18
Physics Takes the Cake
You have invited friends and family to a celebration, maybe a birthday or Independence Day. Then you ask yourself: “Should I buy a ready-made sponge cake, or should I bake it myself?” The first option is maybe the safest bet, but the second will surely communicate more sincerely your love and appreciation for the guests. But, maybe, your experience from prior baking projects has been that your cakes don’t always turn out light and airy. Last time you baked such a cake it came out flat and dense, even though you followed the recipe and treated it with the utmost care, making sure you didn’t disturb or shake the light and tender sponge. You had to rush to the supermarket to get a ready-made sponge. What to do this time? The British physicist Peter Barham has a suggestion for you, claiming that his advice will ensure that your sponge cake does not collapse. Brace yourself: as soon as the cake is ready and baked in its metal spring form, you take it out of the oven and hold it 20–30 cm above the kitchen bench or a table. Then, let it fall flat down. Boom! This, he claims, will ensure that the cake will not collapse. Is he joking!? Does he expect us to trust this advice a few hours before the party? Has he tried this himself? If I try this, should I buy a ready-made sponge cake just in case? The questions and dilemmas pile up, but this claim truly begs for an experiment.
Jokes often have some core of truth, and science jokes are no exception: “If it is green or wiggles, it’s biology. If it stinks, it’s chemistry. If it doesn’t work, it’s physics.” We are chemists and we’re used to getting our hands dirty, but can we trust the physics professor to have actually tested his claim? How many of us haven’t experienced the school science experiment that didn’t work out as expected although the book said it should? With an important cake in the oven, this is not an option. So, we decided to dive head-first and test this claim in a cake-dropping experiment. Perhaps the outcome would result in some jaws dropping as well? The experiment was conducted in a food workshop with 20 participants to witness and taste the result.
The cake drop. Tatu the chef chose a very basic recipe for sponge cake: sugar, eggs, and flour. Sponge cake recipes may also contain milk, and even baking powder to help with the leavening, but we chose to keep it as simple as possible to avoid complicating factors.
The two parallel cakes were baked simultaneously in the same oven. When taken out, one cake was treated with the utmost care and placed carefully to cool in the tin. The other was held horizontally 20–30 cm above the table and dropped flat down, after which it was left to cool. When cold, the cakes were cut in half and the heights measured.
Recipe
Ingredients
250 ml sugar
250 ml eggs (6 medium sized eggs)
250 ml sifted wheat flour + some flour for the cake tin
Procedure
The sugar and eggs were beaten with an electric mixer until a light foam. The flour was added and gently folded in. The mixture was poured into a 32 cm springform cake tin and placed in a convection (hot air fan) oven for 1 minute at 200°C, and then 25 minutes at 180°C.
The recipe was repeated to give two sponge cakes.
The result. The cake that was dropped was clearly higher in the center area compared with the one that was handled with care. The latter had the typical high circumference and pit in the middle, while the one that was dropped had a more even height throughout. In the middle area, the one that was dropped was approximately 20% higher than the one handled with care—quite a spectacular result! Well, not that surprising as we had secretly pretested this in advance. We had safeguarded ourselves and did a corresponding experiment at home, one month earlier. In this experiment, the result was much the same, but with a somewhat less marked difference. In that case, we used some baking powder in the recipe, so the cake handled with care came out quite tall as well, but the one that was dropped came out 15% higher and even had a center area that was higher than around the rim.
We are quite convinced that the British physicist’s claim is indeed correct, but what can be the explanation for this apparently counterintuitive result? This requires a closer look at cake structure and going back to our structural friends, the dispersions (as described in the sausage chapter).
What makes a cake rise? Structurally, a sponge cake is a solid foam—a solid substance with gas bubbles distributed throughout the structure. Just like the inside of the pillow you might be sitting on right now while reading this book. If we simply stir together egg, sugar, and flour and bake it in the oven, we would get a pancake; flat and dense. The foamy cake structure is achieved if we beat eggs together with sugar to produce a liquid foam, full of air bubbles, and then gently fold in the flour to avoid too many bubbles bursting. We still have a fragile liquid foam, but somewhat more thick and viscous. When the cake enters the oven, it rises—even though no baking powder or other leavening agent has been added. The reason for this is that the water from the eggs in the batter evaporates. Liquid water turns into steam. Steam—water in the form of gas—requires more than 1000 times the space compared with liquid water. One teaspoon of water turned into steam would, in the ideal case, expand to more than 5 liters. So, when the water evaporates in the liquid batter, it ends up in the bubbles, who in turn expand radically. The whole cake increases in volume and therefore rises, the internal vapor pressure in the cake keeping it up, competing with gravity, which constantly pulls the whole cake down. The air that we beat into the batter would also expand somewhat when heated in the oven, but this expansion is not much more than 25%, and expansion of air, therefore, has a much smaller effect than expansion of liquid water into steam.

In the early stages in the oven, when the cake is still practically a liquid foam—bubbles dispersed in a liquid—it is very fragile and sensitive to mechanic disturbance. If this wobbly mass gets a nudge, the bubbles of hot steam maintaining the expanded foam might find their way up and out, even leaving behind channels for more steam and gas to escape. This is the moment where the cake requires peace and quiet. However, after a while, the batter solidifies due to chemical reactions facilitated by the high temperature: gelling of starch from the flour and coagulation of proteins from eggs and flour. The liquid foam turns into a solid, but soft and brittle, foam. The cake is still in the oven, but, in addition to the internal pressure of the steam, the solid structures of gelatinized starch and coagulated proteins now hold it up. So now you may safely dance and jump around the cake as much as you wish without the fear of it collapsing.
Then the moment arrives when the cake is ready to be taken out from the oven. How come your beautiful fluffy creation shrinks in front of your very eyes, the high peak in the middle turns into a valley, and the smooth surface of the cake is all wrinkled? The sponge still contains a considerable amount of water. When the cake is taken out, the temperature soon sinks to below 100°C, and the steam still inside the recently expanded bubbles condenses back to liquid water. The recently expanded bubbles experience a vacuum effect; they are sucked together from the inside. What looks like a cake “falling together” is actually a mild implosion. However, the cake is now a solid foam that is more rigid than it was as a liquid foam prior to baking. If we were to bake a bread, the solid foam would be rather strong and elastic, and the crust so sturdy that the bread would not contract that easily, while in a sponge cake the structure is still rather soft and brittle and prone to collapse. Channels that we so much feared would let steam escape if the cake was disturbed in the early stages of baking would now have been much welcome to let air in from the outside as steam condenses and bubbles contract.
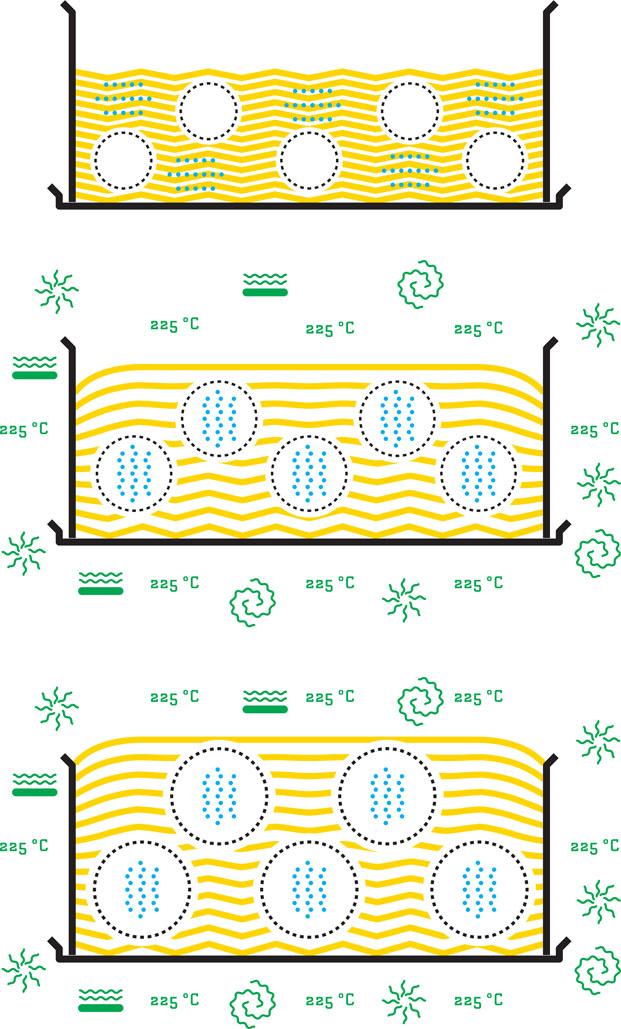
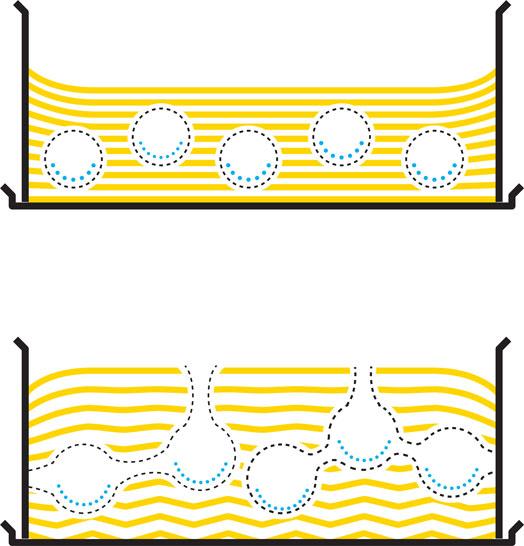
^When water in the cake batter boils as a result of heat transfer from the oven, the air bubbles are filled with steam and expand (left-hand page, from top and downwards). When the cake is taken from the oven, the steam condenses into liquid water. The bubbles—and the cake—contract (right-hand page, top). However, if the cake is subjected to a shock just after baking, cracks will form between bubbles and on the surface of the cake. Air can then pass through these new channels to replace steam that has condensed into water (right-hand page, bottom).
What happens when the cake is dropped? We do not know whether Barham’s advice came after an accident in the kitchen making him reflect upon this, or if the hypothesis came before the experiment. Our tests anyhow support his claim and indicate that he might have found a good way to ensure a high sponge. His explanation for why this works goes as follows: when the cake is taken out from the oven, it is a solid foam: lots of bubbles surrounded by a fragile solid dough (crumb). If this structure receives a physical shock, cracks will appear between bubbles, and on the surfaces of the cake functioning as ventilation channels. Small and rather firm bubbles become interconnected, united into a more open network. Additionally, the steam inside the cake condenses and contracts, but instead of the bubbles contracting with the steam, air from the outside can be sucked in through the cracks to fill the bubbles. Even though the crumb and crust are soft and fragile, they are strong enough to maintain the structure. And you will not have to make double the amount of cream to fill a pit in the middle of the cake.
As to yet, we have not found any scientific studies describing such shock treatments of sponge cakes, so Peter Barham’s explanation should perhaps still be considered as a hypothesis. But it would be interesting if research could document the effect of the cake drop. Pastry chefs and bakers might have a safe route to high cakes, they could make their cakes 10–20% higher without increasing the amount of ingredients, and the customers would have cakes with fewer calories of energy.
This time we must admit that even though it is physics, it still works in real life and not only under idealized and controlled conditions. At least, the times we have tested it. The question remains: would you put your trust in the physics if there was an important occasion at stake…?