14
Nutrition
Catering to a huge, lucrative market, popularizations of health and nutrition have been responsible for a constant flow of useful information as well as a tremendous amount of misinformation. This is why it is wise to take many of the diet products and nutrition books on the best-seller's lists with a grain of salt, while appreciating that publishers are less concerned with what's true than with what sells.
To understand nutrition, you have to understand that it is a combination of the many integrated processes involved in an organism's absorption and use of materials. Each person varies in small ways regarding what nutrients are needed more or less, but we are all similar with regard to the basic biological processes involved in absorbing and using the nutrients we need. Organisms must procure food, which is then broken down to chemical constituents that are absorbed by the cells. Most of the absorption of these nutrients takes place on a molecular level. These atoms and molecules provide the raw materials that constitute, maintain, and run each of us.
The energy produced by the raw materials is usually measured in units known as calories; one calorie is the amount of energy needed to raise 1 g or cubic centimeter (cc) of water 1°C. The calorie units usually referred to in biology and nutrition are kilogram-calories (Kcal); each is 1,000 gram-calories. When calories are discussed below, they are kilogram-calories; that is, the amount of heat required to raise the temperature of 1,000 grams (one kilogram of water from 14.5–15.5 °C).
Breaking down the nutrient materials we call food involves a series of processes that in the aggregate are known as digestion. Digestion is the physical and/or chemical breakdown of food into sizes small enough to be passed across cell membranes. Our food is the result of other organisms' procurement of nutrients from the environment. Eating other organisms, or their parts, saves us considerable time and energy.
Not all organisms eat other organisms to survive. Green plants and a number of additional life forms survive without consuming other organisms. Animals, however, could not exist without the green plants that synthesize the majority of molecules that are passed on to other organisms. (In addition, it should be remembered that green plants generate oxygen and consume carbon dioxide, while most animals consume oxygen and generate carbon dioxide).
Of the four basic macromolecules found in living things, proteins, carbohydrates, and fats are so abundant, readily accessible, and nutritionally useful that they have become the primary molecular units representing the food in our diets. Nucleic acids, the fourth group of macromolecules, are not abundant enough to be an important dietary item. Of course, water and many minerals are also important and will be discussed in this chapter.
PROTEINS
Proteins are one of the most plentiful organic substances in animals. Some are among the largest molecules that exist naturally in living things; some are also quite small. While they are widely used as structural materials to form muscles and organs, they also form hair, fingernails, feathers, and scales.
The protein we eat has to be digested to produce more readily absorbable chemical constituents. Proteins are compost of subunits, amino acids, arranged in a pattern specific to each distinct protein type. The cells in human tissues are able to synthesize only 10 of the 21 amino acids required in our proteins. The other 11 are obtained from plant and animal tissues present in the diet; these are known as the essential amino acids.
When arranged in their specific patterns, the amino acids make thousands of different kinds of proteins. All amino acid molecules contain one amino group (-NH2). They also contain one carboxyl group (-COOH), which is a characteristic of organic acids. Each of these groups is connected to a carbon atom. Two amino acids are held together by a specific type of covalent bond (resulting from a shared pair of electrons) called a peptide bond. Peptide bonds are formed by joining the amino group of one amino acid with the carboxyl group of the other, through the elimination of water. This is known as a dehydration or condensation reaction.
Along with removing excess simple sugars and processing them into glycogen, which is discussed below in the carbohydrate section, organisms also process fatty acids and other lipids, as well as proteins. Animals store amino acids, proteins, and other nitrogenous compounds that can be used for energy when carbohydrates and fat supplies are exhausted. The first step in converting the nitrogenous compounds into glucose involves removing an amino group (-NH2), a step called deamination. During deamination the amino group is converted into ammonia (NH3), a waste product that is converted into a less toxic molecular form and then excreted.
CARBON
Molecules based on carbon are called organic molecules. The properties of carbon make it one of the most important elements, upon which almost all molecules composing living organisms are based. This element is extremely versatile since a seemingly endless variety of molecules can be formed around it. Four atoms may bond to each carbon atom at any given time; carbon atoms will bond with many other elements. Carbon atoms can join to one another to form long chains. These chains may have additional carbon chains branching from them. Occasionally the ends of the carbon chains join, forming rings. It seems that carbon is the only atom that forms enough different, complex, stable compounds to be the base for the variety of molecules found in living organisms.
FUNCTIONAL GROUPS
Several different functional groups, such as the amino group and the carboxyl group mentioned above, are used to categorize the wide range of carbon-based molecules into their molecular “families.” Each functional group causes the molecule to behave in a specific manner. Members of each molecular family predictably behave in certain ways, taking part in chemical reactions no matter what the rest of the molecule is like. For instance, when a molecule has the carboxyl group, it is called an acid because it is a proton donor, releasing hydrogen ions when in water. Some of the groups that identify important organic molecular families are presented in Figure 14.1.
MONOMERS AND POLYMERS
All the molecules without any carbon are termed inorganic. Of the organic molecules, which are those with carbon, the smallest molecular units are called monomers. When these monomer units are linked together, the molecules are called polymers. The polymers are sometimes called macromolecules. They can be long, straight, or branched chains. A myriad of different polymers, or polypeptides when speaking of proteins, can be formed from the relatively few monomer units that exist. Some proteins consist of just one polypeptide chain. Others are made from two or more polypeptides linked together.

Figure 14.1 Representations of some common organic functional groups.
These macromolecular, polymeric compounds, which compose the bulk of organic matter in animal cells, are important constituents of cell membranes and connective tissues between tissues and organs. Proteins are important to both plants and animals because they are vital to the plasma membranes and intracellular organelles. During certain stages in some plants' life histories, such as in the seeds of legumes (the plants in the bean and pea family), the protein concentrations reach very high levels.
In addition to their importance in the structural components of animals, there are many proteins that have other functions. For instance, there are a number of types of proteins that are important in blood. Some help in transporting calcium ions, and others are vital in the clotting process. Others transport amino acids and various chemicals to parts of the body requiring them at any given time. Antibodies are largely made of proteins.
Enzymes, another group of proteins, have been shown to have specific shapes that enable them to affect all the chemical reactions that occur inside cells. Many of the chemical reactions that break down food molecules require specific enzymes. They accomplish this by attaching themselves to certain molecules to bring them together in an orderly manner so that the chemical reactions can proceed step by step. The enzymes that bring molecules together, as well as break them apart, are catalysts. Stated slightly differently, enzymes are catalysts made of protein.
The body doesn't need much protein each day. The Recommended Dietary Allowance (RDA), published by the National Academy of Sciences in conjunction with the National Research Council, states that an adult needs about 60 g of protein per day, which is about two ounces. (If you want to calculate this, multiply 0.8 g of protein times the number of kilograms you weigh; 1 kg = 2.2 lb.) A 1-year-old needs about 25 g; otherwise symptoms of protein deficiency may occur. Depending on the degree of the symptoms, a protein deficiency may be diagnosed as kwashiorkor. Adults with this disease slowly lose their muscle tissue. The process can be reversed by addition protein to their diet. Children suffering from kwashiorkor may recover if treated in time but they may never grow as tall as they might have otherwise, and their learning abilities may be diminished.
CARBOHYDRATES
Carbohydrates form another class of organic compounds. They are composed of carbon, hydrogen, and oxygen, usually with the ratio of two hydrogen atoms to one oxygen atom to one carbon atom. Carbohydrates (people often call them carbs) are rich in energy, but organisms cannot survive long on a diet consisting of just carbohydrates. The same is true of most diets consisting of just carbohydrates and fats; these too would eventually prove fatal because neither of these nutritional groups contain the nitrogen necessary to create the amino acids that form the proteins. Unlike most animals, plants have the capacity to combine inorganic nitrogen, usually in the form of nitrate, with carbon from the carbohydrates they manufacture through photosynthesis. (Photosynthesis is the process by which plants use light energy to convert water and carbon dioxide into carbohydrates.) From these, plants make their own proteins.
SIMPLE SUGARS
Carbohydrates all have a very similar molecular arrangement, though they range in size from the smallest simple sugars, monosaccharides, to the larger polysaccharides, which form such molecules as starch and cellulose. The length of the carbon chain in simple sugars varies, depending on the specific sugar involved. Some contain just three carbons. Those containing three carbons are the trioses, and those with four carbons are tetroses. The trioses, pentoses (five carbons), and hexoses (six carbons) are biologically important because they are monomers most often involved in the complex, polymeric carbohydrates.
DIGESTION
Food is chewed with teeth in the mouth, where the processed food is mixed with saliva, which contains amylase, a starch-digesting enzyme. Saliva is produced and released by salivary glands. Once chewed, food is swallowed. Swallowing involves the passage of food through the throat, via the esophagus, to the stomach, by a wave of muscle contractions known as peristalsis. The stomach is located in the upper portion of the abdominal cavity. The stomach's muscular lining creates an environment where the already chewed food is mixed more and where further mechanical breakdown contributes to the foods' digestion. As the stomach processes food, it temporarily stores food while readying it for further digestion in the small intestine. This assembly line method from the mouth with the teeth for chewing, salivary glands for the manufacture and release of saliva, the tongue for manipulating and helping to position a bolus to be swallowed so the chewed food can go down the esophagus to the stomach, with different roles in digestion, improves the efficiency of digestion by making discontinuous feeding possible.
The mucous that the stomach secretes protects the stomach's lining from the gastric juice that the stomach secretes. Gastric juice is a mixture of hydrochloric acid and digestive enzymes. Once the stomach's contents are reduced to a soupy mixture, they pass through the narrow muscular passageway, the pyloric sphincter, into the first portion of the small intestine, known as the duodenum. Digestive enzymes from the pancreas and the gallbladder are released into the duodenum, from which the contents move on through the rest of the small intestine, where most of the remaining digestion and nutrient absorption occurs.
Much of the nutritional contents of the small intestine are absorbed into the bloodstream through the intestinal villi (tiny, finger-shaped structures in the mucous membrane). Therefore, the blood passing through the capillary beds surrounding the intestines contains high concentrations of simple sugars, amino acids, and other absorbed molecules. It is imperative to rapidly bring the high concentrations of these compounds to a lower level, otherwise the well-being of an animal may be radically affected. After passing through the small intestine, the remaining intestinal contents pass to the large intestine, which is also called the colon, where water absorption occurs before the indigestible wastes pass through the terminal portion of the intestine, the rectum, and out the anus. These wastes are the feces, or excrement.
Immediately after passing from the stomach and the intestine, the nutritionally enriched blood goes to the liver, which removes most of the excess simple sugars, converting them to the insoluble polysaccharide glycogen. Converted simple sugars are stored in this more complex, less reactive form until they are needed. When the blood leaves the liver, it contains a concentration of glucose that is only slightly higher than the normal level found throughout the rest of the vascular system. This sugar is then taken up as it passes through the rest of the body.
The liver stores glycogen until the liver's storage capacity exceeds its limit. Then, any excess glucose gets converted into fat, which is stored in fatty tissues located throughout the body. Therefore, the liver helps to maintain a stable blood sugar level, regardless of how much carbohydrate is consumed. If a period elapses during which an individual is unable to obtain the proper carbohydrates, then the liver converts some glycogen into glucose and releases it into the bloodstream. However, the human liver stores only about 24 hours worth of glucose. When the total amount of glycogen stored in the liver is depleted, the body begins converting its stored fat into glucose.
In addition to the liver, the pancreas also helps to control the blood sugar levels via the hormones released. Insulin decreases the blood's glucose concentration, and glucagon increases the bloods glucose concentration.
ISOMERS
Some of the simple sugars have the same number of carbon, oxygen, and hydrogen atoms. But their atoms have different spatial arrangements, resulting in properties specific to each. Such compounds that are composed of the same kinds and numbers of atoms but are arranged differently, and therefore have different properties, are known as isomers.
Isomers can be classified into different categories. Some differ in the grouping of their atoms. For instance, a molecule may have an –OH group attached to the last carbon, making it an alcohol, or an oxygen atom may be bonded between the two carbons, making it an ether. Figure 14.2 shows the alcohol and ether isomers of C2H6O.
The examples in Figure 14.2 are called structural isomers because they differ in basic molecular structure. Geometric isomers also differ in the spatial arrangement of their atoms because the carbon-to-carbon double bonds cannot rotate, as they would be able to if they were single bonds. Two examples of geometric isomers are provided in Figure 14.3; both have the chemical formula C4H4O4.
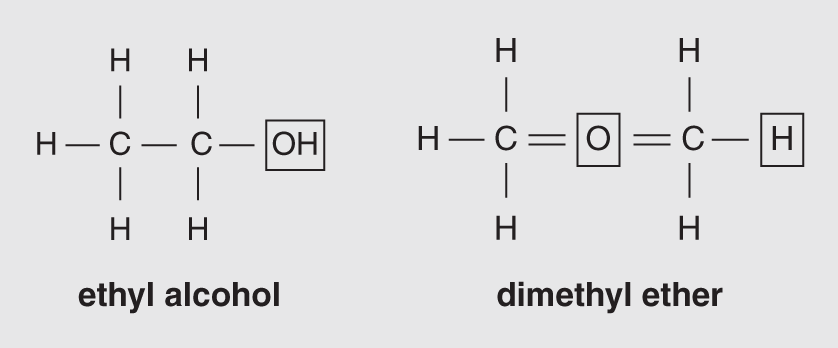
Figure 14.2 These two isomers of C2H6O are structural isomers.
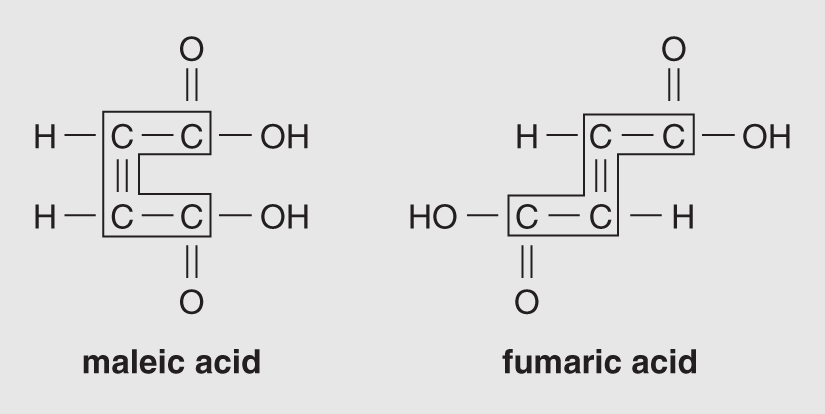
Figure 14.3 These two isomers of C4H4O4 are geometric isomers; note the double bond between the two carbon atoms.
Another type of isomer can be explained in terms of a mirror image. While some mirror image isomers are functionally equivalent to one another, others represent molecular rearrangements that are distinct both structurally, in that they are arranged differently from one another, and chemically, in regard to how they react to each other. Two such optical isomers, as they are called, are presented in Figure 14.4; both have the chemical formula C3H6O3.
Figure 14.5 shows examples of isomeric hexoses – the three monosaccharide molecules each have the chemical formula C6H12O6. Although these three sugars are shown with straight carbon chains, they occur in aqueous environments with all six carbons attached in a ring. The ring structure of glucose is illustrated in Figure 14.6. The other monosaccharides with chains of five or more carbon atoms usually become rearranged into the ring structure when they are dissolved in water, as is the case in living organisms. A graphic way to illustrate these structures, both for simple sugars and for more complex sugars, which are composed of strings of ring structures, is illustrated in Figure 14.7.
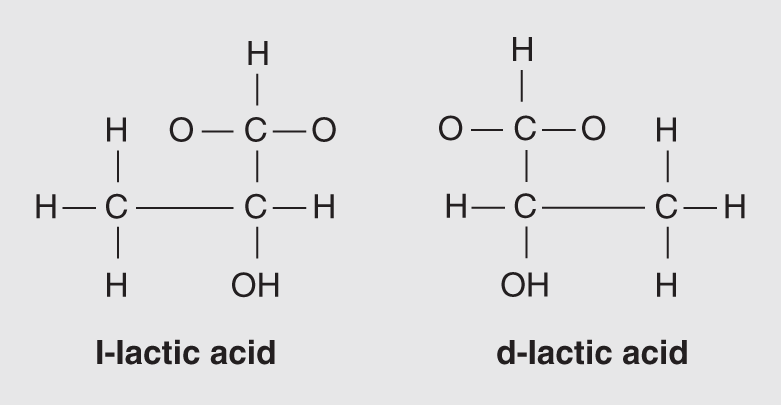
Figure 14.4 These two isomers of C3H6O3 are optical isomers.
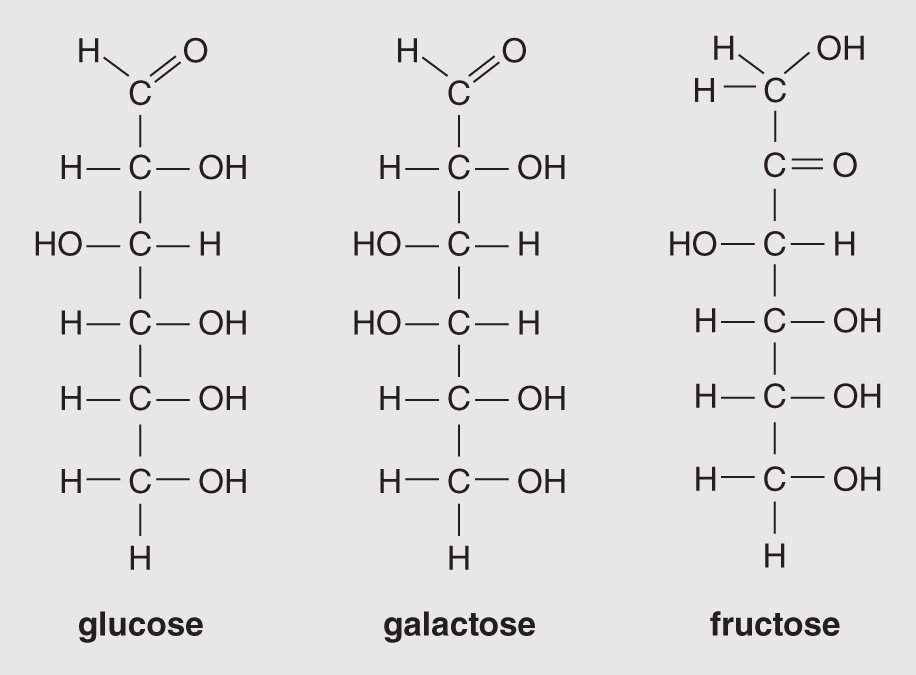
Figure 14.5 Isomers of C6H12O6; these three are isometric hexoses (six carbons).
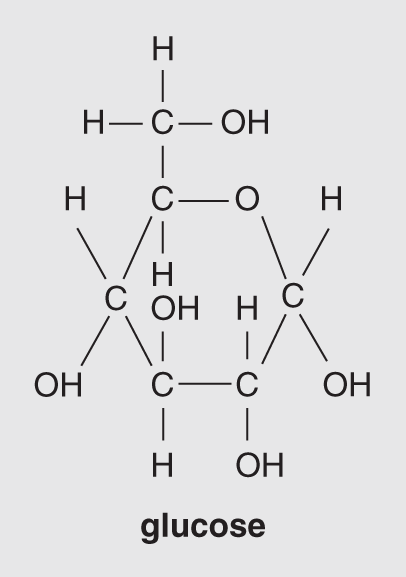
Figure 14.6 Ring structure of glucose (C6H12O6).
When two simple sugars are bonded together, the product is a double sugar, or disaccharide. The bonding process is similar to that of amino acids described above. It involves the removal of a molecule of water and is called a dehydration reaction (or condensation reaction). Such reactions form the disaccharides sucrose, maltose, and lactose as well as the polysaccharides such as glycogen, starch, and cellulose. The dehydration reaction involving two molecules of glucose is illustrated in Figure 14.8.
After each monomer loses an atom or two, the remaining part is the residue. Condensation reactions are reversible; when a water molecule is added between the monosaccharide residues, they will split by hydrolysis, which means water breaking. The carbohydrate molecules that cannot be broken down into smaller units by hydrolysis are monosaccharides. By a series of condensation reactions, many monosaccharides can be joined into polysaccharides, some of which are of great biological importance. Starches, for example, are composed of long chains of glucose. Many plants store excess sugar molecules as starch. Another polysaccharide found in most animals is glycogen (see Figure 14.9), which is similar to starch but has more branching sequences and is one of the main carbohydrates in animal tissues. It is made and stored in the liver and muscles, where it is available as an energy source.
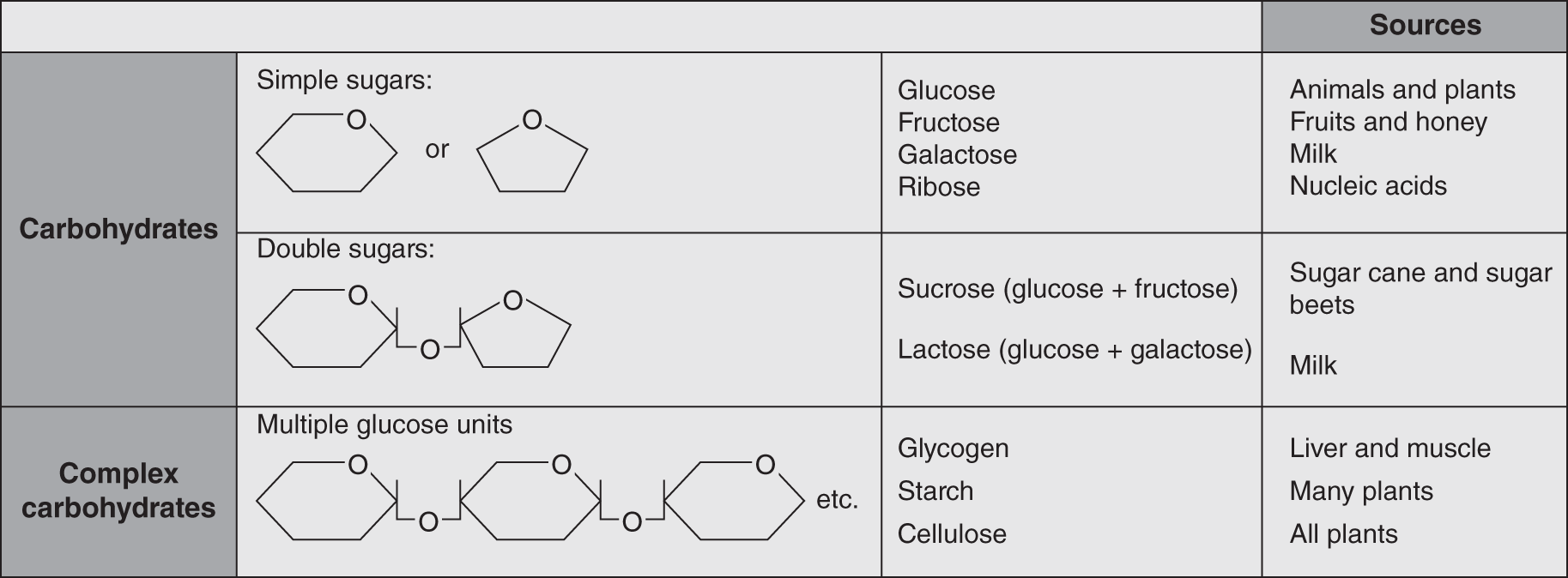
Figure 14.7 Some common simple, double, and multiple molecules composed of sugar ring units, with examples and sources.
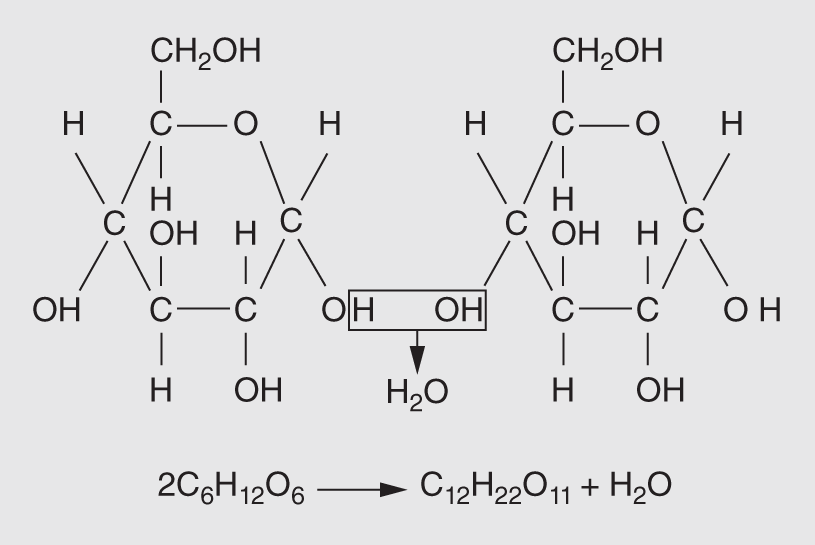
Figure 14.8 Sucrose, our common table sugar, is a disaccharide composed of a glucose and fructose unit. Cells use the energy in the chemical bonds linking the hydrogen and carbon atoms.
Another biologically important complex polysaccharide is cellulose, which supports the bodies of higher plants. Like starches, cellulose is formed by polymerization, the bonding together of small molecules to form long chains. Both starch and cellulose are formed by large numbers of glucose rings that are bonded together, but the precise arrangement of the two polysaccharides differs slightly.
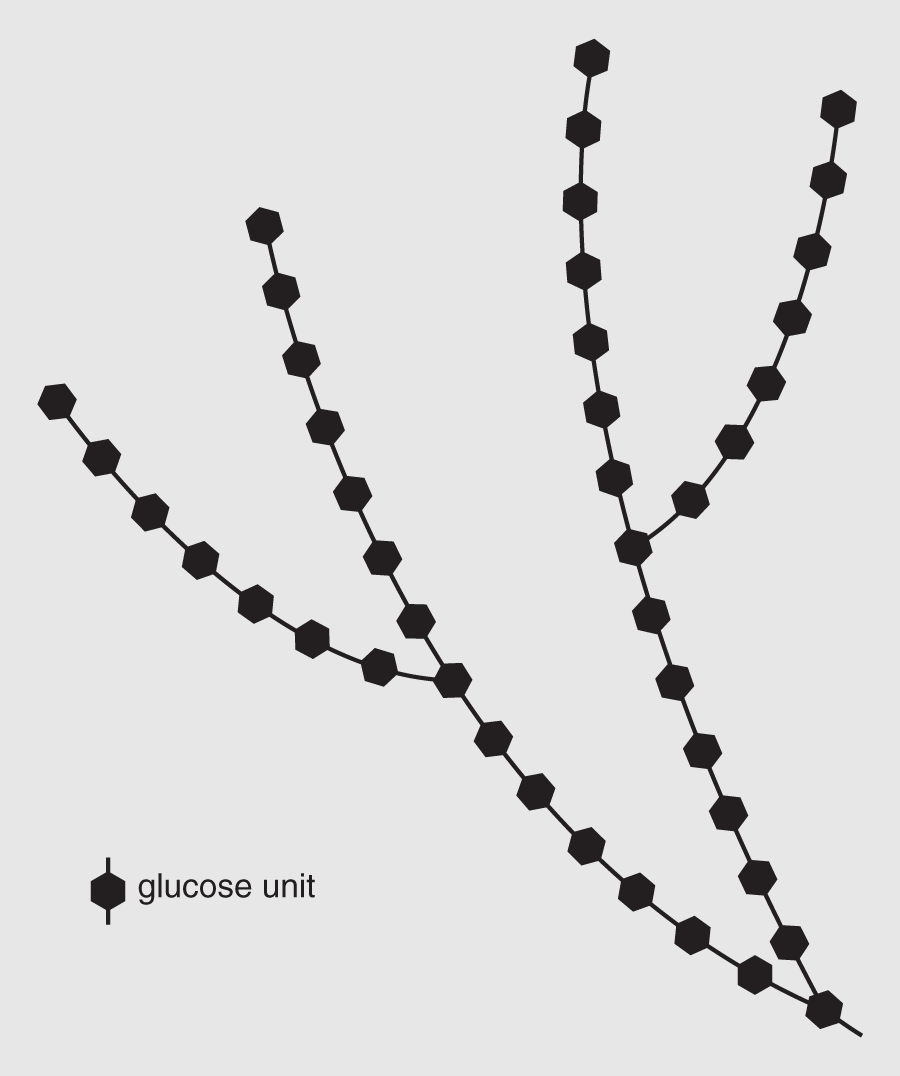
Figure 14.9 Glycogen is composed of long branching chains of glucose molecules. Strings of glucose units are often stored in this form.
LIPIDS
Another group of biologically important compounds are the lipids, which like carbohydrates, are composed largely of carbon, oxygen, and hydrogen. Lipids are defined by their solubility, being insoluble in water and soluble in nonpolar organic solvents such as benzene, chloroform, and ether. Because of their insolubility in water, lipids are vital in the composition of cell membranes. Lipids are also important because they store energy in a concentrated form without interfering with the body's water balance, unlike many energy-rich compounds such as the smaller carbohydrates that dissolve in water and therefore affect the cell's osmoregulation.
Proportionately, lipids contain much more hydrogen and much less oxygen than do carbohydrates. Because of the many energy-rich carbon-hydrogen bonds, lipids have slightly more than twice the number of calories as equal amounts of carbohydrates. The major types of lipids are the fatty acids, triglycerides, phospholipids, and steroids.
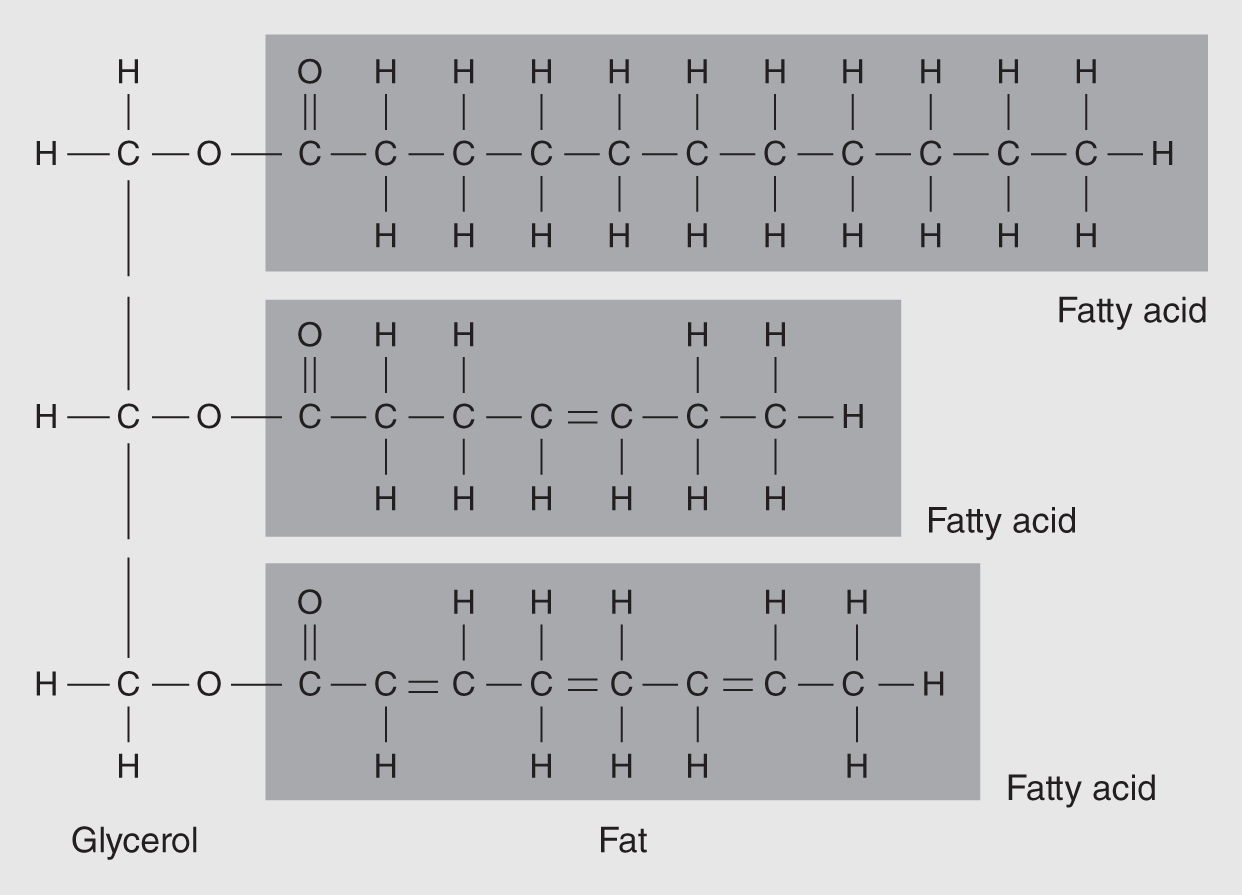
Figure 14.10 Each fat molecule is composed of a glycerol unit to which are attached three fatty acid molecules. (The fatty acid units are usually much longer than those shown here.)
Fats, the most widely known group of lipids, are composed of glycerol and fatty acid subunits (see Figure 14.10). Glycerol is a three-carbon compound that is quite similar to the simple sugars. A fatty acid consists of a chain of carbon atoms, usually 14–22, with hydrogen atoms attached throughout except at the end, where there is a carboxyl group (-COOH). This makes the compound an organic acid and, in this case, a fatty acid. A glycerol combines with three fatty acid molecules, making fats and oils; the technical term for this type of lipid is usually either triglycerol or triglyceride.
Fatty acids are another type of lipid. When a fatty acid doesn't have any double bonds between the carbon atoms, it is called a saturated fatty acid because they cannot take up any more hydrogen atoms. That is, they are already saturated with as many hydrogens as possible. Such fatty acids are found mainly in animal fats. Those with double bonds between some of the carbon atoms can still take up more hydrogens if the double bonds are converted to single bonds. The adding of more hydrogens is called hydrogenation. Fatty acids containing some double bonds are called unsaturated fatty acids. They are found mainly in plants.
Food labels listing the ingredients often term an oil or fat hydrogenated when hydrogen atoms have been added to the fatty acids to thicken the consistency of the product. Although this makes the product less runny, it also makes it less healthy. Doctors used to say saturated fatty acids are bad for you. They also said unsaturated fatty acids appeared to help protect people from heart and arterial disease such as atherosclerosis, which results from the accumulation of fatty material inside the arteries. These deposits cut down the blood flow and can increase blood pressure. Should a piece of the fatty deposit break off, it might lodge in a blood vessel in the heart or brain, causing a heart attack or stroke.
Decades ago consumers were told margarine (made from plant oils, such as canola oil, palm fruit oil, and soybean oil) was healthy and butter (made from cream or milk; it is a dairy product) was bad for us. This was when doctors focused on saturated and unsaturated fatty acids. The question about butter versus margarine took a turn. It's now more complicated and the answer is no longer clear. It turns out, more important than the question of saturated versus unsaturated fats, is the question of trans fats versus saturated fats. Trans fats (trans-unsaturated fatty acids are also called trans fatty acids) raise the concentration of low-density lipoprotein (LDL, also called bad cholesterol) and they reduce high-density lipoprotein (HDL, also called good cholesterol) in our blood.
It was later found that saturated fats do not affect good cholesterol (HDL), yet they raise bad cholesterol (LDL) in our blood. Because margarine contains relatively high amounts of trans fatty acids, margarine was deemed to be bad (or at least not good) for us. And in comparison, butter was no longer so bad.
These two lipoproteins (LDL and HDL) carry cholesterol to and from blood cells. Their levels are now often checked in blood tests. Doctors check these lipoproteins because their relative proportions have been implicated in heart disease. LDL takes cholesterol to your arteries. Too much LDL appears to cause cholesterol to accumulate in and on the interior walls of arteries. This reduces the size of the opening or bore (the hole) through which blood flows. That means the heart has to work harder to pump enough blood through the body. This increases blood pressure. This is not healthy. This can also lead to “hardening of the arteries” (atherosclerosis).
Phospholipids are another kind of lipid that comprises an important component of cell membranes. Each phospholipid is composed of a glycerol, two fatty acids, and phosphoric acid, and in most cases a nitrogenous compound is also attached.
Steroids are another important family of lipids, which comprise many hormones, vitamins, and other body constituents. Each steroid has four joined carbon rings. Cholesterol, which is a steroid, is notorious for being the substance that is laid down inside atherosclerotic arteries. Other important steroids are cortisone, estrogen, progesterone, and testosterone. Synthetic steroids that mimic the male sex hormone, testosterone, known as androgenic anabolic steroids, are used by many athletes to build muscle mass. As men age, they produce significantly less testosterone, and therefore they tend to lose much of their muscle mass. Because researchers feel using these compounds can lead to dangerous side effects, their use is carefully controlled in many countries, and the International Olympic Committee also prohibits their use for a host of reasons. The illustrations of cholesterol and testosterone in Figure 14.11 show the basic steroid construction.
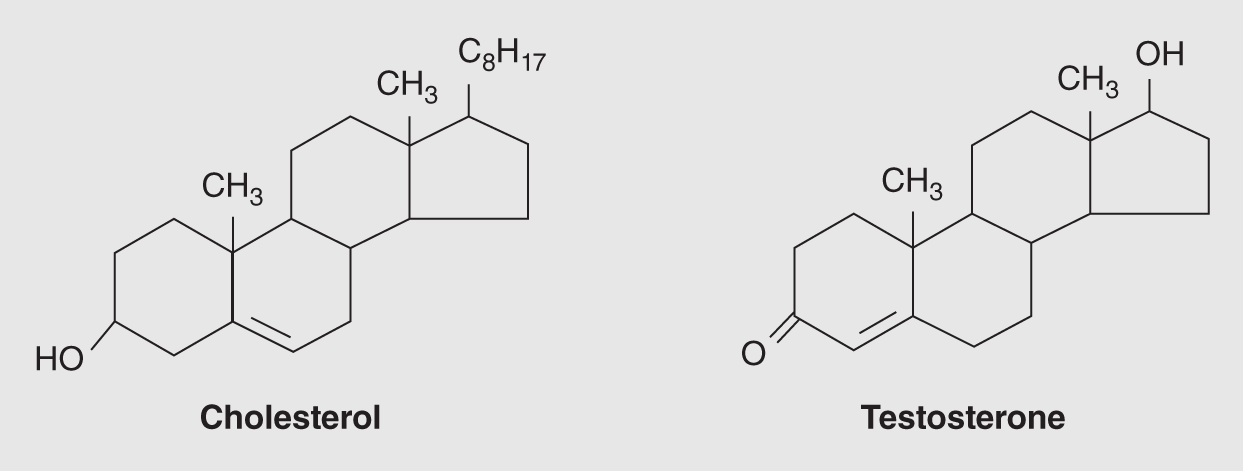
Figure 14.11 Ring structures of two well-known steroids.
VITAMINS
Vitamins are organic molecules that are vital in small quantities to all living organisms. Many participate in biochemical reactions; for instance, some help to activate specific enzymes. Such vitamins are coenzymes. Other vitamins act as catalysts by assisting either in reactions that produce energy or in the manufacture of such chemicals as hormones. Catalysts accelerate chemical reactions without being permanently changed by the reaction. Other vitamins help activate reactions that synthesize tissues, break down waste products and toxins, or help remove toxic substances.
Vitamins are classified into two groups: fat-soluble vitamins and water-soluble vitamins. Water-soluble vitamins, when consumed in the diet in quantities greater than required, are readily passed through one's system and excreted, mostly through the urine. These include the various B vitamins, such as thiamine (vitamin B1) and riboflavin (vitamin B2), and vitamin C. The fat-soluble vitamins are A, D, E, and K. Most vitamins can be extracted from plants and animals, being concentrated especially in their most metabolically active tissues. Table 14.1 provides a listing of the water-soluble and fat-soluble vitamins.
Table 14.1 Water-soluble and fat-soluble vitamins.
| Name | Sources | Function | Deficiency symptoms | Daily need |
| Fat-soluble | ||||
| Vitamin A | Dairy products, egg yolk, green and yellow vegetables | Healthy skin, resistance to infection, formation of visual pigments | Scaly skin, susceptibility to infection, night blindness | 5,000 IUa |
| Vitamin D | Fish, liver, milk, sunlight action on the skin | Calcium metabolism | Rickets muscular weakness | 400 IU |
| Vitamin E | Green, leafy vegetables | Involved in electron transport chain | Anemia, male sterility | 15 IU |
| Vitamin K | Leafy vegetables | Blood clotting | Excessive bleeding | Unknown |
| Water-soluble | ||||
| Vitamin B, (Thiamine) | Organ meats, whole grains, green vegetables | Involved with krebs cycle, normal growth and metabolism, appetite, nervous stability | Beri-beri, irritability, loss of appetite | 1.5 mg |
| Vitamin B2 (Riboflavin) | Dairy products, meat, green vegetables | Normal growth and metabolism, healthy skin, part of electron carrier FAD | Dermatitis | 1.8 mg |
| Niacin | Whole grains, meat | Healthy skin, cell respiration, part of electron carrier NAD | Pellagra, nervous disorders | 20.0 mg |
| Vitamin B12 | Liver | Red blood cell maturation | Pernicious anemia | 0.003 mg |
| Vitamin C (Ascorbic acid) | Citrus fruits, tomatoes | Ground substance in cells | Scurvy, anemia, hemorrhage | 45 mg |
a IU = International units.
MINERALS
There are about 25 different minerals that are required for life to exist. Some of these are needed in relatively large amounts, while others are needed in only trace amounts. Although carbon, hydrogen, oxygen, and nitrogen are three of the main elements occurring in the organic molecules so vital to plants and animals, many other elements are necessary for chemical reactions and the construction of specific molecules. One example is magnesium which, though needed only in trace amounts, is an absolutely necessary component of chlorophyll. Likewise, iron is an important part of the hemoglobin molecule found in red blood cells; iron helps carry oxygen to all the cells in the body. Although the daily number of grams of iron typically equals about 18, only about 0.015 g of zinc are required. Individual differences in people's requirements can vary from 20 to 50 times. Other minerals that are essential for maintaining human health include calcium (health of bones, blood clotting, function of muscles and nerves), chlorine (important in plants, used to open and close pores in leaves, and used in digestion as part of stomach acids), cobalt (part of vitamin B12, used in plant cell division), copper (important in many enzymes, needed for respiration), iodine (important for proper function of thyroid gland), manganese (important in many plant and animal enzymes, necessary in photosynthesis), phosphorus (required in photosynthesis, necessary in animal bones), potassium (necessary in plant and animal cells, and needed for plants to grow and reproduce), and sodium (required for nerves to function, also required to pump ions in and out of membranes). Plants also require specific minerals above minimum and below maximum levels.
FIBER
Much has been said about the importance of fiber, the indigestible part of the vegetable matter one consumes. Fiber consists in large part of cellulose and lignin, two polymers that human digestive systems are unable to digest. We get relatively large amounts of these materials in our diet when we eat vegetables, fruit, and cereals.
The value of fiber lies in the passage of our digesting food through the digestive tract. Peristalsis works best when there is much food in the intestine. However, if by the time everything we eat reaches our intestines, it is soft and without bulk or substance, then the residues will remain there longer than normal. The extra time promotes more bacterial action than usual, which results in more gas than usual, and the added bacterial action may also lead to diarrhea, or constipation. Research indicates that years of this type of digestion can significantly increase the chances of developing colon cancer. A colonoscopy is a simple procedure where the doctor periodically checks the inner lining of the colon for polyps, and if any are found, they are immediately removed, so they won't become cancerous. Cancer cells that develop in the colon and rectum are called colorectal cancer cells. The photo on the cover of this book is a colorectal cancer cell. I chose this because I appreciate that learning biology can be a pain in the butt. Most physicians recommend getting colonoscopies every several years starting when you are around 50 years old, or earlier if a close relative had colon cancer when 60 or younger.
KEY TERMS
| alcohol group | keto group |
| aldehyde group | kwashiorkor |
| amino acid | large intestine |
| amino group | lipid |
| amylase | methyl group |
| anus | minerals |
| calorie | monomer |
| carbohydrate | monosaccharide |
| carboxyl group | nucleic acid |
| catalyst | organic molecules |
| cellulose | optical isomer |
| cholesterol | pancreas |
| coenzyme | pentose |
| colon | peptide bond |
| condensation reaction | peristalsis |
| covalent bond | phosphate group |
| deamination | phospholipid |
| dehydration reaction | polymer |
| digestion | polymerization |
| duodenum | polypeptides |
| enzymes | polysaccharide |
| esophagus | protein |
| essential amino acid | pyloric sphincter |
| ester group | rectum |
| fats | residue |
| feces | saliva |
| fiber | saturated fatty acids |
| fructose | small intestine |
| functional groups | starches |
| galactose | steroid |
| gall bladder | stomach |
| gastric juice | structural isomer |
| geometric isomer | tetrose |
| glucose | triglyceride |
| hexose | triglycerol |
| hydrogenation | triose |
| hydrolysis | unsaturated fatty acids |
| inorganic molecules | villi |
| isomer | vitamin |
SELF-TEST
Multiple-Choice Questions
Proteins
- Nutritional energy is measured in ___________, one of which is equivalent to the amount of energy needed to raise one cc of water one degree centigrade.
- calories
- moles
- Kelvin
- amperes
- volts
- Breaking down the materials containing nutritional value, which we call food, involves a series of processes that in the aggregate are known as ___________.
- digestion
- moles
- calories
- nutrition
- proteination
- Proteins are made of ___________.
- monosaccharides
- disaccharides
- simple sugars
- cellulose
- amino acids
- Amino acids contain one ___________ and a carboxyl group.
- amino group
- monosaccharide
- simple sugar
- phospholipid group
- cellulose
- Enzymes are a group of ___________ .
- essential amino acids
- monomers
- proteins
- lipids
- a, b, and c
- Most amino acids can be manufactured from smaller constituents, but others have to be taken in the diet already intact; these are called the ___________ .
- enzymes
- catalyst
- proteins
- essential amino acids
- none of the above
- A protein deficiency may be diagnosed as ___________ .
- scurvy
- rickets
- polio
- kwashiorkor
- none of the above
Carbohydrates
- Most diets consisting of just ___________ and ___________ can eventually prove fatal.
- fats and proteins
- fats and carbohydrates
- carbohydrates and proteins
- essential amino acids and fats
- essential amino acids and carbohydrates
- Carbohydrates with their same basic molecular arrangement range in size from the smallest ___________ to the larger ___________.
- simple sugars, polysaccharides
- monosaccharides, polysaccharides
- simple sugars, starch
- monosaccharides, cellulose
- all of the above
- The length of the carbon chain in simple sugars varies, depending on the specific sugar involved. Some contain just three carbons: these are the ___________; those with four carbons are ___________; five carbons ___________; and six carbon sugars are ___________.
- trioses, tetroses, pentoses, hexoses
- tetroses, pentoses, hexoses, trioses
- pentoses, hexoses, trioses, tetroses
- hexoses, trioses, tetroses, pentoses
- none of the above
- Compounds having the same molecular weight and molecular formula as one another, or, in other words, having the same number of atoms of the same kind but arranged differently, are known as ___________.
- isomers
- tetroses
- hexoses
- triomers
- none of the above
- When two simple sugars are bonded together, the product is a ___________.
- monosaccharide
- disaccharide
- trisaccaride
- quatrosaccharide
- none of the above
- When two simple sugars are bonded together, the bonding process involves the removal of a molecule of water and is called a ___________.
- condensation reaction
- dehydration reaction
- hydrolysis reaction
- a and b
- a and c
- After a dehydration reaction, the remaining part of each monomer is called a ___________.
- condensate
- dehydrate
- hydrolate
- monomate
- residue
- Dehydration reactions are reversible. By adding a water molecule between the monosaccharide residues, they can be torn apart. Such a reaction is known as ___________.
- dehydration
- hydrolysis
- demonomerization
- a and b
- a and c
- By a series of dehydration reactions, many monosaccharide molecules can be joined into long chains, creating complex carbohydrates, which are known as ___________.
- disaccharides
- trisaccharides
- sucrose
- maltose
- polysaccharides
- ___________ is composed of long chains of glucose.
- sucrose
- maltose
- galactose
- fructose
- starch
- A polysaccharide found in most animals is ___________, which has more branching sequences than starch, and is one of the main carbohydrates in animal tissues. It is made and stored in the liver and muscles where it is available as an energy source.
- starch
- cellulose
- glycogen
- fibrinogen
- none of the above
- A biologically important molecule that is a polysaccharide formed by polymerization, that supports the bodies of higher plants, is called ___________.
- starch
- cellulose
- glycogen
- fibrinogen
- none of the above
Lipids
- The group of biologically important compounds that is defined by its solubility, being insoluble in water and soluble in nonpolar organic solvents such as benzene, chloroform, and ether, is known as the ___________.
- carbohydrates
- proteins
- lipids
- a and b
- a and c
- The major types of lipids are the ___________, ___________, ___________, and the ___________.
- amino acids, fatty acids, triglycerides, and steroids
- amino acids, triglycerides, steroids, and phospholipids
- amino acids, phospholipids, steroids, and fatty acids
- fatty acids, triglycerides, phospholipids, and steroids
- all of the above
- Fats are one of the most widely known groups of lipids. They are composed of two different types of subunits: ___________ and ___________.
- triglycerides and phospholipids
- fatty acids and steroids
- glycerol and steroids
- glycerol and phospholipids
- glycerol and fatty acids
- Glycerol is a ___________-carbon compound that is quite similar to the simple sugars.
- one
- two
- three
- four
- five
- A fatty acid consists of a chain of carbon atoms usually consisting of ___________ carbon atoms, with hydrogen atoms attached throughout, except at the end where there's a carboxyl group.
- 0–4
- 4–10
- 10–14
- 14–22
- 22–50
- Fatty acids without any double bonds between the carbon atoms are called ___________.
- unsaturated fatty acids
- polyunsaturated fatty acids
- saturated fatty acids
- polysaturated fatty acids
- none of the above
- Those fatty acids with double bonds between some of the carbon atoms can still take up more hydrogen atoms if the double bonds are converted to single bonds. The adding of more hydrogen atoms is called ___________.
- bonding
- hydrogen bonding
- hydrogenation
- a and b
- a and c
- Fatty acids containing some double bonds are called ___________.
- saturated
- unsaturated
- polyunsaturated
- polysaturated
- none of the above
- Trans fats raise ___________ and they reduce ___________. HDL is high-density lipoprotein; it is also called good cholesterol. LDL is low-density lipoprotein; it is also called bad cholesterol.
- LDL, HDL
- HDL, LDL
- bad cholesterol, good cholesterol
- good cholesterol, bad cholesterol
- best answer is both a and c.
- The important group of lipids composed of glycerol, fatty acids, phosphoric acid, and, in most cases, also with a nitrogenous compound attached is known as the ___________ .
- triglycerides
- steroids
- phospholipids
- fatty acids
- none of the above
- The ___________ are another large family of lipids comprising many hormones, vitamins, and other body constituents. They have four carbon rings, which are joined.
- triglycerides
- steroids
- phospholipids
- fatty acids
- all of the above
Vitamins, Minerals, and Fiber
- Some vitamins act as ___________ by accelerating certain chemical reactions without being permanently changed by the reaction.
- minerals
- fats
- steroids
- hormones
- catalysts
- Vitamins are classified into two groups: the fat-soluble vitamins and the water-soluble vitamins. Excess fat-soluble vitamins consumed in the diet become stored in one's fatty reserves rather than being passed through one's system. Examples of fat-soluble vitamins are ___________.
- vitamins A, B, C, and E
- vitamins A, B, E, and D
- vitamins B, C, D, and E
- vitamins A, C, D, and K
- vitamins A, D, E, and K
- The following mineral is an important component of chlorophyll:
- calcium
- copper
- cobalt
- manganese
- magnesium
- The following mineral is an important component of hemoglobin:
- iodine
- chlorine
- potassium
- magnesium
- iron
- ___________ refers to the indigestible part of the vegetable, fruit, and grains one consumes.
- minerals
- fats
- chlorophyll
- fiber
- hemoglobin
- After digesting food passes through the stomach and small intestine, it passes through the ___________.
- pancreas
- gall bladder
- large intestine (colon)
- esophagus
- duodenum
ANSWERS
- a
- b
- e
- a
- c
- d
- d
- b
- e
- a
- a
- b
- d
- e
- b
- e
- e
- c
- b
- c
- d
- e
- c
- d
- c
- c
- b
- d
- c
- b
- e
- e
- e
- e
- d
- c
Questions to Think About
- Why is it necessary for food to be digested?
- Describe some simple sugars and explain their nutritional value.
- Why are proteins important in a diet?
- What is the role of lipids in one's diet?
- Why do blood tests often check LDL and HDL levels?
- What are vitamins and why are they important?
- What is the difference between a vitamin and a mineral?
- What is thought to be the value of fiber in one's diet?