4
Mussels on Acid
We were sitting at a small Norwegian seafood restaurant with pints of beer and a pot of mussels in front of us. Life couldn’t have been better: the company was good, beer gently foaming and the hot steaming mussels better than ever. Indeed, we were convinced that the mussels that we had this night, cooked in local beer, were more tender and moist than their comrades that we had tasted before, who had met their fate in steaming white wine. Usually, observations made over a pint of beer are not considered the hallmark of scientific validity. However, even after a good night’s sleep the impression persisted of having had unusually succulent and tender mussels the night before. Surely, they were freshly caught from the Atlantic, the convivial atmosphere and nice people around the table had added to the overall experience. The question was thus: would our observation at the restaurant stand up to a replication study under more neutral circumstances? There are obviously a number of possible factors behind our informal observation, so we had to narrow it down to one single research question: the effect of using beer when steaming mussels. A question of chemical nature. How do beer and wine differ as liquids for steaming mussels, and what effect might they have on the final product? The first thing coming to mind was the difference in acidity. Wine is more acidic than beer as the pH of beer usually ranges between 4 and 4.5 whereas the pH of wines is lower, between 2.9 and 3.9. Hence, wines ranger from slightly to considerably more acidic than beers, and it is well known that pH has an effect on both chemical reactions during cooking and the structure of food.
Protein-rich foods such as fish or eggs cooked in acidic media, such as wine or water with a splash of vinegar, end up harder or even rubbery unless the cook is not careful in controlling the cooking time. Those who like poaching their eggs might have accidentally poured too much vinegar into the cooking water, resulting in a hard and dry surface compared with the softer center parts. Or you might have had fish with a dry and rubbery texture if you have cooked it a bit too long in white wine. This happens because both heat and acids promote coagulation of proteins, the main component in the structure of eggs and meats (this also may occur during smoking, since smoke also give acidic conditions in the water phase of the food). So, combining two treatments that both promote coagulation requires more careful control to ensure that the proteins do not coagulate too hard and squeeze out the water bound in the food to form denser, tougher, and drier protein networks than desired.
Our theoretical pondering seemed convincing enough, so we decided to follow the idea of difference in pH of cooking media being responsible and test it in one of our food workshops. Thus, we had to design a model experiment that would not reveal the origin of each sample to the taste panel.
Chef Tatu made two identical portions of fish stock used for steaming two portions of mussels, the only thing different being the acidity during cooking. Both stocks ended up with the same amount of citric acid, but in one parallel the acid was added before cooking the mussels, while in the other, the mussels were cooked and the acid was added afterwards. Thus, both portions had the same amount of acid when served, but only one portion of the mussels had actually experienced acidic conditions during cooking. This way, we reckoned, the panelists would not perceive any taste difference in terms of acidity, and the main difference would be any changes occurring in the mussels.
Steamed mussels
Ingredients per portion
50 g olive oil
150 g shallot onions, finely chopped
36 g garlic, thinly sliced
30 g parsley, chopped
1 l good fish stock
2.5 kg mussels
2.5 g citric acid
Procedure
The shallots and garlic were sautéed in oil and the fish stock was added. Citric acid was added to one of the portions. The two portions of mussels were steamed in their respective stocks for 6 minutes, after which citric acid was added to the second portion. Parsley was added; the two portions were given codes for blind tasting and served.
The workshop participants were asked to rate the two portions in terms of tenderness, chewiness, acidity, and finally, which they would prefer. While we summarized the results, the panelists were allowed to enjoy the rest of the mussels together with fresh bread and aïoli. After all, it is no bad thing to keep your taste panelists happy!
The difference between the two batches of mussels was surprisingly clear. Two out of three considered the mussels cooked without acid to be more tender. The differences in chewiness or tartness were less clear cut, but the results still pointed toward the same conclusion: cooking in acidic medium gave both a less tender and chewier texture. The more tender mussels were considered more pleasant by 2/3 of the participants.
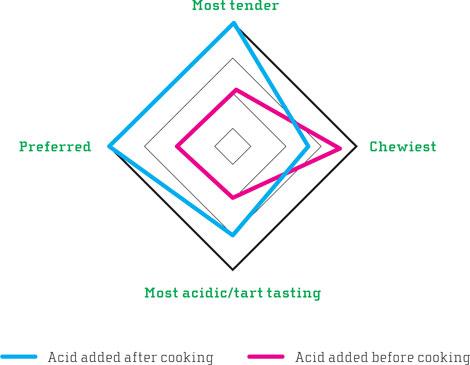
^The diagram shows how the panel of 2g participants judged the mussels. One portion was steamed with citric acid, and the other had citric acid added after steaming.
Which recommendations should we give based on this experiment? At the very least, if you want to cook your mussels in white wine, you might want to pick a wine with moderate acidity. But beer or good fish stock may also be good alternatives.
Acids in cooking. Protein chemistry plays a key role when it comes to structural changes of food during cooking. When protein-rich foods such as fish, meat, or egg are exposed to heat, the natural structure of proteins is disturbed. The protein molecules denature and eventually coagulate, resulting in changes such as firmer structure and changes in appearance. In addition to heat, there are at least three other strategies to changing the protein structure in food: we can use acids to “cook” protein-rich foods. An example is the Peruvian dish ceviche, where the cook sprinkles lime juice over raw fish cut in small pieces, or the Nordic pickled herring, where herring or Baltic herring is “cooked” in vinegar, often with added spices and herbs. The acid in lime juice or vinegar affect the structure of the fish meat: the almost transparent and soft structure turns opaque and takes a firmer “cooked” structure. The second strategy is cooking with ethanol, or, in everyday language, alcohol. In some recipes for carpaccio, which is based on thin slices of raw meat, we are asked to add a dash of brandy or other strong spirit. Doing the same with salmon, and adding some salt and sugar, as well, we get gravlax. The spirit does not only add flavor, but the alcohol also gives a more tender meat because high concentrations of ethanol make proteins denature. The third strategy is described in the chapter on foams, such as when we converted the clear fluid of egg white into an opaque and stiff foam by sheer physical movement of a whisk. The change of appearance from clear to opaque indicates that we have actually “cooked” the egg white proteins by our vigorous whisking, spreading and stretching the egg white protein bundles around the surface of air bubbles.
Combine any of these treatments and you get new kinds of solid structures because you expose the raw material to more than one treatment. What we observed in our mussel experiment can also be seen when you poach an egg. Recipes for poached eggs recommend a little vinegar in the cooking water. The egg white coagulates faster and you get a characteristic firmer structure on the outer layer of the egg white. Without vinegar you simply get a more classic soft-boiled egg. Thus, playing with acid and combining it with heat or other methods that affect protein structure adds diversity to the culinary structures of everyday food. But be aware that combining such strategies can also result in overcooking your mussels, such as using a too acidic wine or not reducing the heat treatment when combining it with acid.
Acids can also result in less pleasant surprises when cooking. We experienced this when baking a traditional Finnish spice commonly made for Christmas. The cake batter contains a special mixture of gingerbread spices as well as a significant amount of lingonberries, a tart and somewhat bitter wild berry commonly used in the Nordic countries.
Spice cake with lingonberries
Ingredients
100 g butter or margarine
2 dl brown sugar
3 eggs
2.5 dl flour
2 tsp baking soda
1 dl mashed lingonberries
1–2 tsp cinnamon
1–2 tsp ginger
0.5 tsp cloves
Procedure
1. Whisk butter/margarine and sugar into a dense foam.
2. Whisk the eggs into the butter/sugar foam one by one.
3. Mix baking soda and spices into the flour and fold it gently into the foam.
4. Add lingonberries and transfer to a 1.25 l springform pan.
5. Bake at 175°C for 45 minutes or until the cake is ready (poke a knitting needle or wooden cocktail stick into it. If the batter doesn’t cling to the stick, it should be ready).
The unexpected happened when we, in a hurry, added the ingredients in a different order than instructed by the recipe; the flour and lingonberries changed order. Rather than beating sugar and butter with eggs, adding flour and finally lingonberries, we beat sugar and butter with eggs, added lingonberries, and stirred in the flour at the end. The acidic lingonberries made a significant part of the egg proteins, which were supposed to maintain a foamy and spongy structure, denature already in the cold batter—well before the cake went into the oven. This coagulation should not take place until after the cake is in the oven. The foam is a delicate structure, and the batter must first be allowed to rise (by effect of the leavening agent and water evaporating), and thereafter solidify into a firm but porous cake. But since some of the egg proteins had already coagulated in the batter due to the acids from the berries, there was not much flexible protein network left to form a structure around the bubbles in the foam. The hard work of beating and whisking air into the sugar, butter, and eggs had been in vain. Ready baked, the cake came out as a sad, flat, and crumbly specimen. Damn acids! Luckily the cake was served to a chemist colleague, so one positive side effect of the accident was the pleasure of a discussion about protein chemistry.