
5
Sausage Engineering
How many sausage recipes could there be in the world? Bratwurst, chorizo, kiełbasa biała, frankfurter, chipolata, salami, blood sausage, seitan sausage, baloney. The sausage is part of culinary heritages all over the world, and has since prehistoric times been a method to prepare and preserve meat, making use of all parts of the animal. Salted meat, offal, or blood is mixed with spices and perhaps some cereals, stuffed into casings made from various natural materials such as stomach, lungs or intestines of cattle, pigs, or sheep. The resulting neat packages can be dried, smoked, fermented, cooked or a combination of these treatments, to be enjoyed at a later point. Even after new storage techniques were invented such as ice houses, fridges, and freezers, the sausage was not abandoned—perhaps because it is such a practical technique to deal with meat, and the end result so delicious. On the contrary, the idea of sausage has been carried on and developed further by modern home and professional butchers, and later in large scale by the food industry. And the fact that more and more sausages based on vegetarian protein sources are making their way to the consumers is another sign that the popularity of sausages seems to increase rather than decrease.
No doubt that the sausage is an important part of the world’s food heritage. For example, in the United States, the National Hot Dog and Sausage Council reported that only during summer, the three-month-long peak hot dog season, 320 million American citizens typically consume 7 billion hot dogs. Around 20 hot dogs per person. Also, Europeans and those in Nordic countries love sausages. The Finns eat approximately 20 kg sausages per capita a year, which is about half a barbeque sausage every single day for every Finn on earth. Although Norwegians are not the winners in this somewhat peculiar sausage consumption competition, they are reported to consume around 115 sausages per capita annually, one sausage every third day. Of course, many are not sausage fans, so if we remove these from the statistics it is obvious that those who eat them have one or two per day, around the year. Consequently, which type of sausages we make or eat is no small issue, and worthy of closer inspection.
Mixtures of things that do not mix. Substances that make up food come in various forms, or states (phases) of matter: solid, liquid or gas (plus a couple of other, rather rare ones of little relevance to home cooking).
Food commonly consist of a mixture of substances in various states, and more often than not it is a mixture of substances of rather different natures that do not normally mix. Oil and water are two liquids that will not dissolve into each other but remain separate even after vigorous mixing, returning to separate layers shortly after you stop mixing. To get these two mutually opposing materials to form a fairly stable mixture, an intermediary is required: an emulsifier. The emulsifier does not make the oil dissolve in water, but makes sure they are kept together; we have an emulsion. Emulsifying agents are usually molecules with both water-loving (hydrophilic) and fat-loving (lipophilic) properties in different parts of one and the same molecule. Examples of such emulsifiers are lecithin found in, for example, egg yolks and soybeans, phospholipids that make up the cell membranes of cells, certain proteins and many more.
What about making your sausages at home? Although most of us let the food industry make our sausages, stuffing one’s own sausages at home has become increasingly popular. It is not particularly difficult and can be done with relatively simple means. All you need is meat (or some plant protein such as seitan), casings and grinding and a stuffing attachment for your kitchen machine. All of it is available from the shelf or fridge in any well-stocked supermarket and a kitchen equipment shop.
Regardless of type, sausages are all complex mixtures where fat and water form a delicate and mutual relationship. The key ingredients of a typical sausage are meat, fat, and water. Making good sausages, be it in industry or at home, is based on quality raw materials and the building of a stable emulsion where fat droplets are dispersed in a water-based continuous phase (see info box and diagram). In this mixture, the basis is a water-based, protein-rich mixture into which fat is emulsified into oil droplets or solid fat particles. Some sausages have a coarse structure with noticeable bits of meat and fat, such as salami or the Spanish-cured salchichón. However, if we want smooth and juicy sausages, the fat droplets need to be really small in size to give that creamy sensation on the tongue rather than clumps of fat rolling around in the mouth. Furthermore, small fat droplets are better retained in the sausage during cooking, rather than leaking into the pan, taking with it the good flavor and juicy texture into the water. Too little fat will give hard, crumbly and dry sausages. In order to get a juicy and smooth texture it is recommended to use at least 20% fat.
In sausage mince, the most important emulsifying agents are the meat proteins, with both water soluble and fat-soluble properties. They make sure that the fat particles don’t separate out. In addition to the casings you will at least need meat/protein, fat, salt, and water to make a good sausage. All of these four play their own important roles in achieving a good texture. If one is missing, you will probably fail, or you might struggle convincing others that you have made a sausage. The bulk of the sausage is meat, which provides the important proteins that emulsify the fat particles as well as water. In some recipes the water bound up in the meat is not sufficient, and you are asked to add some additional water. Upon cooking, the proteins coagulate to form a firm but nicely elastic structure in the ready-cooked sausage. Salt helps dissolve some of the proteins in the water which in turn helps the fat becoming evenly dispersed and emulsified in the mass/dough.

Mayonnaise is an emulsion classic, where small oil droplets are surrounded by lecithin from the egg yolk and evenly distributed in water from lemon juice or vinegar. In that case, the oil is the dispersed phase while the water is the continuous phase, both still being liquid. We have a water-in-oil (w/o) emulsion. Milk and cream are emulsions of the same kind, where either oil droplets or fat particles (depending on the temperature) are dispersed in water. The same applies to butter and margarine, but in those cases water droplets are dispersed in oil or fat: an oil-in-water (o/w) emulsion. Many such mixtures consisting of otherwise non-miscible or difficult-to-solve substance pairs exist (see the diagram). Examples are suspensions (solid particles floating in a liquid), foams (gas bubbles trapped in a solid or liquid) and gels (liquid bound in a solid network). When whisking double cream, three difficult-to-mix materials dance together in an airy whipped cream while the whisk gives the rhythm: air, water, and fat. A general term for such mixtures of immiscible substances is dispersions. Indeed, we should all praise the chef who can organize mutually repellent molecules into a wide range of different structures for our mouth to enjoy!
But why introduce all these new and foreign words? Well, because they give us a language that allows us to talk about food as general structures, and thereby make sense of the food from a structural perspective. A chef can, for example, use the term “emulsion sauce” as a general term for sauces with certain properties (Béarnaise, Hollandaise and so forth). The language that follows with this is then a tool that helps us in understanding and dealing with them in practice, such as understanding why the sauce occasionally splits, how to keep it stable and so forth. ×
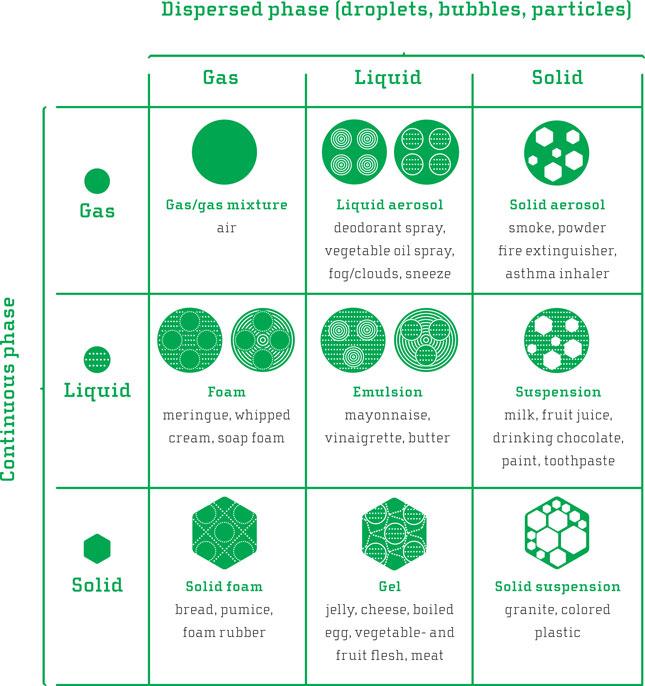
^ Diagram with possible dispersions. Both components of a dispersion (the dispersed and continuous phase) can be either gas, liquid, or solid, giving nine possible combinations. The gas mixture in the upper left-hand corner is the only exception, not being a dispersion but one single homogeneous phase. Thus, there exist eight basic dispersions.
The Food and Agriculture Organization of United Nations, FAO, have much information on their web sites, including detailed descriptions of what happens during the various steps in sausage making:
1. During grinding the meat, muscle- and connective tissue fragments are mixed to form a homogeneous mass.
2. Addition of salt and water causes meat fragments to swell. Furthermore, part of the proteins will dissolve in the salt water to form networks in the liquid—a gel. Other parts of the muscle proteins remain intact and fairly unaffected, floating in the water to form a suspension.
3. Fat is then added, resulting in an emulsion in which the complex water-meat mixture described above forms the continuous phase and fat taking role as dispersed phase.
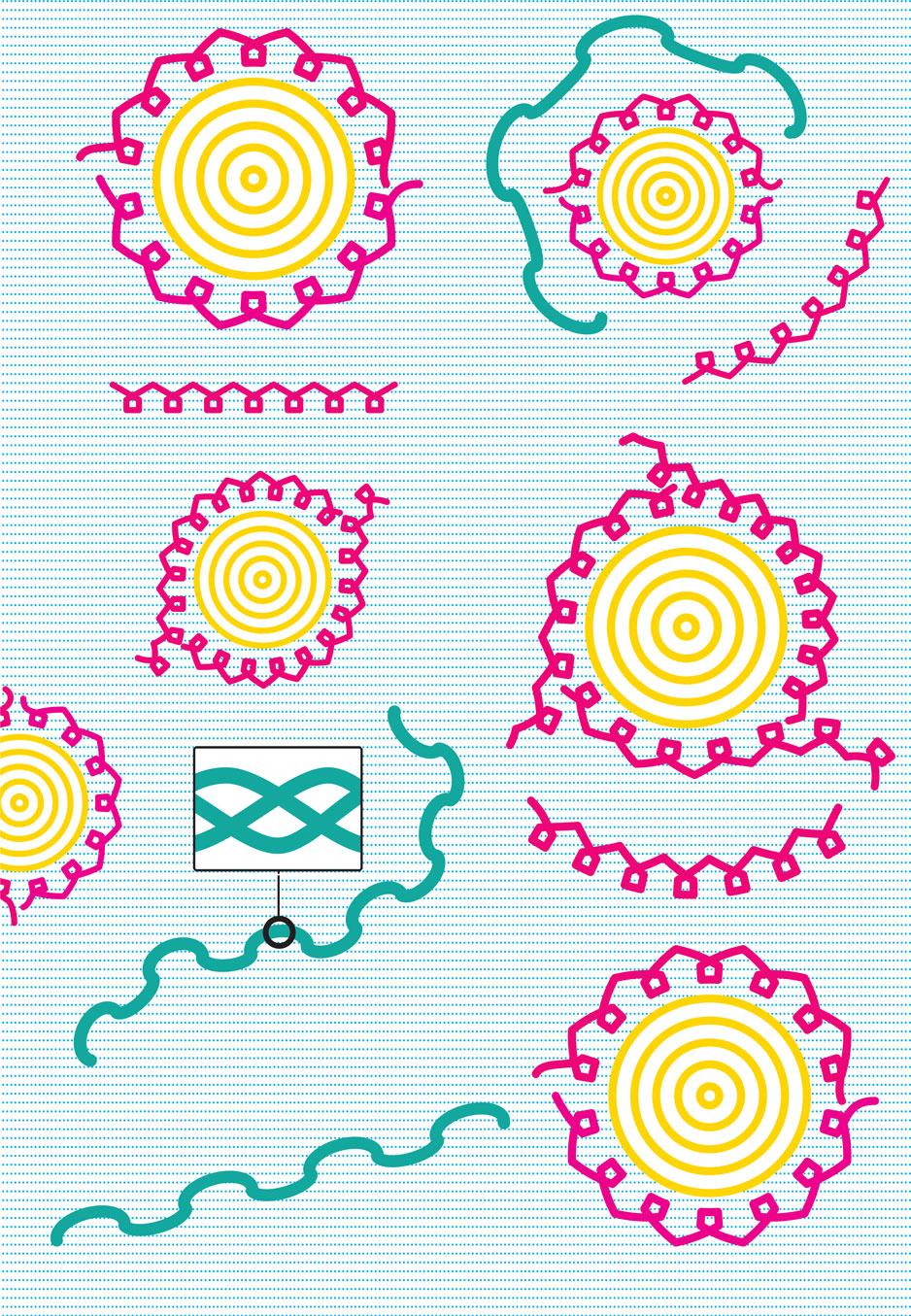
4. During heating the protein molecules go through structural change to coagulate and firm up to form a rigid network: The soft and pliable sausage mass turns into cooked sausage. The connective tissue fragments mainly consist of the protein collagen with a triple helix structure. Upon heating, this untangles into single strands of gelatin proteins which dissolve in the hot water, eventually to form a gelatin gel when the sausage is later cooled (see figure). The more connective tissue in the meat, the higher more gelatin in the cooked sausage.
The process gives a product that consists of several states of matter and various structures at the same time, giving the texture and mouthfeel we have learnt to enjoy. A sausage is at the same time an emulsion, a suspension, and a gel. Sometimes there are air bubbles or -pockets in the structure, so foam can also be said to occur in some sausages. Evidently, foods are often not only dispersions, but mixtures of dispersions.
Sausage on the rocks. In some sausage recipes, especially from old cookbooks, a rather specific and peculiar advice is given. Namely that the water should be added in the form of ice. In more modern recipes this is not usually mentioned, but the advice to keep the ingredients cold during the mixing is not uncommon. Why not just use water from the tap? This is one of those questions that begs for an experiment to be done during one of our scientific food workshops. Why ice? Why cold? Is it simply because the raw materials are easily perishable, or could there be another reason?
We prepared two batches of basic pork sausages with only four ingredients: meat, fat, salt, and water. The sausage batches were identical except for the water temperature. In one batch water was added as crushed ice while the other batch got room-tempered water. The outcome was then blind tasted by the nine participants who were to evaluate, without mustard or any other condiments, which sausage was most juicy, which had the smoothest texture and which was most salty. Naturally we also asked them to point out which of the two they would prefer. We also collected data of somewhat “harder” nature: the weight of the samples before and after cooking, and the temperature of the sausage mass at the end of mixing.
The temperature measurements showed that ice cooled the sausage mass effectively, with a temperature difference of more than 10°C between the sausage masses after mixing (5.5°C vs. 16.9°C). Visually the samples appeared to be similar, and this difference in temperature during preparation seemed to be the only difference between the two sausage batches. However, during cooking the two behaved rather differently: starting with 160 g samples, we saw a clear weight difference between the final, steamed, sausages. The sausages with ice had lost about 5 g while the sausages with room-tempered water had lost more than double of the amount. The sausages also tasted differently, as two-thirds of the participants considered the ice versions to have smoother texture. Eight out of nine considered the sausages prepared with room-tempered water as saltier, and some said that the saltier sausages gave a stronger burst of salty water when biting into the sausage. When it comes to juiciness and preference, the two performed equally, or the difference was too small to discern.
The conclusion from our informal experiment was that the water temperature indeed can make a difference when you make sausages. The weight and texture differences may be due to a stronger emulsion/gel and thus better water-holding capacity of the meat proteins in the colder mass. A more stable emulsion and gel may also explain the difference in saltiness and burst of salty juices, since a weaker dispersion will more easily release water.
Sausage On The Rocks
Ingredients per batch
600 g finely minced pork meat
190 minced pork fat (lard)
125 g water (as crushed ice or room-tempered)
16.5 g salt (1.8 %)
Procedure for the two batches
Room-tempered water |
Water as crushed ice |
|---|---|
1. Meat and fat were mixed in a kitchen machine using the K-beater. 2. After two minutes water was added (22°C), and mixing continued until a total of 15 minutes. 3. The temperature was measured (16.9°C). |
1. Meat and fat were mixed in a kitchen machine using the K-beater. 2. After two minutes crushed ice was added (0°C), and mixing continued until a total of 15 minutes. 3. The temperature was measured (5.5°C). |
For both batches
4. Four portions of each 160 g was rolled into 36 mm diameter sausages shaped cylinders using plastic wrap.
5. The sausages were coded so the taste panel should not know which was from which batch. They were then cooked in steam oven at 80°C for 22 minutes.
6. The sausages were weighed.

^The table shows the participants’ verdict in a blind tasting of sausages made with water added as crushed ice and room-tempered water, respectively. Numbers refer to the number of votes; the starting weight for all sausages was 160 g
The temperature issue is also mentioned in the technological sausage literature, where it is recommended that the temperature of the dough/mass should be kept below 12°C during mixing. There are two common reasons given to back up this recommendation: manufacturing hygiene and cooking loss. Our results thus were in accordance with the observations made in the food industry: lower dough temperature gave lower cooking loss.
However, why one should keep the temperature low to minimize cooking loss has two competing explanations in the literature, of which one or both might be correct. The first is about overheating. During grinding and mixing, “hot spots” with considerably higher temperature than the rest of the mass might occur. Indeed, one of the most prominent proteins in meat, myosin, denatures at 40°C. And if the protein is denatured, it will have reduced capacity to form a water-holding gel or stabilize the fat-in-water emulsion. But if the temperature is kept low, the risk of such premature protein denaturation due to “hot spots” is minimized. The second explanation concerns the fat: the harder fat (cold fat is harder than warm fat) will result in smaller fat particles. Thereby, the fat is distributed more evenly throughout the mass, and the emulsion is more stable. A more stable emulsion does not so easily leak fat nor water.
Which of the explanations is the most plausible, or which reason has the greater effect on the final product, is still a matter of debate among meat scientists. However, there is no problem agreeing on the fact that temperature has an effect on the final quality of sausages. In the discussions following our experiment, we agreed that this debate among sausage scientists is of utmost importance and that there is indeed need for more research on this area. So, for now, we will have to live with the fact that we know that this works, but not exactly why. We cross our fingers that the sausage scientists will work hard and do quality research in the near future.