

I have found that anyone interested in aromatherapy will inevitably want to learn about the chemical composition and properties of essential oils. I’ve always relied on my intuitive understanding of nature and its products, and had never planned to undertake a study of chemistry. But this is a very exciting time in aromatherapy. The essential oil industry is expanding rapidly, and with the explosion in popularity of aromatherapy more and more oils are being scrutinized by science. As various essential oils become the subject of increasingly penetrating research into their composition, traditionally accepted properties are confirmed, common constituents are identified, and previously unknown constituents and actions are demonstrated. Eventually I realized that it was imperative for me to have a rudimentary understanding of essential oil chemistry, which, while intimidating, actually led me to a deeper understanding of aromatherapy. I discovered that the hard, technical science of chemistry was a complement to my softer, intuitive, and spiritual understanding of aromatherapy.
Essential oils are compounds made up of aromatic molecules. Organic chemistry examines the compounds made up of carbon, hydrogen, nitrogen, and oxygen atoms, the bonds between carbon atoms and other carbon atoms, and the bonds between carbon atoms and hydrogen atoms. These are the basic molecular building blocks of living organisms. In the same way that we have cells, tissues, and systems in our bodies, we also have atoms, molecules, and compounds, which range from minute to visible in size.
Carbon atoms bond together in chains. These chains can be structured in a straight line, branched, or formed into a ring.
An isoprene is a branched structure composed of five carbon atoms, onto which hydrogen can easily bond. Isoprenes form the basic building blocks of aromatic molecules.
Terpenes are molecules made up of carbon and hydrogen atoms. As a circumstance of their chemical structure, terpenes — particularly monoterpenes — are vulnerable to oxidation. Indeed, oxidation is the main cause of spoilage in essential oils, such as the citrus oils, that are rich in monoterpenes.
A monoterpene is a molecule composed of two isoprene units, or 10 carbon atoms joined head to tail. Monoterpenes occur in almost all essential oils. Limonene, for example, is a monoterpene that occurs as a major constituent of lemon oil, as well as occurring in smaller quantities in many other essential oils.
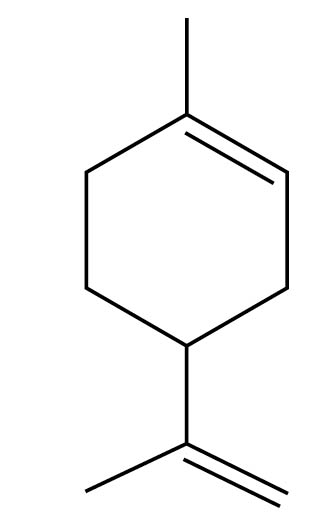
Limonene
Sesquiterpenes have three isoprene units, or 15 carbon atoms joined together head to tail. This makes a denser molecular structure, which causes sesquiterpenes to evaporate more slowly than monoterpenes. Sesquiterpenes are a common constituent in essential oils and make very significant contributions to their odors. The zingiberene in ginger oil is an example of a sesquiterpene.
The other basic building block for essential oil molecules is the joining of six carbon atoms in a ring structure, called a benzene (aromatic) or phenyl ring.
When other atoms bond to these two basic structures, we have what are called functional groups: special arrangements of atoms in a molecule that are subject to characteristic chemical behavior. The chemical behavior and effect of an essential oil can be predicted through an understanding of this chemical structure. (The chart found here outlines oils in the most common functional groups.)
The composition of an essential oil from a plant of a single species always follows the same general pattern, but it will differ in detail from sample to sample. The chemicals most dominant in a particular essential oil will indicate the probable action of that oil. The activity of certain essential oils is dominated by the properties of one class of chemical compounds. This may be a result of a quantitative dominance of a particular compound (or class of compound). Another possibility is that the compound has such a high level of activity that even small amounts suffice to dominate the character of the essential oil, as with lemon.
Alcohols and phenols are similarly structured molecules. Both are common constituents in essential oils, but with distinctly different chemical behaviors: A phenol is highly subject to oxidation, whereas an alcohol is highly resistant to it. Thus a phenol will darken, or redden, with age. Both alcohols and phenols will form esters in reaction with organic acids.
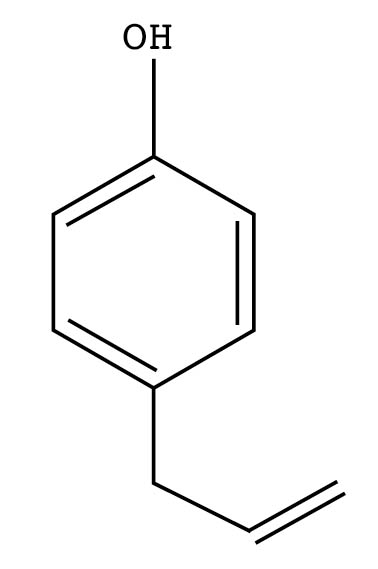
Alcohol
Most alcohols that occur in essential oils possess soft, sweet, herbaceous, or woody odors. You will recognize the ol ending in many common constituents, such as geraniol, linalol, menthol, santol, and so on.
A phenol odor is typically medicinal in character, as with thymol, a major constituent of both thyme- and oregano-type oils, and carvacrol, a constituent that occurs widely in oils of plants from the Labiatae family. Phenols can also smell pungent and spicy, as does eugenol, the characteristic scent of clove. Eugenol occurs in many other oils as well, including cinnamon, rose, and ylang ylang.

Phenol
Esters are the result of a chemical reaction between organic acids and alcohols or phenols, and are widely represented in essential oils. They usually provide fruity notes: Examples include the benzyl acetate in jasmine, ylang ylang, and neroli oils; the geranyl acetate in geranium, citronella, lavender, and petitgrain; and the linalyl acetate in bergamot, lavender, and clary sage.

Ester
Just as organic acids react with alcohols to form esters, the reverse will also occur. High-ester essential oils that contain a proportion of dissolved water from the distillation process can develop acids. The increased acidity that will develop in “moist” ester-containing oils presents an unpleasant sour smell, and is equated with spoilage.
Amines and imines are a subgroup of naturally occurring esters that lend an unpleasant note to some odors. Amines account for a sharp chemical odor in some citrus oils, as well as ylang ylang, jasmine, and tuberose. The imine indole lends a heavy animalistic note to jasmine and orange flower. Skatole, an imine highly present in civet, has more of a role in perfumery. The powerful fecal odor of skatole, when used with great discretion by a highly skilled blender, can actually add beautiful fragrance effects.
Organic Acid + Alcohol = Ester
| Functional Group | Effects of Corresponding Oils |
| Alcohols (monoterpenes) | Largest user-friendly group; balancing |
| Phenols | Aggressive antibacterials |
| Esters | Safest of all the essential oils; can be used “neat” (undiluted on the skin) |
| Aldehydes | Calming to the emotions, irritating to the skin |
| Ketones | Toxic, powerful, aggressive against mucus and abnormal cell growth |
| Ethers | Soothing to the digestive tract but will irritate in a bath |
| Sesquiterpenes | Most soothing and calming |
Aldehydes occur in nature as minor constituents in citrus and other essential oils, as well as major constituents in a few tropical oils. Naturally occurring aldehydes include the citrals neral and geranial, found in lemon and lemongrass oils, and citronellal, found in citronella and eucalyptus oils. Aldehyde odors range from sharp and lemony through floral to intensely green. A wide range of aldehydes is manufactured synthetically for use in perfumery.
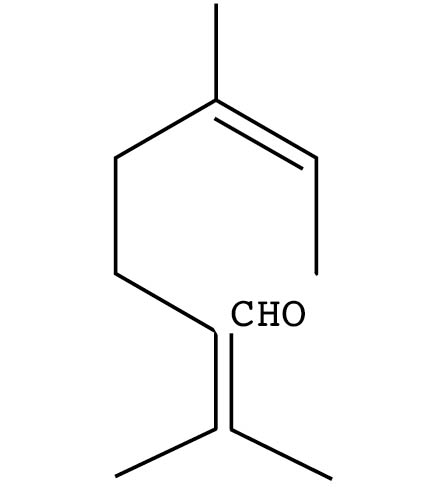
Aldehyde
The family of ketones exhibits a broad range of odor types. For example, methyl heptenone is a ketone occurring in lemongrass and litsea cubeba oils that gives a green, oily, rather coarse odor. Methyl amyl ketone is a minor constituent of clove oil that provides a fruity odor, and menthone is a ketone found in peppermint and other mint oils that provides a fresh, dry odor.
Note: For the purpose of consistency, the spelling ketone is used throughout this text. However, the spelling cetone (or ceton) is also correct, and often used in aromatherapy references.

Ketone
Ethers are relatively stable compounds, thus essential oils that are rich in ethers (such as aniseed and fennel) are particularly stable oils. Ethers contribute a variety of odors, from light and sweet to pungent and medicinal. Anethole, estragole, methyl para-cresol, and safrole are naturally occurring esters that contribute to the warm, sweet odors of licorice-like scents, including basil and tarragon.

Ether
| Chemical Family | Essential Oil |
| Phenols | |
| Syzygium aromaticum | Clove |
| Cuminum cyminum | Cumin |
| Origanum vulgare | Oregano |
| Satureja montana | Savory |
| Thymus vulgaris | Thyme |
| Ethers | |
| Artemisia dracunculus | Tarragon |
| Ocimum basilicum | Basil |
| Pimpinella anisum | Aniseed |
| Foeniculum vulgare | Fennel |
| Alcohols (C10) (monoterpenes) | |
| Aniba rosaeodora | Rosewood/Bois de Rose |
| Citrus aurantium | Neroli |
| Citrus bergamia | Bergamot |
| Thymus vulgaris ‘linaloliferum’ | Sweet Thyme |
| Pelargonium x asperum | Geranium |
| Mentha x piperita | Peppermint |
| Melaleuca alternifolia | Tea Tree |
| Melissa officinalis | Melissa |
| Origanum vulgare | Wild Marjoram |
| Origanum majorana | Sweet Marjoram |
| Daucus carota | Carrot Seed |
| Cupressus sempervirens | Cypress |
| Santalum album | Sandalwood |
| Oxides (1.8 Cineol) + Alcohols (C10) | |
| Eucalyptus globulus | Eucalyptus |
| Eucalyptus radiata | Eucalyptus radiata |
| Ravensara aromatica | Ravensara aromatica |
| Myrtus communis ‘linalol’ | Myrtle |
| Lavandula latifolia | Spike |
| Oxides (1.8 Cineol) + Alcohols (C15) (Sesquiterpenes) | |
| Melaleuca viridiflora var. rubriflora | Niaouli/MQV |
| Oxide (1.8 Cineol) + Ester | |
| Laurus nobilis | Bay Laurel |
| Oxide (1.8 Cineol) + Ketone | |
| Rosmarinus officinalis ‘cineol’ | Rosemary |
| Esters | |
| Lavandula angustifolia ssp. angustifolia* | Lavender |
| Lavandula angustifolia | Miller maillette floris |
| Ammi visnaga | Ammi |
| Salvia sclarea | Clary Sage |
| Juniperus communis | Juniper |
| Cistus ladanifer | Rockrose/Cistus |
| Chamaemelum nobile | Roman Chamomile |
| Pelargonium x asperum | Geranium |
| Ester + Alcohols | |
| Cananga odorata | Ylang Ylang |
| Ester + Lacton | |
| Inula graveolens | Inula/Sweet inula |
| Ester + Diceton | |
| Helichrysum angustifolium | Helichryse/Everlasting/Immortelle |
| Aldehydes | |
| Aloysia triphylla | Lemon Verbena |
| Cymbopogon citratus | Lemongrass |
| Eucalyptus citriodora | Eucalyptus citriodora |
| Cymbopogon nardus | Citronella (Ceylon) |
| Cymbopogon winterianus | Citronella (Java) |
| Cinnamomum zeylanicum | Cinnamon |
| Melissa officinalis | Melissa |
| Ketones | |
| Rosmarinus officinalis ‘camphor’ | Rosemary |
| Rosmarinus officinalis ‘verbenon’ | Rosemary |
| Eucalyptus dives | Eucalyptus |
| Salvia officinalis | Sage |
| Cuminum cyminum | Cumin |
| Hyssopus officinalis | Hyssop |
| Hyssopus officinalis ‘decumbens’ | Hyssop |
| Thuja occidentalis | Thuja cedar |
| Terpenes (C10) Carbon + 10 bonds | |
| Myristica fragrans | Nutmeg |
| Pinus spp. | Pine |
| Pinus spp. | Turpentine |
| Citrus limon | Lemon (peel) |
| Citrus aurantium | Orange (peel) |
| Pistacia lentiscus | Mastic |
| Hydrocarbons | |
| Hypericum perforatum | St.-John’s-Wort |
| Sesquiterpenes (C15) | |
| Apium graveolens | Celery seed |
| Matricaria recutita | German Chamomile |
| Nardostachys jatamansi | Spikenard |
| Achillea millefolium | Yarrow |
| Sesquiterpenes (C15) + Ketone | |
| Artemisia arborescens | Blue Artemis |
|
*Other species of lavenders that are not in the ester group: Lavandula latifolia L.F. Medikus floris Lavandula x intermedia Lavandula hybrida abrialis (10% camphor) Lavandula hybrida rosso Lavandula hybrida var. reydovan (monoterpenic alcohols) Lavandula stoechas (70–80% cetones) Lavandula hybrida super linalol does contain esters. | |