6

Mini-skeletons
To a Tudor physician covered head to foot in thick clothing doused in vinegar, and wearing a beaked mask, avoiding disease carried by airborne vapours was a serious business. Little was known about the vectors of disease, and little about how to cure it, and absolutely nothing was known about the microscopic world that was its cause. When Queen Elizabeth I of England was ‘diagnosed’ with smallpox in 1562, she was given the most advanced medical treatment known to the physicians of England at that time: being wrapped from head to foot in a red blanket. Apparently it worked, though no one could be sure why. The tiny virus that carries the smallpox disease is no more than 400 nanometres in maximum length, and there are one million of those nanometres in a single millimetre. The smallpox virus then, is much, much too small to see with the naked human eye.
What then, is the smallest object that the naked human eye can resolve? In good natural light, the eye can see objects somewhat less than 1 mm in size, and probably about one-tenth that, which is around 100 000 nanometres. Human vision can struggle to see the breadth of a single strand of hair. That is nowhere near small enough to resolve the smallpox virus. But it does mean that long ago, people had already recognized tiny, millimetre-long objects swimming in their drinking water. The indigenous Mogollon people of New Mexico, for example, noticed the minuscule ostracods (seed shrimps) that inhabited their lakes and streams, and depicted them ‘swimming’ on their pottery 1000 years ago. To see beyond the breadth of a hair, into a world of unseen skeletons, humans had to wait another 600 years, for the invention of the microscope.
A World Unseen
Magnifying glasses and eyepieces have been used since classical times, and what is perhaps more surprising is that no one thought to put two of them side-by-side, and make the first spectacles, until the 13th century. In optics, the early advances were made by both Italian and Dutch engineering wizardry. Spectacles were being used south of the Alps in the 14th century, and they had probably been invented in northern Italy towards the end of the previous century. Tommaso da Modena’s painting of Cardinal Hugh de Saint-Cher in 1352 is the earliest depiction of someone reading and writing with the aid of spectacles. By the late 16th century, the Dutch spectacle makers Zacharias and Hans Jansen had gone one step further, and put several lenses in an extendable tube to make a microscope. It seems that people were using these devices to explore the world of both the very small and the very distant: at about the same time in Florence, Galileo Galilei was experimenting both with microscopes and telescopes. The Jansens’ microscope could magnify perhaps nine times, while that of Galileo could manage 30 times. His microscope was elegant as well as functional. Conceived by Galileo, but engineered by Giussepe Campani, it was made of cardboard, wood, and leather, elaborately decorated, and inserted into an iron mount with three legs. But the great genius was too concerned with tracing the motions of celestial bodies to work out the exquisite detail of the insects he could see through his new magnifying device. It was his friend Johannes Faber who coined the name ‘microscope’. On to the stage now enters Dutchman Antonie Von Leeuwenhoek, and, in England, Robert Hooke—one of the founding fathers of the Royal Society.
Antonie von Leeuwenhoek’s contribution to microscopy is immense. From humble beginnings—his father was a basket maker and his mother from a family of brewers—he is the first person in history to systematically document the microscopic world. His interest developed from cloth, or rather a need to interrogate the integrity of that cloth using better lenses. And he developed an improved technique for making these, using tiny glass spheres that gave his microscopes the capacity to magnify 275 times—perhaps even more—representing a technological leap forward akin to the invention of the scanning electron microscope in the 20th century. Leeuwenhoek was the first to observe bacteria, and the first to observe myriad mobile single-celled organisms. His findings were so revolutionary that for a time the Royal Society in London doubted him, dispatching a group of ‘wise men’ to visit him in Delft, only to confirm his findings. Leeuwenhoek lived a long life, dying at the age of 90, but never giving away the secret of his microscope design.
At the same time that Leeuwenhoek was observing microorganisms in Holland, Englishman Robert Hooke was working on his book Micrographia, and labouring with a compound microscope—one using several lenses—that was probably inferior to that of his Dutch contemporary. Hooke, too, was a man of humble beginnings, the son of a Church of England curate on the Isle of Wight in southern England. He was a true polymath, with an eclectic array of skills and friends—which included Sir Christopher Wren, who built St Paul’s cathedral in London—but he infamously argued with Isaac Newton over who had conceived the idea of gravity. If Newton won that battle, Hooke’s impact on science is nevertheless huge, from ‘Hooke’s law of elasticity’ mastered by all high school students, through sundry other discoveries that are testament to his multifaceted genius.
Micrographia, or some Physiological Descriptions of Minute Bodies made by Magnifying Glasses with Observations and Inquiries Thereupon was published in 1665. It was a revelation. For the first time in history, the general reader could study the exoskeleton of a flea, a spider, and an ant, or gaze into the eye of a fly. To keep his tiny subjects still while he drew them, Hooke even resorted to the influence of alcohol, plying his ant with brandy to keep it still for an hour. Published just before the Great Fire of London in 1666, the book also found its way into the diary of Samuel Pepys who, completely absorbed by it until the early hours of the morning, wrote that it is ‘the most ingenious book that ever I read’.
The ‘Ur Animals’
The thread of scientific discovery now passes to Germany, and the invention of the word that is used to describe these many tiny organisms, the ‘Urthiere’, being made from ‘ur’ primitive, and ‘thiere’ animals. For some 150 years after his correspondence with the Royal Society, the tiny organisms observed by Leeuwenhoek, and referred by him as ‘very many little animalcules’, were thought to be minuscule but fully formed animals. Leeuwenhoek himself had described one of these animalcules as being a tiny oval-shaped form that had two little legs poking out near the head. In 1765, German anatomist Heinrich August Wrisberg coined the term ‘Infusoria’ for these organisms, and as late as 1838, the distinguished German naturalist Christian Gottfried Ehrenberg still considered them as tiny animals, publishing that year Die Infusionsthierchen als vollkommene Organismen (which roughly translated means ‘The infusion-animals as complete organisms’). At about the same time, though, Frenchman Félix Dujardin had already observed that some of these organisms were made of a single cell.
In 1818, ‘urthiere’ mutated into its Greek form ‘protozoa’—a name meaning exactly the same, and one that has stuck fast in science down to the present day. Though German scientist Georg August Goldfuss coined the name, it was his compatriot, Carl Theodor Ernst von Siebold, who established its use for single-celled organisms. Siebold, incidentally, was also the scientist who established the single grouping for those animals with an exoskeleton and jointed limbs—the arthropods. The term ‘protozoa’ is now used mostly informally for a range of unicellular eukaryotic (i.e. nucleus-bearing) organisms that show functions that mimic animals: that is, they can move, and they can prey on each other.
In the early 19th century, one of these groups of protozoans, the foraminifera, was about to take centre stage in both oceanography and geology. Their fossils, abundant in ancient mudstones and limestone accumulating over millions of years, could be used to recognize different time intervals in rocks, whilst in the modern oceans their patterns of distribution could be used to map water masses of different salinity, temperature, and depth. Foraminifera possess one key feature. For more than 500 million years they have been making hard exoskeletons: a shell that protects their body from the elements. Their skeleton is sometimes almost literally homemade, stuck together from bits of sediment. Mostly, though, the foraminifer skeleton is formed from calcium carbonate, which the organism extracts from seawater. These shells have been preserved in their countless billions in rocks over hundreds of millions of years, reflecting, as time passed, the evolution of thousands of different species, which are characteristic of different ages of rock. Foraminifera are close relatives of the amoeba too, and so they are sometimes called the ‘amoebae with a shell’.
An Amoeba within a Shell
Hooke and Leeuwenhoek established the foundations for the study of microscopic organisms. The mass manufacture of microscopes in the 19th century then brought that world much more widely within the reach of ‘parlour microscopists’. During that century too, the biological relationships of many of the microorganisms that make tiny skeletons began to be recognized for the first time.
Born in 1802 and brought up along the Atlantic coast of France, Alcide d’Orbigny would, from an early age, be seen with his brother Charles collecting the local beach sands. From these he would extract the shells of many tiny organisms, including foraminifera; d’Orbigny became fascinated with these minute spiral forms that had long been classified as tiny cephalopods, relatives of the much larger and extinct ammonites. As a young man, d’Orbigny set out to examine what these tiny shells might be, travelling first to Paris to study the collections of the famous naturalist Jean-Baptiste Lamarck. In 1826, d’Orbigny published a major study, Tableau méthodique de la classe des Céphalopodes, in which he coined the name foraminifera, which literally means ‘hole-bearer’. By studying their minute forms—he eventually described over 690 different species—he had recognized that foraminifera have holes in their shells, and thus they were significantly different from their supposed larger cousins, the cephalopods, though at that time he still classified them within that molluscan group. The holes are indeed very important to foraminifera, because it is through these openings that thin strands of cellular material stream out from the shell to make pseudopodia (sometimes called ‘false legs’), structures that foraminifera use to reel in food particles from their surrounding environment. It would be another decade though, before the true affinities of foraminifera were recognized, as unicellular protozoans.
Foraminifera have made a bewildering range of skeleton shapes that sometimes defy their classification as ‘micro’. The shells of the largest of these, the Nummulites that lived over 50 million years ago, sometimes reach 16 centimetres in diameter, or about 300 times larger than the typical foraminifera. Compared with the size of an average domestic cat, a scaled-up Nummulites-sized cat would loom 100 metres into the air above you. This ability to change the size and shape of the shell identifies one of the key features of foraminifera, in that they are almost infinitely adaptable to a great range of aquatic environments on Earth. Nummulites adapted rapidly to fill vacant marine ecological niches following a geological catastrophe on Earth 55 million years ago, one that, for a time, exterminated coral reef systems worldwide.
Foraminifera are amongst the ultimate survivors. The earliest forms may have lived in the Precambrian seas more than 550 million years ago, and certainly from the early Cambrian onwards their tiny shells can be found fossilized in rocks. They live in a great range of aquatic ecosystems today, from freshwater lakes to the very deepest parts of the ocean, including the Marianas Trench of the West Pacific, where they thrive many kilometres below the sea surface. The foraminifer shell is a marvel of engineering too, even more so when you consider that it is made by an organism comprising just a single cell. The form of these shells has kept evolving through time. The marvellous World Foraminifera Database—yes, there is such a one—records 38 151 species of foraminifera as of January 2017, 8981 of them living, the rest being extinct as fossils.
The simplest of their skeletal designs are single-chambered sacs, tubes, or globular structures, while in some the tubes just keep growing, spiralling around to mimic the shape of a tiny, coiled serpent. These skeletons may be made of a resistant organic material, and sometimes sediment grains are embedded into this. Others stick sediment grains together to make a ‘crazy paving’-style shell, as in the foraminifer Reophax.
As time went on, foraminifera evolved the ability to make their shells from calcium carbonate, extracting it from seawater to make thousands of different shapes. Some of the most ancient of these are called the fusulinids, which made multichambered shells that could resemble rice grains in shape. They became so common in marine sediments of the Carboniferous and Permian periods that they are widely used to make precise time zonations for rocks of that age.
The most diverse of living foraminifera are a group called the rotalinids. They are in the sands that Alcide D’Orbigny collected on the French coast: in typical sands that one can use to build sandcastles on a summer’s day. The rotalinids make multichambered shells from calcium carbonate too, some types living on the sea bottom and some floating high in the water. They add chambers to their shell by stretching out their pseudopodia to make a protective cyst within which the new shell is formed. There are thousands of living sea-bottom rotalinid species, but only a few species—perhaps 40—that permanently float as plankton. Many of the floating forms have shells that look like tiny balloons stuck together, the most famous of these being Globigerina (Figure 29), the skeletons of which amass to form oozes on the deep ocean floor. Although the number of these planktonic species is very small, they are all highly prized by scientists. This is because certain of them live in the cold, almost freezing surface waters of the polar oceans, others in the warm waters of the tropics, and yet others in the temperate zones in between. Change in these patterns of ocean temperature over geological time has largely been worked out by scientists studying the distribution of these fossils in ancient strata. To do this, though, their modern patterns had first to be discerned.
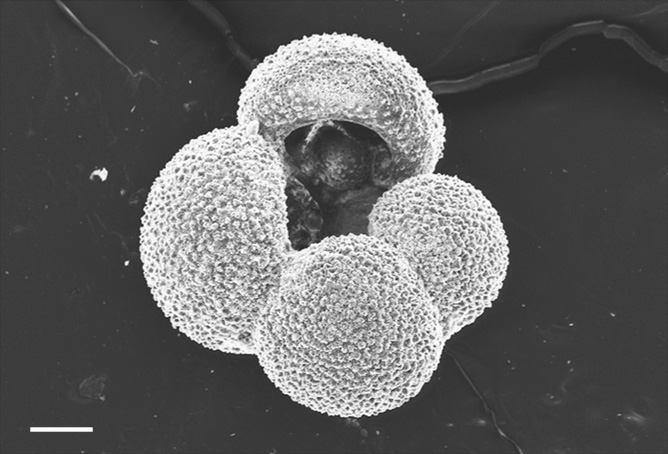
Figure 29. The planktonic foraminifera Globigerina bulloides. The scale bar is one-tenth of a millimetre.
Challenging Times
The 19th century was an age of great discovery for biology, led by explorer-scientists such as Humboldt, Darwin, and Wallace. And towards the end of that century, building on the work of these giants, the Royal Society in England equipped the HMS Challenger to undertake the world’s first global oceanographic survey. Challenger was not the first ship to survey the seas. During the American Civil War of the 1860s, Union forces conducted surveys down the east coast of North America. And, during that decade too, dredges from 270 fathoms by the USS Corwin in the Florida straits revealed a profusion of life that would soon help overturn the prevailing idea of a dead ‘azoic’ deep ocean.54 In 1867, Scotsman Charles Wyville Thomson on the Royal Navy’s HMS Lightning turned up ocean life in dredges from more than 600 fathoms off the Faroe Islands. HMS Challenger was therefore not the first to explore the deep oceans, but she was certainly among the most intrepid, and was the first to make a truly global survey.
Challenger was not originally equipped for science. Built in 1858, her 17 guns immediately identified that she was a warship, a steam- and sail-powered corvette in Britain’s Royal Navy. She had seen active military service, being involved in operations in Mexico and the Pacific during the 1860s. But, with 15 of her 17 guns removed, the space could be converted into purpose-built scientific laboratories, one of these being for natural history. Sadly, little of Challenger’s structure remains. She was sold for scrap in 1921 and broken up for her copper bottom. But her figurehead was preserved, and it welcomes visitors to Britain’s National Oceanography Centre in Southampton. Challenger’s scientific legacy remains as a wealth of materials from her 69 000-nautical-mile, 4-year journey that began in 1872. In the process, Challenger used both shallow and deep sea dredges to collect biological specimens from the oceans. Often these dredges were cast so deep into the sea, kilometres below the surface, that they returned to the surface crumpled, damaged by the immense pressures at these depths. But they mostly returned with a wealth of marine life, and amongst that life were myriad tiny creatures with hard skeletons. Two brothers were now set to describe these tiny creatures in enormous detail.
Born in the northeast of England at about the same time that foraminifera were first being recognized as protozoa, George Stewardson Brady (born 1832) and Henry Bowman Brady (born 1835) are two of the great names of early micropalaeontology, though almost entirely forgotten to most of science today. Still, they lend their name to a medal—The Brady Medal—presented once a year on behalf of the Micropalaeontological Society in London to honour an esteemed micropalaeontologist. The medal is lovely: cast in bronze and sculpted by famous artist Anthony Stones—a past president of the Royal Society of British Sculptors—it depicts the profiles of the two brothers on one side and, fittingly, a microscope on the other.
The Bradys were not by formal training natural historians. Henry was trained as a pharmaceutical chemist, whilst George followed his father into medicine. By the 1870s, though, when the chief scientist on HMS Challenger, Charles Wyville Thomson, was looking for someone to describe the microfauna, it was Henry who received the foraminifera, and George who took the tiny arthropods.
In science, ideas and observations are sometimes ‘cooked’ quickly whilst others simmer slowly. The Bradys were simmerers. Working with microscopes that would look primitive today, they set about systematically documenting the Challenger microfauna, producing beautifully detailed drawings of the foraminifera and ostracods that had been scooped up from the oceans. They produced two huge published volumes that proved to be scientifically durable, and that still grace the shelves of scientists today. The Brady brothers had provided the first detailed inventory of the microfauna of the oceans, from many different localities around the world. This formed the basis for recognizing that different water masses could be characterized by the organisms that lived in them—and that, on dying, fell to the sea floor, to become entombed in the accumulating strata. This is an idea now used widely by oceanographers as they study changes in ocean circulation.
‘The uninitiated may be excused for wondering why men of ability should spend a considerable part of their lives in studying creatures so insignificant in size and so generally harmless to mankind, as the Entomostraca’, as George’s obituary in the proceedings of the Royal Society for 1922 recorded. It went on to say ‘That it may be observed that, as in [the] old Camden's phrase, “many a little makes a mickle” and as little grains of sand may make a mountain, so the stupendous multitudes in which some of the entomostracan species occur make them indirectly yet ultimately important contributors to human food and comfort’. These words are a fitting epitaph for any scientist who studies small objects, from microfossils to fundamental particles. But they have a particular resonance now, as we begin to understand the tremendous importance of these tiny organisms so beautifully and precisely recorded by George and Henry. They occur in numbers in the oceans sufficient to underpin some of the major biological cycles on Earth, such as those that control the availability of oxygen, or the carbon cycle.
The tiny copepods studied by George Brady are perhaps the most abundant microplankton today. Typically just a few millimetres long, their arthropod bodies resemble those of the ostracods (Figure 30). But their exoskeleton is not toughened with calcite, and so after death their bodies rot, leaving almost no trace in the fossil record. They feed on phytoplankton in the surface waters, whilst their sea-bottom dwelling relatives mostly chew through organic detritus at the seabed, though some have parasitic or predatory lifestyles. In the Southern Ocean around Antarctica, copepods have become so super-abundant that, like krill, they are a primary food source for animals living in these cold polar waters.
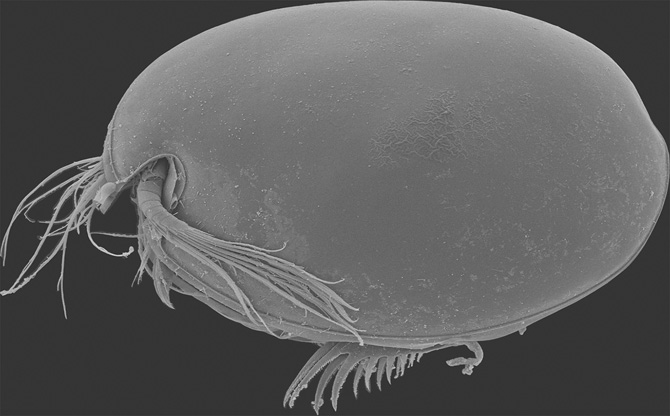
Figure 30. The ostracod Vargula hilgendorfii, from Tateyama, Japan. Specimen is 2.1 mm long.
The planktonic foraminifera live in waters that range from polar to tropical. Some of these species are ‘sunbathers’, favouring the warm surface waters of the tropics, like the elaborately named Globigerinoides sacculifer. Others favour cold waters, like the unpronounceable Neogloboquadrina pachyderma—its second name an allusion to having a shell surface that looks like an elephant’s (pachyderm) skin. Still others chase the seasonal blooms of plankton in the mid-latitudes, like Globigerina bulloides. After death, their remains rain down to the seabed, to contribute to the slowly growing strata of the deep ocean, forming a permanent archive of ocean conditions at the time they were living. As time passed, over tens, hundreds, thousands, and ultimately millions of years, the patterns of ocean currents and climate changed above them, the changes being faithfully recorded in these tiny skeletons.
Ancient Mysteries and Whirling Dervishes
How far back does this archive of micro-skeletons go? Across the world in rocks older than the Cambrian System, older than 541 million years ago, it is difficult to find fossils. This seeming absence of fossils puzzled Darwin, who wrote ‘Consequently, if my theory be true, it is indisputable that before the lowest [fossil-bearing] strata, long periods elapsed, as long as, or probably longer than, the whole interval from the [Cambrian] to the present day.’ In these Precambrian rocks, organic fossils visible to the naked eye were later recognized: so too, eventually, were skeletal fossils quite invisible to the naked eye. But Darwin, even had he suspected their presence, was in no position to undertake the difficult and dangerous process necessary to release them from the rocks in which they are entombed. To extract these long-entombed miniature skeletons, one must use hydrofluoric acid, while dressed head to foot in protective clothing, rather like the Tudor physicians of long ago. Like smallpox or the plague, hydrofluoric acid is not to be toyed with. Although not the very strongest of acids,55 it is highly toxic if swallowed or spilled onto skin, and causes severe burns. When the acid has done its work on the rock, though, the tiny fossils are released and can be examined by microscope. What, then, can be seen?
Ranging in size from a few tens of microns to a tenth of a millimetre in diameter, they have a variety of shapes. Most of the very ancient forms look like simple spheres made out of some complex, tough organic substance—so tough that it will resist the acid while the rock dissolves around it. The general term for them is acritarchs, coined by the palaeontologist William Evitt in 1963, a name that means, in effect, ‘we don’t know what these organisms are’. We now know they go back an awfully long way into the Earth’s deep past. A few years ago, convincing examples were dissolved out of South African rocks all of 3.2 billion years old.56 These were just simple spheres (later ones could develop different shapes, or spine-like projections), but some of these pioneers were giants as such microfossils go—up to a third of a millimetre across. Their affinity, as with later acritarchs, was unknown. But they meant that, way back then, there were organisms, almost certainly single celled, that had learned to grow a kind of armour. What for?
Simple survival is the likely answer. Microscopic organisms today have, individually, a precarious existence. There is generally something out there that regards them as food (often another microbe), and so their lifespan is typically terribly brief. One way to circumvent this is to reproduce extremely quickly, so that many new individuals are continually recruited, to offset the carnage. But another way is simply to lie low at times when the going gets particularly tough. For some microscopic organisms that today live as microplankton, such as the dinoflagellates, this may involve literally growing a tough outer envelope, termed a cyst, and sinking to the sea floor. There, they wait, more or less inanimate, until better times come, when they emerge from their cysts, which are left discarded in the sea floor muds, and get back to whatever is normal life for them. We assume that at least some of the mysterious Precambrian acritarchs, whatever they were, were doing something similar. Some have also been interpreted as egg cases, as the sheaths of microbial cells, and even as fungal spores.
When Darwin was writing On the Origin of Species, the tiny organic skeletons of acritarchs were unknown to him. minuscule organic-walled structures such as the pollen of modern flowering plants had already been observed in the 17th century using microscopy, though, and by the 19th century German botanist Heinrich Göppert was recognizing pollen fossils. By the end of that century the use of fossil plant pollen and spores to identify time zones in ancient rocks was becoming widespread, particularly in strata that were coal bearing, and these microfossils helped trace the evolution of plants.
Bill Evitt also correctly identified that some of the organic-walled microfossils in his samples were closely related to the cysts of dinoflagellates, protozoans that are abundant in the oceans today, with perhaps over 2000 different species. The fossils of dinoflagellates represent only their recalcitrant ‘resting cysts’ or dinocysts—perhaps analogous to the acritarchs—though only a fifth of the species of living dinoflagellates produce such structures. This might provide a clue to the obscure fossil record of dinoflagellates, as possible chemical traces of them have been detected in early Cambrian rocks from more than 500 million years ago, whilst the fossils themselves only appear in the Triassic Period, 300 million years later. This might mean that early dinoflagellates did not make cysts, and so could not be fossilized—or, alternatively, some of the enigmatic acritarchs might have been in reality dinoflagellates.
The dinoflagellates are extraordinary unicellular organisms, which, rather like microscopic versions of the scary protagonists of John Wyndham’s novel The Day of the Triffids, have some characters of plants and some of animals. They bear two flagellae, one of which possesses a whip-like motion that both propels and rotates the cell like a whirling dervish, whilst the other flagella acts like a rudder. Hedging their bets, some use photosynthesis for their food supply, whilst others ingest food particles, displaying a range of complex feeding behaviour. Some engulf other cells using a flexible cell wall. Others are like tiny vampires, sucking away the contents of other cells via a straw-like device called the peduncle. Still others extrude a pseudopodia-like structure, the pallium, which flows around cells digesting their contents. The most famous relationship of dinoflagellates is their symbiosis with corals and many other organisms, where they live within the cells of their hosts, providing a food supply, and utilizing nutrients in return. This is a vital symbiosis, and when the dinoflagellates are expelled from corals, as happens during periods of intense environmental pressure, the corals ‘bleach’, losing their vivid colours and, starved of the dinoflagellates’ food supply, eventually die.
Only some dinoflagellates form a protective outer skeleton in life. Some of those that do not make such an outer skeleton make an internal skeleton instead, this time of silica, with two star-shaped silica spicules located near the cell nucleus. These structures have been found as fossils too. Of those dinoflagellates that have an external skeleton, this is embedded within the cell covering as a series of overlapping structures made of cellulose, which is the stuff of cotton and paper. The skeletons that make up the resting cysts of dinoflagellates, which typically find their way into the fossil record, are made of a different material, a complex biopolymer called dinosporin that has some similarities to the resistant material in the walls of spores and pollen. The cysts may also be mineralized with calcium carbonate or silica.
Dinocysts are formed as part of the sexual cycle of the dinoflagellate (some acritarch cysts may have been similarly formed), and their structure can reflect changes in the water’s nutrient supply, salinity, and temperature. The cysts are elaborate structures, with various projections and a kind of a trapdoor that is used by the organism when it finally emerges. This emergence can be as much as a century later (though most sojourns are much briefer) and so the cysts are as much like the suspended animation capsules of science-fiction films as they are skeletons.
Plankton in Glasshouses
In the original Star Trek episode ‘The Devil in the Dark’, Captain James T. Kirk and the crew of the Starship Enterprise encountered a strange silicon-based life form on the planet Janus VI that could burrow through rock. The science-fiction writer Isaac Asimov, similarly, invented animals he called ‘siliconies’, based on the way that silicon, like carbon, can form long-chain compounds—but not quite as well as carbon, so the siliconies were rather feeble, if charming, interstellar creatures. On Earth, silica is used by life forms—but not to form whole organisms based on exotic chemistry. It is remarkably widespread as a skeleton-building material, and may be the oldest medium from which the skeletons of animals are made.
Silica, usually in the form of quartz, makes up most of the sand grains on a typical beach, is a common component of many rock types, and is the main component of glass. It has the chemical formula SiO2, or one atom of silicon combined with two atoms of oxygen. It is very slightly soluble in water, and is present in the Earth’s oceans as silicic acid (H4SiO4), being bought in by rivers carrying materials weathered from the land, by submarine volcanic activity, and by dissolving pre-existing silica at the seafloor. So, although the idea of a skeleton made from a brittle substance like glass may seem a little strange, silica is a very widespread and readily bioavailable material.
The silica found in the skeletons of sponges and microplankton is not the simple form, as is present in crystals of quartz. Rather, it is biogenic (or opaline) silica, which is hydrated and amorphous, and similar to that in the gemstone form called opal, which is given its treasured opalescence by the water present in the mineral structure. Opaline silica is likely to be present on other planets too, having been recognized on the surface of Mars, near to volcanic rocks, and being regarded there as an indicator of hydrothermal conditions—that is, a mineral product of the chemical interaction between water and hot reactive rocks.
On Earth, the earliest group of organisms to use silica to make their skeletons may have been a motile group of protozoa called the choanoflagellates. They are considered to be the nearest living relatives of animals, with strong similarities to the flagella-bearing cells of sponges, a relationship already noticed in the 1840s by Félix Dujardin, a largely self-taught French biologist who nevertheless became a Professor at the University of Toulouse, and who contributed much to the understanding of protozoans. Alas, choanoflagellates have no fossil record, but this particular trail of evolution that led to sponges may have developed during the late Precambrian.
It is another group of protozoans that provides a longer-lived record of silica skeletons: the radiolarians (Figure 31). They are cousins of those shell-bearing amoebae, the foraminifera, and like them are widespread microorganisms in the oceans. All radiolarians are planktonic; they are not fussy eaters, their food supply including other protozoa and tiny zooplankton. Like foraminifera, too, they can stream their cell material as ‘axopods’ to protect their delicate skeletons, catch prey, and dispose of waste materials. But unlike foraminifera, they form their skeletons from hydrated silica, perhaps from within the sheath of cell material that coats them. Why would such a small organism spend energy to build such an elaborate skeleton? A clue to this lies in the complex structure of radiolarians, the single cell of which is differentiated into inner and outer parts that control different functions. The inner part, the central capsule with the organelles and nucleus, is rather like the command centre of the cell. The outer froth-like envelope, which also contains the cell material that streams out to make the axopods and rhizopods—the same kind of structure as the pseudopodia of foraminifera—both makes this tiny animal buoyant and helps it catch prey. In turn, the outer skeleton of the radiolarian covers all of these tissues, and it often has a further protective layer of spines. Internally too, there are further layers of skeleton, with bars and beams extending inwards towards the centre of the structure that support the complex functions of the cell.
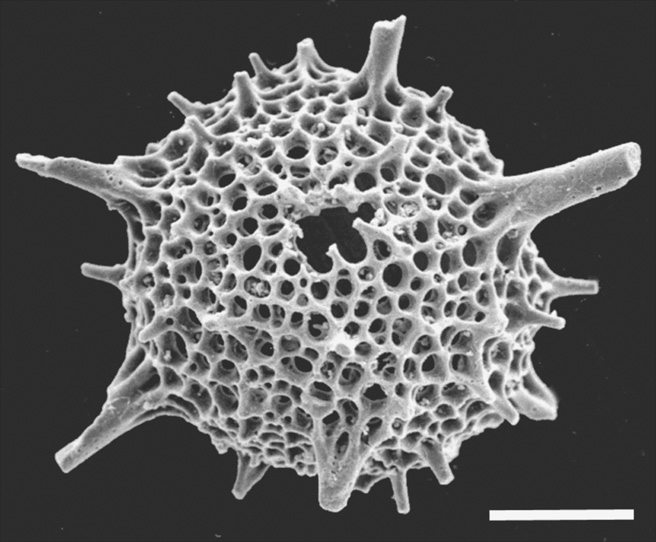
Figure 31. The radiolarian Inanihella sagena from the Silurian of Herefordshire. Scale bar is 100 microns.
These elaborate skeletons—which often appear like tiny glass chandeliers—are hence amongst the most exquisite structures produced in nature, and a brief scan of the Internet will reveal that, minuscule as they are, they have inspired many artists, including the architects of the Skejby Hospital in Aarhus, Denmark. They inspired German scientist Ernst Haeckel too, who was the first scientist to bring the beauty of their skeletons to a broader audience through the 35 copper plates of his monumental work Die Radiolarien, published in 1862. Imbued with a love of both science and the arts, through his dual inspirations Alexander von Humboldt and Johann Wolfgang von Goethe, Haeckel is one of the key figures of 19th-century biology, bequeathing to us such terms as ‘ecology’ and ‘phyla’, and widespread concepts from school biology classes such as ‘ontogeny recapitulates phylogeny’ and the ‘phylogenetic tree of life’. But like some 19th-century thinkers, he had a darker side too, believing in a hierarchy of humans, with ‘higher’ and ‘lower’ races. Little of this, luckily, spills over into the splendid illustrations of life that filled the pages of his Kunstformen der Natur (‘Nature’s Works of Art’).
A little like the young Japanese scientist we met in these pages, Gengo Tanaka, searching for his bioluminescent ostracods at the side of the harbour at the Aitsu Marine Station in the summer of 2016, Ernst Haeckel’s love of radiolarians also began at the seaside, on a visit to Messina in Sicily in 1859. The harbour there contained an abundance of radiolarians in the surface waters, and Haeckel became captivated by their intricate forms. Better still, they satisfied his two great passions, science and art. Little wonder then that HMS Challenger’s radiolarians were delivered to him to describe.
Most radiolarians live in the surface waters of the oceans, and many contain symbiotic algae—including dinoflagellates—that are photosynthetic. But some are found kilometres below the sea surface, as in the Marianas Trench. Like other plankton, they are important in the chemical cycles of the oceans, most notably that of silica. As they die, their skeletons sink into the deep ocean, to be preserved as siliceous sediments at the seabed. They therefore help to maintain a balance between the silica entering the oceans and that leaving. Until the emergence of diatoms in the Jurassic Period, radiolarians were the most important component of this cycle. The silica cycle, too, is linked to other ocean cycles, particularly that of carbon, as both radiolarians and diatoms are involved in the export of organic material from the surface waters to the deep oceans.
Yet other plankton form their skeletons from silica, but these are photosynthesizing forms that take us into the realm of tiny plants. Chief amongst these for their sheer numerical abundance and importance for aquatic food webs are diatoms. Diatoms are algae. They are a highly diverse group with perhaps 100 000 living species, more than all of the different types of vertebrate skeletons put together. In the oceans they are so numerous that they are probably responsible for nearly half the primary production—the basal food supply; this makes them, with the coccolithophore alga, the foundations of the entire ocean ecosystem. Some are adapted to freshwater, and they can be abundant in lakes and rivers. The diatom skeleton is made of hydrated silica, and is called the frustule. The silica of the frustule is made within the cell and extruded into the exoskeleton. Diatoms are typically very tiny, sometimes just a few microns in size, though some species reach gigantic—for them—proportions of a millimetre or more. Although there are a multitude of different shapes, diatoms come in two basic designs, elongate ‘pennate’ forms, and those that are circular, a little like microscopic hatboxes.
Diatoms compete with other skeleton-bearing algal plankton in the oceans for space. Where food is scarce they are outcompeted by the coccolithophores. But, they have supplanted the radiolarians as the key component of the silica cycle and there is evidence too that radiolarian skeletons may have become more delicate and less robust since the Cretaceous, as a result of increased competition for oceanic dissolved silica.
Perhaps the most enigmatic silica skeletons of all, and certainly the rarest, are those of the silicoflagellates. In contrast to the thousands of species of radiolarians and diatoms, silicoflagellates make up just a few species in the oceans and their opaline skeleton is internal, providing a framework to support the cell. Though they are also a part of the silica cycle, their delicate skeletons are much less abundantly preserved in siliceous sediments, forming perhaps 2% of this material. Propelled along by their single flagellum, silicoflagellates can photosynthesize like other algae, but strangely they may also form pseudopodia, perhaps to extract nutrients from seawater. Although there are only a few species of silicoflagellate, their skeletons can take many shapes, from tuning forks to triangles, making them like the ‘snowflakes’ of the ocean. Like other skeletal-bearing plankton, their different morphologies reflect changes in the ocean waters in which they live, and hence they too are skeletal archives of past oceanographic and climate change.
The Coccospheres
Sometimes the surface of the ocean turns a milky white colour from blooms of plankton that make their skeletons from calcium carbonate. Such blooms are seen in the late spring of the Celtic Sea between Britain and Ireland, for instance, or in the western approaches to Cornwall. They are large enough to be seen from space, and the plankton within them may reach millions of cells per litre of seawater. The most important of these carbonate-producing planktonic organisms, in terms of the amount of carbonate they generate each year, are the coccolithophores, tiny algae that characteristically bear two flagellae, and a third structure called the haptonema, which resembles a flagellum but is coiled. These are amongst the tiniest of the skeleton-constructing microorganisms on Earth, though they compensate in the length of their names, not least in that coccolithophores make an exoskeleton called a coccosphere (Figure 32), and each individual plate of that coccosphere is called a coccolith.
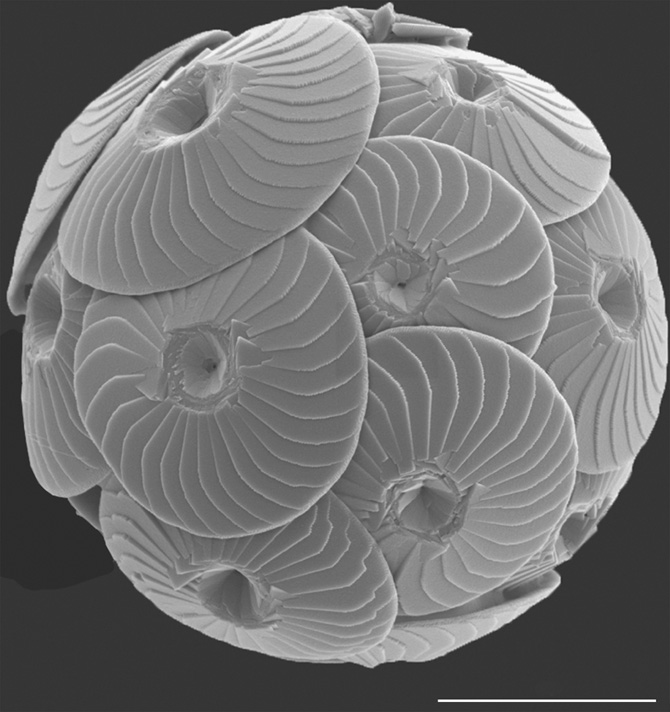
Figure 32. A coccosphere of Calcidiscus leptoporus subsp. quadriperforatus from a plankton sample collected in the Mauretania upwelling, Atlantic Ocean. Scale bar is 5 microns.
Although the whole coccosphere is typically just a few tens of microns in diameter, their numbers can add up to something much larger. During the warm climate conditions of the late Cretaceous, when there were no large polar ice sheets and sea level was much higher, much of the world’s continental shelves were submerged beneath a deep sea. In these warm seas coccolithophores proliferated in numbers so great that their skeletons now make up the chalk cliffs of southern England and many other places. If you take some of this chalk rock and crush it between your fingers, you can then mix this with water and smear the mixture onto a glass slide. With a microscope you will see (just—for they are very tiny, and an electron microscope is needed to see them in detail) that it is largely made of coccolith plates, with some foraminifera and other calcareous fossils such as ostracods. It is this ability to bloom in such numbers, and their wide distribution in the oceans, that make coccolithophores so important for the life-support systems of Earth.
The skeletons of coccolithophores were noticed for the first time in the Cretaceous chalk rocks of Rugen Island, in northern Germany, by Christian Gottfried Ehrenberg in 1836. Ehrenberg was one of the pioneers of the microscopic world—and one who lived dangerously. As a young man he had taken part in an expedition to Egypt and the Middle East funded by Friedrich Wilhelm III and led by the charismatic but dubious Heinrich von Minutoli. It was a disaster, with fever killing several at the outset, and Minutoli soon abandoned the party to pursue his own individual travels. Ehrenberg was the only scientist to survive the gruelling 5-year journey, and was so disheartened57 at the end that he left for others the study of the 80 000 specimens that had been collected.
His journey through the world of the very small, though, bore greater fruit. As a student he had discovered that fungi were not the spontaneous products of rotting vegetation, as was then thought, but instead reproduced via spores.
Initially, Ehrenberg thought these strange discs of the chalk to be inorganic, but later linked them to the ‘animalcules’ that he saw teeming in the Mediterranean, and realized that their remains, accumulating on the sea floor over immense spans of time, could eventually build up a layer of rock a kilometre thick.
Coccoliths were later collected during the Challenger expedition, and recognized to be the skeletons of microscopic algae. Each individual coccolith plate is produced from within the algal cell and then stuck on to the coccosphere from its inner surface. Why such a tiny photosynthetic alga should produce such an elaborate skeleton is unknown, but each coccolith resembles the shape of a Spartan shield, and when locked together in the exoskeleton they form a minute but formidable shield wall that must confer some protection to the cell.
Coccolithophores may be tiny, but their impact on the Earth is colossal. The oldest coccolith fossils are found in sedimentary rocks of the uppermost part of the Triassic Period, from a little over 200 million years ago. In the Palaeozoic oceans there were, perhaps with a few exceptions like planktonic ostracods, no oceanic microplankton that made their skeletons from calcium carbonate. This major pathway in the global carbon cycle became established midway through the Mesozoic Era, with the emergence of both the coccolithophores and the planktonic foraminifera as major players in the biosphere. Why is this skeleton transport system so important for life on Earth?
The export of these skeletons from the sea surface to the deep ocean is part of an ocean-wide process called the ‘biological pump’. It occurs through the life and death of organisms but it is in fact a chemical pump, one that moves carbon in various forms from the sea surface to its depths, and back to the surface again. The carbon in ocean waters exists in organic form in the bodies of organisms, within their shells, and is dissolved as inorganic carbon in seawater itself, while the carbon accumulating at the seabed comprises the bodies of dead organisms and their shells. When a coccolith makes its skeleton near the sea surface it extracts dissolved carbon from the water and as the sea surface interacts with the atmosphere above, it modifies the content of carbon dioxide in the air, drawing down some of that carbon to maintain an equilibrium between ocean and atmosphere. After death, the coccosphere dissociates and the coccoliths (and their carbon) are dissolved back into the seawater or sink to the seabed. Those that become preserved in sea bottom sediments help to balance the amount of carbon that goes back into seawater and ultimately the amount in the atmosphere.
It is a delicate balancing act. Sometimes—as at present with humans burning fossil fuels—the amount of carbon released to the atmosphere exceeds the amount that can be quickly captured by the skeletons of the calcifying plankton, or that can be stored in their organic bodies. When this happens, atmospheric carbon dioxide increases, and so too does the uptake of carbon dioxide into seawater, which reacts to produce carbonic acid, with the subsequent release of hydrogen ions. That makes seawater less alkaline, and it is this process that is called ‘ocean acidification’. Even then, the oceans do not become acidic—otherwise, they would rapidly become lifeless—because the carbonate that has accumulated at the seabed from generations of calcifying organisms simply dissolves back into the water to maintain the pH balance. This is one of many balancing acts that sustain the life of the oceans over very long time frames.
As well as helping to maintain a seawater chemistry that is just right for life, coccolithophores are probably responsible for generating about half of the ocean’s primary food supply. They make their ‘edible’ tissues during photosynthesis, and a by-product of that activity is that they yield oxygen to the atmosphere. They are therefore—together with the diatoms—an integral part of the Earth’s respiratory system. These skeleton-bearing microorganisms are not just a major component of the biosphere, but are also essential for maintaining its life support systems.
From the time of the Tudor physician to the present, our understanding of the world of the very small has grown enormously. The invention of the microscope, at the time that plague was still ravaging much of Europe, identified a new world of skeleton-making organisms, and the oceanic voyages of the 19th century showed their importance across a range of different Earth processes. These tiny skeletons and those of macroscopic animals like bivalves conceal more secrets that, once properly decoded, provide a long and detailed archive of oceanographic and climate change on planet Earth. That record provides us with vital clues that—if acted upon—can help humans mitigate the effects of future climate change. We will examine this record—after, though, exploring the world of aerial skeletons.