
9
The Art of Heating
Cooking very often implies heating, that is, increasing the temperature of the food. Temperature as phenomenon basically comes down to one thing: movement. In gases and liquids, such as the hot air in an oven or water in a pan on the stovetop, the temperature directly reflects how fast the molecules are moving. In solids, where the molecules are kept firmly in their places not moving around (in fact, this is what makes an object or substance a solid), the temperature reflects how intensely the atoms or molecules vibrate. So, whenever we measure temperature in a material, we usually measure movement at the molecular level. Cook with heat therefore implies transferring energy to the food. The source of this energy can be the surface of a skillet, the water or gravy in a pan, the air in an oven, the radiating elements of a microwave oven, the coals of a barbecue grill or the rocks in a cooking pit.
The choice of energy source will contribute to how the food ends up: a pork chop can be simmered, shallow fried, oven baked, or grilled. If you ask a physicist, they would probably say that it is this transfer of energy that is called “heat.” Such thermal energy spontaneously spreads from a place with high temperature to a place with lower temperature. According to physics, this energy transport can occur in two different manners: conduction and radiation. Additionally, energy transfer in cooking can benefit from a couple of other concepts: convection and condensation. These four concepts can be quite useful when we talk and write about food and cooking, so let’s try to put some meaning into them.
Conduction—when hot and cold surfaces touch. One of the most striking experiences of conduction that probably every human being at some point experiences is when you burn your hand as you accidentally touch the ovenproof dish in your oven, or place your hand on the hot skillet. Conduction occurs when two objects are in direct contact with each other and one has higher temperature than the other. The molecules in the skillet, which is of solid metal, are vibrating intensely. When these come in contact with the surface of some food, or your hand, they collide with high intensity into the molecules on the surface of the food (hand), thus transferring some of their energy by setting the molecules of the food into motion. If the food remains in the hot skillet, a domino effect occurs from the outer parts inwards. This domino effect, a gradual conduction between outer and inner parts of the food, is one reason for the food eventually becoming hot all the way through. Substances that are efficient heat conductors are consequently efficient for heating food. A metal, YES. Styrofoam, NO.
Some metals are better conductors than others. If you have had the pleasure of stirring a cup of hot tea or coffee with a silver spoon, you might have noticed that the spoon feels hotter than your everyday steel spoon. This is because silver conducts heat much more efficiently, by this domino effect conduction, through the length of the spoon to your fingers. On the other hand, when one of our prehistoric forefathers made a primitive skillet by placing a flat rock on top of a burning fire, it was rather inefficient compared with the metal pans used in the later metal ages. S/he might be able to heat the rock to fry one side of the meat, but the rock is not very efficient in continuously transferring the heat from the fire to the food, so s/he would have to move the meat around to new spots every time the meat was turned.
Liquids and gases are comparably poor conductors of heat, one reason being that the distance between the molecules is larger than in solids. So, the collisions between the cooking medium (gas or liquid) and the food are necessarily less frequent compared with cooking on a solid surface such as a metal. It takes longer to heat food in an oven, where the heat transfer medium is air, compared with having the food in direct contact with the hot metal surface of a skillet. Since water has much higher density than air, water is a much more efficient heat transfer medium; the molecules are more densely packed and collide more often with your food. So, cooking potatoes in water is usually quicker than baking them in the oven, even though the temperature in the oven might be much higher than in the water, boiling at 100°C.
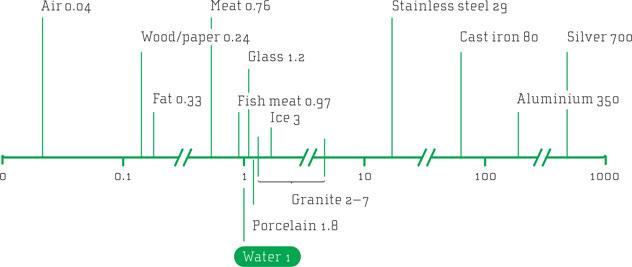
^From air to silver: The table shows relative conductivity for various materials relative to water. Metals are good conductors, but even among these the differences are large.
A barbecue radiates. Feel the sun warming an otherwise chilly, spring day, or feel the warmth sitting beside your wood-fired oven. In both cases, you experience thermal radiation warm your cold toes. Heat through radiation is different from conduction in that there needn’t be any physical contact between what is hotter and colder. In radiation, energy moves via electromagnetic waves, a form of light. If we leave out microwaving for a while, it is basically infrared radiation we use when we use radiation for cooking. The radiation is absorbed at the surface of the food, where molecules are set into motion, and the food is heated. The energy source (the coals in a barbecue, the hot walls or broiling elements of an oven) throws packages of energy around in all directions. When a molecule at the surface of the food is hit by one of these, it is energized and starts vibrating or moving around faster. An example of a food that is cooked purely by infrared radiation is the Finnish way of cooking salmon called “loimulohi,” flame-roasted salmon. You fasten a salmon fillet to a plank by driving wooden plugs through it and then place the plank upright by the side of the fire. If you don’t place it too close to the fire, the only heat reaching the fish is the radiation. The hot air from the fire move upwards and will not reach the food (see convection below).
Microwave cooking also relies on radiation but in a somewhat different fashion than infrared radiation from hot bodies. While infrared radiation affects basically all types of molecules and materials, microwaves are more selective. They are particularly efficient in setting water molecules, and, to a certain degree, fat molecules, into rotating movement. Consequently, almost all of the infrared radiation is absorbed at the surface layers of the food, while microwaves may travel several centimeters into the food before being absorbed. Thus, food that is microwaved is heated just as much from the inside out as from the outside in. When a molecule absorbs an energy package in the microwave frequency range, it is set into intense motion. This results in small areas of very high temperature. Therefore, you would want to wait for a while for the energy to spread from the hot spots to the cooler regions in your food via conduction.

In his book, What Einstein Told His Cook, the American chemistry professor asks: On a really hot day, would it be possible to fry an egg on the bonnet of a car? The answer would be “probably not.” The reason is the fact that as soon the egg lands on the bonnet, the surface will be cooled while the egg becomes hot. But since there is no other heat source than the sun radiating energy onto the bonnet, you end up with the egg partially fried. However, if you opened the bonnet and cracked the egg straight onto the engine block, chances would be considerably better for frying the egg all the way through, especially if the engine was running. Having the engine running would be similar to cooking on the stovetop, the combustion process constantly feeding energy to the metal. The amount of metal in the engine block is also much higher than in the bonnet, more energy would have been stored in the material, which would in turn be transferred to the egg to cook it. ×
Convection—boiling and baking. If you burn yourself holding your hand over a candle you experience energy transfer via convection. The phenomenon occurs when a liquid or gas of higher temperature moves to a place of initially lower temperature. In the case of the candle, warm gases flow upwards from the flame to reach your hand, after which your hand becomes heated by conduction when the hot gas comes in physical contact with your skin. Since convection is all about “hot molecules moving to new places,” it can only occur in media that are able to flow: liquids such as water or oil, or gases such as air or steam. Since these “hot molecules” have high energy, they move about or rotate in high speed, and when they collide with “colder” and slower molecules, some of their energy is transferred via conduction. Convection processes are common phenomena both in nature and in everyday life at home. While heating water for your pasta, you may see flows and currents in the water even though the pan stands perfectly still on the hotplate: hot water from the bottom rises and pushes colder water to the side, and so the circulation goes. Warm air rises while cold air sinks both as a meteorological phenomenon as well in your own living room.
The difference between conduction and convection can be illustrated using a dance floor as metaphor. Envisage a dance floor where some (colder) couple dance slowly cheek to cheek while other (hotter) couples dance a more intense swing, as shown in the illustration. The red steps illustrate fast swing, “hot molecules.” The blue pairs dance slowly and are “colder.”
During conduction, the cold couples surrounding the hot couple are affected, inspired, as they come in contact with the latter. After a while, the cold couples become hotter, while the originally hot couple has shared some of its energy and become somewhat cooler. This illustrates conduction in both solids (all maintain their original positions, but can still rotate on the spot and bump into each other) and in liquids and gases (the ones surrounding can move around when pushed by the “hot” couple).
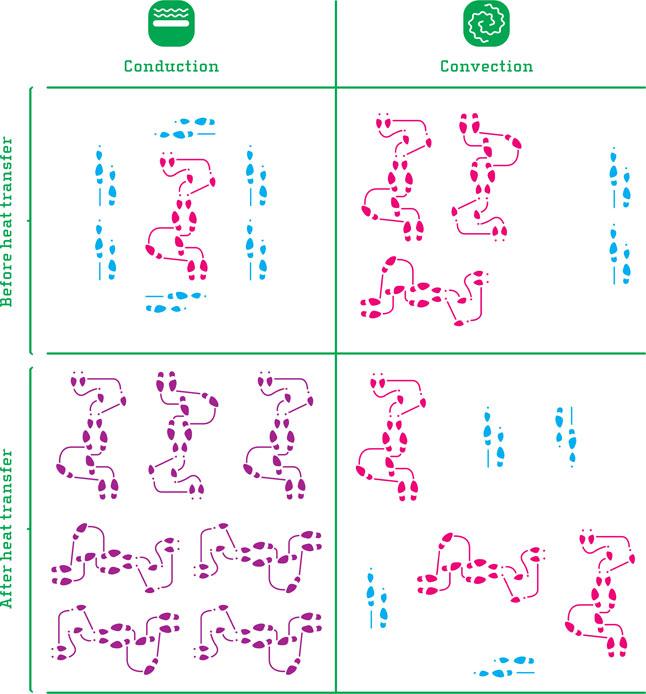
^Conduction and convection illustrated by hot and cold couples on the dance floor. Red steps represent hot molecules, blue represents cold and purple represents temperatures between hot and cold.
Convection is illustrated by a room with one cold end, inhabited by couples dancing slowly, “cold molecules,” and the other end dominated by more energetic dance. Very similar to a hot oven standing in an otherwise cold room. Since molecules by nature move randomly and are reflected when they bounce against each other or a surface, they will in time distribute evenly across the room. We move toward an even temperature in the whole room for two reasons: 1) the various couples will over time distribute evenly throughout the room. 2) The “hot” couples will eventually bounce into the “cold” ones, sharing their energy (this is consequently conduction).
The most striking practical example of convection when cooking food is when you turn on the fan in your oven; indeed, ovens with such fans are called “convection ovens.” Many ready-made foods have instructions that you should heat at different temperatures depending on whether you use the fan or not, 200°C in ordinary ovens or 180°C with the fan running. This reflects the fact that the fan sets the air into motion to increase heating through convection. Even without the fan, convection also occurs via hot air moving out from the hot surfaces, pushing aside less hot air.
When barbecuing/grilling, at least over a campfire, you have the choice of holding your hot dog on the side of the coals or above them. Everyone having roasted hot dogs by a campfire has experienced that holding them above the coals is more efficient. Hot air moves upwards from the fire, and the hot dogs experience convection in addition to radiation. This is a more intense way of heating, which often results in a hot dog with a burnt surface while still cold in the middle. Apparently, sometimes convection can be a bit too effective way to transfer energy. However, if you hold the hot dog by the side of the fire or coals, which is also recommended as you avoid transfer of soot particles from a burning fire, you would be heating by radiation from the flames or embers, just like cooking Finnish loimulohi.
Condensation—steam in a closed pan. Rather than plunging your potatoes into hot water, you may place them on a grate with just a little water in the bottom of the pan. You place a glass lid on top, turn on the power. Your potatoes are ready almost just as quickly as when boiling them in water, without many visible signs of anything happening in or around the potatoes, possible except for some water dripping off the potatoes. How come?
Let’s return to the burnt hand because that experience is quite a powerful association for many of us. If you place your hand in front of the spout of a kettle with boiling water, you may be badly burnt. You will not only experience convection and conduction from 100°C air and steam reaching your hand transferring energy by contact (conduction), but in addition the steam, water in form of gas, will condense back to liquid water on your skin. It requires a large amount of energy to make water evaporate; indeed, it takes seven times more energy to evaporate water (already holding 100°C), as it takes heating the same amount of water from room temperature to 100°C. All the energy that went into evaporating the water is returned to your skin when the steam reaches your hand and condenses to become small drops of liquid water. This comes in addition to the hot water transferring its heat energy by ordinary conduction from temperature difference, like if you had stuck your finger into hot water. This is also the reason that you are more severely burnt if you come in contact with steam at 100°C compared with dry air of the same temperature. After all, you can stick your arm into an oven holding 200°C without getting burnt, but holding your hand just above a boiling pan of water will cause severe burns at “only” 100°C. However, what you actually see coming out of the kettle spout, or of a boiling pan, is not steam. Water vapor is a colorless and invisible gas. What you see is liquid water, steam that has already condensed into miniscule drops on its way out in the kitchen. If you look closely at the tip of the spout, or just above the boiling water, you would see (apparently) nothing. That area is where the water vapor is, and that is the place where the energy transfer to other bodies in the most intense.
When you steam food, you make use of this evaporation–condensation energy. Steam moves by convection from the boiling water below your food, hitting the surface of the food to release both condensation energy as well as some heat energy through direct contact, conduction. Consequently, steaming is quite efficient and competes well with boiling it in water even though steam is much less dense than liquid water. But don’t lift off the lid to have a look every second minute because this will release the efficient steam out into the room. A transparent glass lid is a good thing for the curious cook. If the water in your pot is boiling but you don’t see white clouds, you can be fairly certain that the pan is full of invisible steam condensing on your food to cook it.
Cooking with heat. It is very seldom that only one form of heat is in action when cooking. Two exceptions mentioned are the Finnish “flame-roasted” loimulohi, and microwaving, where radiation is the only source for heating. But even in these cases, conduction, convection, and condensation occur inside the food: the heat spreads and the temperature evens out throughout the food. Usually, several or all forms of heat are in action at the same time.

How often should you turn a steak in a skillet? This important question arises when pan frying, because there the heat transfer takes place both via conduction and convection: while the heat is conducted from the hot surface of the skillet to the food, energy is transferred by convection from the hot surface of the steak to cooler, surrounding air. The steak also cools via evaporation of water from the steak surface, the opposite of condensation. So at least three different heat transfer mechanisms are involved in the frying of a steak (in practice, there will also be radiation from the skillet surface onto the meat). Traditionally, many of us have learnt to turn the steak once during cooking—when you see juice seeping out on the top. But lately, chefs have started turning it more frequently, as often as every 30 seconds. An explanation for the superiority of this new technique lies in the optimization of heat transfer. If you turn the steak only once, the hot surface of the turned steak will cool down longer and more compared to the frequently turned steak, and the total cooking time is increased compared with the frequently turned steak. When turning frequently, the hot surface cools more evenly, avoiding significant temperature differences between the outer and inner parts. Furthermore, it has been found that the layer 1–3 mm beneath the surface is heated to about 10°C higher if turned only once compared with the frequent-turned steak, risking overcooking of the outer parts. In other words, flipping your steak frequently would both reduce cooking time and promote a more evenly cooked steak. ×
In a coal barbecue, the coals are very hot and the heat transfer occurs via radiation plus some convection by hot, rising air. But where the food is in direct contact with the grates, conduction occurs between the metal and the food. This heating is sufficiently intense to give the barbecued food its appealing grated pattern, also giving a more complex and appealing flavor. If you have a gas barbecue, the radiation is not as intense as in the glowing embers, and you get comparably more convection compared with radiation. The resulting process is somewhere between a coal barbecue and baking in an oven, particularly if you keep the lid on. In an oven, the surfaces are not as hot as the embers of a barbecue, so the radiation from the walls are less intense. However, the air in the oven is hot and kept locked inside the confined space, and, therefore, the most important factor when cooking in an oven, unless you have the grill function turned on, is convection in air.
Although it might seem self-evident, it is worth reflecting upon the fact that heat almost always moves from the outside of the food inwards when we cook, the seemingly only exception being microwaving. If we use high temperature when cooking a large piece of food, the outer parts will be overcooked before the heat reaches the inner parts. Keeping the temperature lower will make the cooking take longer, but the domino effect of heating the food throughout will have more time to occur without the outer parts becoming overheated. This is one of the main principles behind the sous vide technique, where the food is vacuumed and left in a water bath of desired temperature for a long time, allowing the heat to penetrate the food more gently. Chemical reactions are allowed to proceed, giving good flavor and tender meat. We will, however, not go into sous vide cooking here as great sources to information about this are only a few clicks away on the web (we recommend Douglas Baldwin’s book and web page or the grand cookbook, Modernist Cuisine—see the bibliography). In the illustrative table we have connected the various heating techniques to the various forms of heat and given each its own icon.
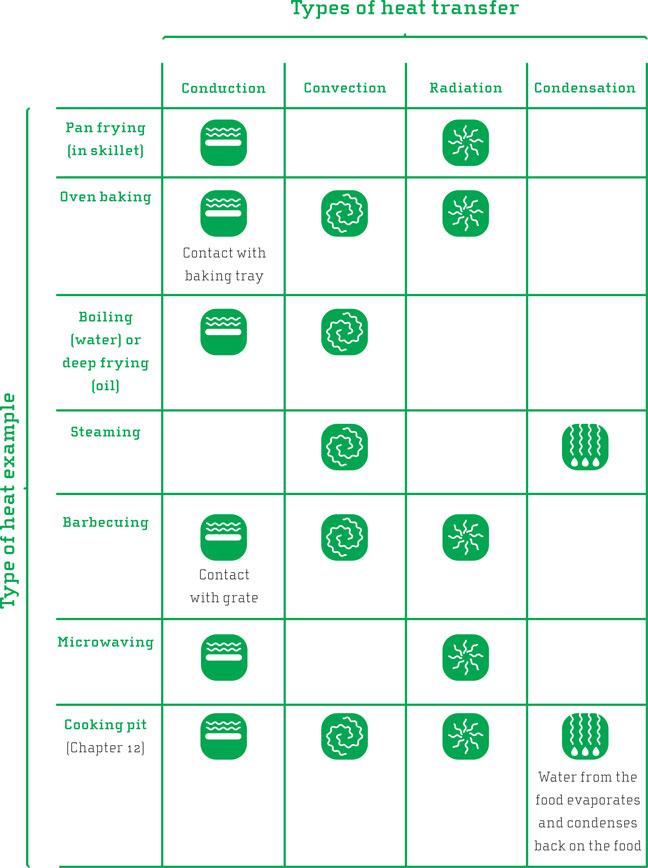
^The table shows the different mechanisms for transfer of thermal energy, be it frying, deep frying/boiling, baking, grilling, microwaving, or using a cooking pit.