.4 Wide Hybridization, Embryo-Ovule Rescue and Haploid Production
5.4.1 Embryo-Ovule Rescue
Plenty of evidence points to wild lentils as a useful source of untapped genetic variations for important agronomical traits, disease resistances, and abiotic stress tolerances. Several attempts have been made to introgress desirable traits from wild lentils to the cultivated gene pool with the eventual aim of selecting promising recombinants from backcrossing to advanced cultivated accessions (Gupta and Sharma, 2006, 2007; Fiala et al., 2009; Gupta et al., 2012; Tullu et al., 2013; Singh et al., 2013). Indeed, introgressed populations have shown transgressive segregants which surpass their parents in those traits, indicating the creation of new recombinant progeny that may be utilized in breeding programs. Among cultivated and wild lentil species, crosses are easy to make within the primary, and to some extent, between the primary and secondary gene pools. (Gupta and Sharma, 2007; Singh et al., 2013). Although some genotypic variation for the success of crossability with cultivated lentils exists even within these closer gene pools (Gupta and Sharma, 2007; Singh et al., 2013, 2017), the search for significant variation for selective purposes may need to go much wider and hence into the tertiary and quaternary gene pools. These more distantly related gene pools contain more desirable chromatin for stress resistances, which is lacking or easily broken in cultivated varieties (Gupta and Sharma, 2007; Singh et al., 2013; Tullu et al., 2013; Dadu et al., 2017).
Interspecific hybrids of cultivated x L. orientalis (primary gene pool) and L. odomensis (secondary gene pool) have been successfully developed through conventional hybridization techniques (Vandenberg and Slinkard, 1989; Fratini et al., 2004; Gupta and Sharma, 2007; Singh et al., 2013). However, the hybrid fertility may be affected by altered chromosomal arrangements (Ladizinsky et al., 1984; Gupta and Sharma, 2007). A far reduced success rate is achieved in obtaining fertile interspecific hybrids from crossing between cultivated lentil and wild species from the tertiary and quaternary gene pools such as L. ervoides x L. nigricans. For example, Goshen et al. (1982) studied interspecific hybrids of cultivated x L. nigricans crosses and found irregularities in meiotic analysis of the hybrids arising from the difference between parental chromosomes for three translocations. Conversely, cytological analysis of 14 populations of cultivated x L. orientalis crosses by Ladizinsky and Abbo (1993) revealed similar chromosome compositions as cultivated lentils.Hamdi and Erskine (1994), Gupta (2003), and Kumari et al. (unpublished) selected promising progenies from a bulk segregating population of cultivated x L. orientalis or L. odomensis crosses that exhibited high variability and high yields. However, the same authors had little success when crossing cultivated lentil with species from the tertiary and quaternary gene pools, likely due to pre- and postfertilization barriers (Gupta and Sharma, 2005). Prefertilization barriers exist mostly due to asynchronous flowering which may be rectified with staggered sowing. On the other hand, postfertilization barriers are mostly due to embryo failure (Gupta and Sharma, 2005), which may be resolved through embryo-ovule rescue techniques. Indeed, conventional hand-pollinated crossing and in vitro rescued embryos remains the only feasible approach for successful interspecific hybrid recovery in many cases (Bridgen, 1994; Sharma et al., 1996).
An embryo-ovule rescue technique is used to overcome interspecific fertilization barriers due to hybrid embryo abortion (Sharma et al., 1996). The first evidence of hybrid embryo abortion of such interspecific crosses in Lens was evident from the findings of Ladizinsky et al. (1985). They reported shrivelled non-viable seeds from wide cross-derived pods, which ceased development 10–16 days after pollination. This is one of the major impediments to uninterrupted gene flow from these wild species into cultivated lentils. To solve this, the embryo-ovule rescue technique was first used to create hybrids between cultivated lentils L. nigricans and L. ervoides (Ladizinsky et al., 1984). Abbo and Ladizinsky (1991) similarly observed hybrid embryo abortion 14 days after pollination in cultivated lentila x L. ervoides and upon examination of the aborted embryos, reported the presence of small cotyledons, shoot and root primordia, and endosperm remnants. They subsequently optimized the embryo rescue techniques to obtain vegetatively normal hybrids.
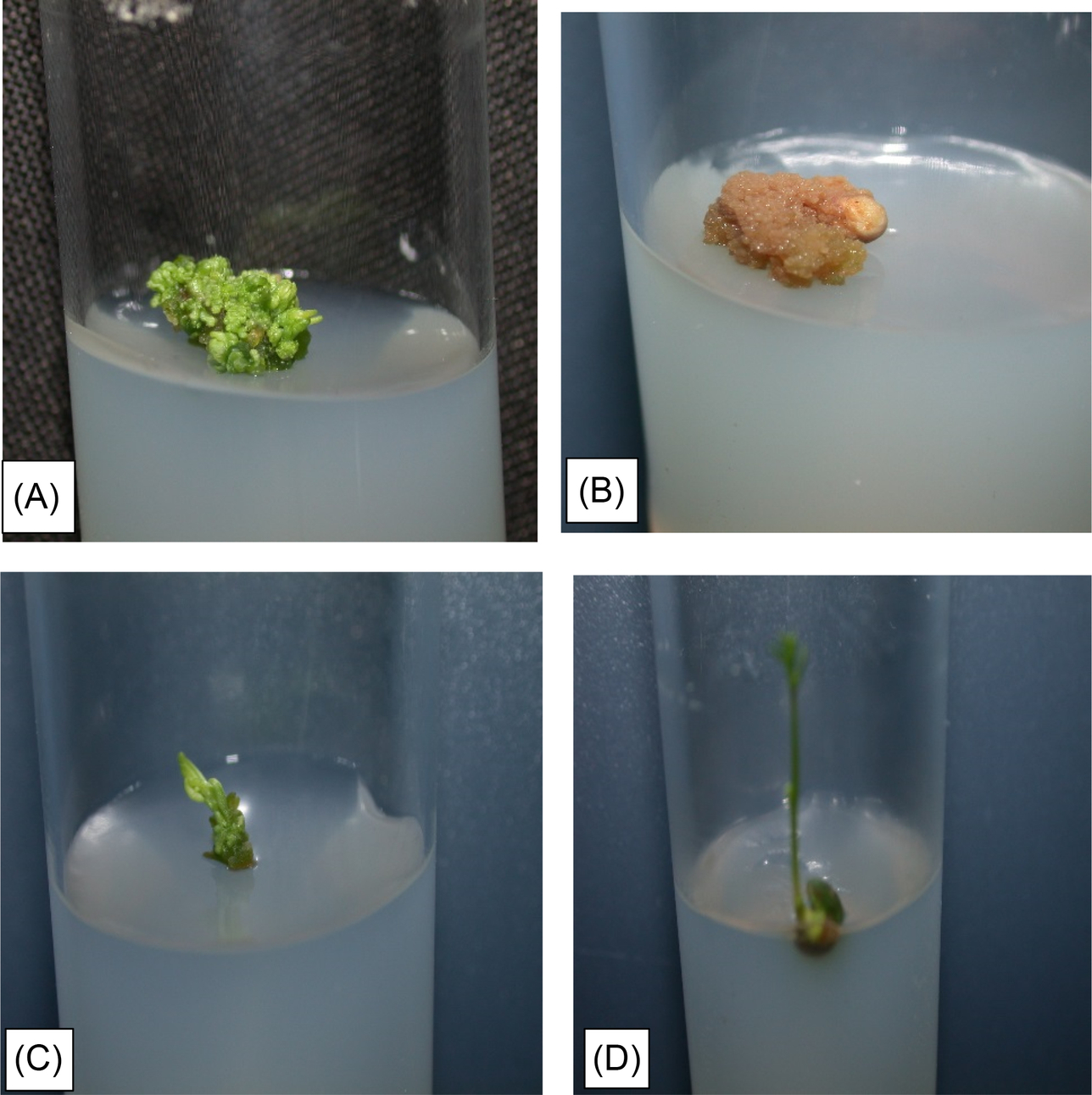
Abbo and Ladizinsky (1994) shed new light in understanding the cause of embryo abortion and suggested that chromosomal aberrations might not be the main reason for F1 embryo abortion of L. orientalis x L. culinaris and L. ervoides x L. culinaris cross combinations. However, they did suggest a strong effect of dominant gene action on embryo abortion, and both dominant and additive gene action was inferred from segregating populations. Ahmad et al. (1995) successfully crossed cultivated lentil with L. orientalis, L. odomensis, L. ervoides and L. nigricans. This was achieved using the plant growth regulator gibberellic acid (GA3) with success rates from 50% to 100%, but with much lower rates of healthy hybrid seeds. Further in vitro culturing of hybrid embryos that did not grow was unsuccessful with death after 3 weeks. Ye et al. (2002) then developed a seed culture based protocol for efficient in vitro propagation of hybrid seed and obtained multiple shoots with the addition of cytokinins such as benzyl adenine (BA), thidiazuron (TDZ), and kinetin. They reported that Murashige and Skoog (MS) media was more optimal for shoot induction than Gamborge (B5) basal media. Among the three cytokinins used, BA and TDZ produced more shoots than kinetin and a higher calcium concentration was beneficial to avoid shoot-tip necrosis. They also successfully induced rooting using MS medium supplemented with Naphthaleneacetic (1-NAA) for more than 50% of the shoots obtained. Later, in order to rescue wide hybrid embryos, a two-step in vitro method was followed by Gupta and Sharma (2005), in which ovules were rescued to obtain viable hybrids of cultivated lentil crossed with L. ervoides and L. nigricans (Fig. 5.2). For this, they used the embryo culture method described by Cohen et al. (1984) with some modifications to the culture media. Ovules of about two-week-old selfed and hybrid crosses were transferred to standard Murashige and Skoog medium (MS stock solutions) + 100 g sucrose + 0.5 mg/L GA3 + 0.5 mg/L BAP + 0.2 mg/L IAA + 6 g/L agar. Later, these were moved for shoot-regeneration to MS media containing BAP (0.5–1.0 mg/L), kinetin (0.5–1.0 mg/L), IAA (0.2 mg/L), sucrose (30 g/L) and agar (6.0–8.0 g/L). These were kept at 25 ± 1°C in the dark for 3 days and then placed under 12 h/12 h daylight/dark. After 7 days, the embryos were excised from the ovules and placed into test tubes containing MS media with eight different types and concentrations of growth-hormones, amino acid sources, and agar. This was done to optimize the MS medium suitable for embryo growth and further differentiation into shoots and roots. Although they observed single and multiple shoot differentiation on the hybrid embryos, the embryos failed to differentiate roots (Fig. 5.3).
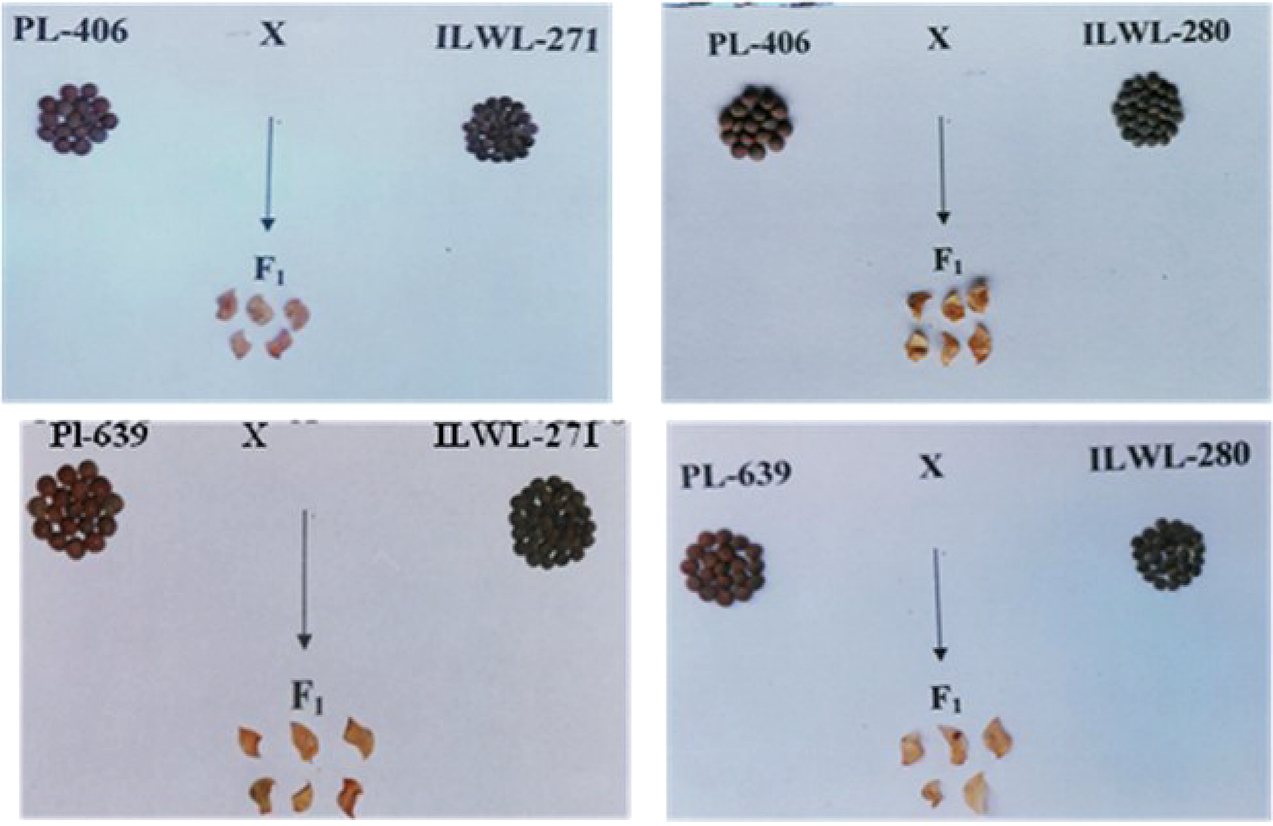
In 2006, Fratini and Ruiz reported obtaining the first successful interspecific hybrids from a cultivated x L. odomensis cross following Cohen et al.’s (1984) embryo rescue protocol. They also proposed a new protocol where ovules were first cultured after 18 days of pollination. The medium was comprised of MS Salts, sucrose (1%), indole acetic acid (1 μM), and Kinetin (0.8 μM). Once ovules had been established in vitro for 2 weeks, the embryos were excised from the ovules and maintained on the same medium. They successfully obtained interspecific plantlets of cultivated x L. odomensis (6), as well as cultivated x L. nigricans (2) and cultivated x L. ervoides (1) and concluded a rescue efficiency range of 50% to 100% based on hybrid recovery per number of ovules cultured. Later, Fratini and Ruiz (2011) published an embryo-ovule rescue method and compared this with Cohen’s method and suggested that the difference in the efficacy of the two methods was due to the media and carbohydrate content used. Cohen et al. (1984) used 10% and 3% of sucrose in the media and Fratini and Ruiz (2006) only used 1% sucrose. One of the most successful attempts to transfer genes of interest from wild to cultivated lentils using the embryo culture technique was reported by Fiala et al. (2009), who used this method to transfer resistance for two races of anthracnose from L. ervoides to cultivated lentils. Individual F2 plants were then raised to obtain F7:8 recombinant inbred lines through single-seed descent (SSD).
Transgenic plant technology, which offers the opportunity to transfer genes of interest from wider sources, is also reliant on successful in vitro culturing of transgenic explants. A regeneration protocol which is efficient and reliable is an important prerequisite for genetic transformation success. Sarker et al. (2003) developed an agrobacterium-mediated transformation system for two lentil microsperma varieties to test the transformation ability of different explants (cotyledonary node, decapitated embryo, immature embryo, and epicotyl). They reported GUS positive regions with the highest percentage for epicotyl explants followed by decapitated embryo. However, they found decapitated embryo to be most useful for multiple shoot formation on MS medium + 0.5 mg/L BAP + 0.5 mg/L Kn + 0.1 mg/L GA3 + 5.5 mg/L tyrosine and with stable expression of the GUS gene in transformed shoots. Recently, the use of a small portion of the embryo axis with the cotyledon as the explant has been suggested as an efficient lentil regeneration protocol (Tavallaie et al., 2011). Bagheri et al. (2012) studied different hormone treatments and explants to determine the best reproducible protocol for in vitro regeneration of an Iranian cultivar. Among 13 different hormone treatments and 4 explants for callus induction and regeneration, modified MS medium containing 1 mg/L α-NAA and 1 mg/L Zeatin (medium E) and decapitated embryos attached to 1/4 of the cotyledon produced the highest fresh and dry weight callus with highest shooting and rooting responses (75%). Recently, Suvorova (2014) successfully used an ovule rescue technique to obtain hybrids from cultivated lentil x L. tomentosus (ILWL 90 and ILWL120) crosses. Three hybrids were recovered from 296 rescued ovules. Hybrids and F2s developed only through the ovule rescue technique from a cross of cultivated and ILWL 90 were either sterile or partly fertile. However, hybrids of cultivated and ILWL 120 crosses were fertile whether obtained through ovule rescue or without ovule rescue, indicating a strong genotypic effect. They were successful in generating breeding recombinant lines of high productivity through selection of plants in F2–F7 generations of this cross. However, concern was raised about the taxonomic status of ILWL 120 as a member of L. tomentosus due to the ease of crossability.
To overcome the rooting issue, Yuan et al. (2011) tested the feasibility of in vivo intergeneric grafting techniques using faba bean as a rootstock for lentil scions. When an accession of each of six wild Lens species was used as the scion in grafts to faba bean breeding line FB50-9 rootstock, successful grafts were obtained for all species with survival to seed maturity rates of between 70.7% and 87.7% except for the L. orientalis accession (55.3% survival). Days to flowering and seed parameters were the same between control and grafted plants, indicating this technique might be useful to considerably speed up efficient interspecific lentil hybrid production with unique wild genetic resources. Khentry et al. (2014) reported successful in vitro propagation of six lentil genotypes (Digger, Indianhead, Nipper, Northfield, ILL 7537, and ILL 6002). In vitro regenerated nodes were transferred to MS medium + 4 mg/L α-naphthalene acetic acid which resulted into about 80% of the regenerated plantlets be normal plants under in vivo conditions. Their finding was useful to report the efficient establishment of roots of normal morphology. More recently, Saha et al. (2015) used an in vitro embryo rescue approach followed by in vivo grafting of the rescued embryo shoots onto faba bean root stocks for the successful production of wide Lens hybrids. Accordingly, they rescued embryos from 14-day-old immature hybrid seeds and cultured them on a medium containing auxin 4-chloroindole-3 acetic acid (4-Cl-IAA) and zeatin combinations that led to proliferation of shoots, and they did not observe any significant difference in shoot elongation for 4-Cl-IAA verses IAA treatment. Subsequent in vivo grafting of hybrid shoots onto faba bean root stocks resulted in large numbers of F2 seed from crosses between cultivated lentil with L. tomentosus, L. lamottei, or L. odomensis.
In summary, regarding shoot and root regeneration from in vitro cultured lentil explants, rooting presents the greatest challenge. Many researchers have reported none or low rooting frequency as an impediment in the regeneration of in vitro cultures (Ye et al., 2002; Sarker et al., 2003; Khawar et al., 2004; Gupta and Sharma, 2005). Mohamed et al. (1992) suggested that cytokinin used in shooting culture media may be associated with no or poor rooting initiation, and this required further exploration. Meanwhile, the use of related genera such as the faba bean looks to be a promising alternative.
Embryo culture also has potential to shorten the breeding cycle as was demonstrated by Gatti et al. (2016) who developed an in vitro-in vivo system using embryo culture along with an SSD method to shorten the breeding cycle in lentil variety development. They collected embryos of microsperma and macrosperma seed types sat 15, 18, 21, and 24 DAP and cultured these on MS medium with five different concentrations of BAP ranging from 0 to 0.25 mg/L. Genotypes, DAP, and their interaction had significant effects on the percentage of germination and shoot formation and indicated that microsperma genotypes are better respondents with an optimized 18 DAP embryo rescue timing for highest shoot production and germination percentage. Furthermore, BAP did not negatively affect embryo-to-plant development, with the ability to produce four generations per year of normal and fertile plants for rapid recombinant inbred lines.
5.4.2 Haploid Breeding
For the fast recovery of fixed lines of desired hybrids, conventional methods used in breeding programs need evaluation and selection of traits of interest in several segregating generations. To reduce the time required, the doubled-haploid (DH) approach has been applied, estimated to shorten varietal production time by five years because homozygous lines may be produced in a single generation (Pratap et al., 2005; Croser et al., 2006; Pratap et al., 2010). This technique utilizes the inherent potential of the cells be able to develop in a haploid plant from either the male or female gametes (n) only. Generally, androgenesis leads to production of haploids from microspores directly or by culturing anthers with microspores (Croser et al., 2006).
The first attempts to assess the potential of haploids were as early as 1964 and 1966, when Guha and Maheshwari (1964, 1966) reported the production of plants using Datura anther culture. Another powerful technique for obtaining barley haploids was demonstrated by Kasha and Kao (1970) using the chromosome elimination technique following distant hybridization. Every crop for which a haploid has been successfully produced has its own protocol, specifically for in-vitro and prepreparation requirements. Croser et al. (2006) have reviewed and summarized attempts done so far to standardize various steps and protocols for different legumes. They reported that haploid production using wide hybridization methods had been used successfully in cereal and potato crops, whereas microspore culture offers potential for producing haploids for most species. They also pointed out the issues that impede efforts in legumes, including their genetic disposition to low numbers of microspores per anther. Therefore, although this technology has tremendous potential to increase the efficacy of breeding programs, there are few successful attempts to produce doubled-haploids in grain legumes (Zhao and Sharp, 1998; Kaur and Bhalla, 1998; Mix and Wang, 1988). Genotypic differences, regeneration conditions, and poor or no in-vitro rooting are some of many factors impacting DH production in lentils (Croser et al., 2006) and wide hybrids (Gupta and Sharma, 2005). Keller and Ferrie (2002) attempted to develop lentil haploidy technology by testing various lentil genotypes with different environments, media, and culture approaches. They also explored many pretreatment conditions, anther culture, and appropriate microspore stages to instigate embryogenesis. Also, researchers at the Crop Development Centre in Saskatoon, Canada, and the Australian Centre for Legumes in Mediterranean Agriculture have attempted to produce lentil doubled-haploids using wide hybridization and tissue culture techniques such as anther culture and isolated microspore culture (https://grdc.com.au/research/reports/report?id=267). Croser and Lulsdorf (2004) reported that one to five days of pretreatment at 4°C triggered an induction response for microspore culture from two Canadian lentil genotypes (CDC Crimson and CDC Robin). However, this led to early embryo development with no plant regeneration (Croser and Lulsdorf, 2004; Croser et al., 2006). Most recently, the GRDC of Australia reported the groundbreaking success of one putative lentil haploid from crosses performed between lentil and field pea and from many wide (interspecific and intergeneric) hybridization attempts (https://grdc.com.au/research/reports/report?id=267). In summary, anther and microspore cultures are suggested as better options than interspecific hybridization for the successful production of lentil double-haploids. However, this will require more species- and genotype-specific research and funding to support scientific progress toward development of a widely implementable protocol.
5.5 Conclusions
To date, improvement for higher yields and resistance to major diseases and pests in lentils has been retarded by a lack of financial investment in R&D. Although most breeding programs around the world recognize that their genetic base is narrow and that wild relative species offer a wealth of genetic material, there has been a lack of investment in the advanced technologies required to enable the movement of this material into the elite primary cultivated gene pool.
Distant hybridization (aided with biotechnological tools like embryo-ovule rescue), together with modern cytogenetic manipulations, has a lot to offer (including the ability to precisely karyotype) to the development of intra- and interspecific genetic populations, genetic maps, association maps, quantitative trait loci, marker-assisted selection implementation in the lentil breeding program, and eventual fast-tracking of the deployment of genes responsible for desirable traits. Also, the application of GISH and FISH in the future may help to characterize apparently similar chromosomes at the constitutive heterochromatin level to aid in future lentil breeding. These technologies are urgently required to fully realize the significance of legumes such as lentils in our sustainable production systems, to guarantee the food security of millions around the world.