Part II
Introduction
Part II of this book will be directly concerned with organic reactions and their mechanisms. The reactions have been classified into 10 chapters, based primarily on reaction type: substitutions, additions to multiple bonds, eliminations, rearrangements, and oxidation–reduction reactions. Substitutions are classified on the basis of mechanism as well as substrate. Chapters 10 and 13 include nucleophilic substitutions at aliphatic and aromatic substrates, respectively. Chapters 12 and 11 deal with electrophilic substitutions at aliphatic and aromatic substrates, respectively. All free radical substitutions are discussed in Chapter 14. Additions to multiple bonds are classified not according to mechanism, but according to the type of multiple bond. Additions to carbon–carbon multiple bonds are dealt with in Chapter 15; additions to other multiple bonds in Chapter 16. One chapter is devoted to each of the three remaining reaction types: Chapter 17, eliminations; Chapter 18, rearrangements; Chapter 19, oxidation–reduction reactions. This last chapter covers only those oxidation–reduction reactions that could not be conveniently treated in any of the other categories (except for oxidative eliminations).
Each chapter in Part II consists of two main sections. The first section of each chapter (except Chapter 19) deals with mechanism and reactivity. For each reaction type the various mechanisms are discussed in turn, with particular attention given to the evidence for each mechanism and to the factors that cause one mechanism rather than another to prevail in a given reaction. Following this, each chapter contains a section on reactivity, including, where pertinent, a consideration of orientation and the factors affecting it.
The second main section of each chapter is a treatment of the reactions belonging to the category indicated by the title of the chapter. It is not possible to discuss in a book of this nature all or nearly all known reactions. However, an attempt has been made to include all the important reactions of standard organic chemistry that can be used to prepare relatively pure compounds in reasonable yields. In order to present a well-rounded picture and to include some reactions that are traditionally discussed in textbooks, a number of reactions that do not fit into the above category have been included. However, certain special areas have been covered only lightly or not at all. Among these are polymerization reactions, and the preparation and reactions of heterocyclic compounds, carbohydrates, steroids, and compounds containing phosphorus, silicon, arsenic, boron, and mercury. The basic principles involved in these areas are of course no different from those in the areas more fully treated.
Each reaction is discussed in its own numbered section.1 These are numbered consecutively within a chapter, each section number is preceded by the chapter number, so that Reaction 16-1 is the first reaction of Chapter 16 and Reaction 13–21 is the 21st reaction of Chapter 13. The order in which the reactions are presented is not arbitrary, but is based on an orderly outline that depends on the type of reaction. Within each section, the scope and utility of the reaction are discussed and references are given to review articles, if any. If there are features of the mechanism that especially pertain to that reaction, these are also discussed within the section rather than in the first part of the chapter where the discussion of mechanism is more general.
II.A. IUPAC Nomenclature for Transformations
There has long been a need for a method of naming reactions. Many reactions have been given the names of their discoverers or those who popularized them (e.g., Claisen, Diels–Alder, Stille, Wittig, Cope, Dess–Martin). In the past, this was necessary because mechanisms were not well understood, and a named reaction was a convenient way to identify certain transformations. Nowadays, the reasons for assigning a name are less clear and there may be as many as 800–1000 named reactions. Some believe that this practice has gotten out of hand, while others believe it to be the best way to organize key reactions. Named reactions are useful to a point, but each name must be individually memorized, and there are many reactions that do not have such names. The IUPAC Commission on Physical Organic Chemistry produced a system for naming, not reactions, but transformations (a reaction includes all reactants; a transformation shows only the substrate and product, omitting the reagents). The advantages of a systematic method are obvious. Once the system is known, no memorization is required; the name can be generated directly from the equation. The system includes rules for naming eight types of transformation: substitutions, additions, eliminations, attachments and detachments, simple rearrangements, coupling and uncoupling, insertions and extrusions, and ring opening and closing. Only the most basic rules are given for the first three of these types, which, however, will suffice for naming many transformations.2
II.A.i. Substitutions
A name consists of the entering group, the syllable “de”, and the leaving group. If the leaving group is hydrogen, it may be omitted (in all examples, the substrate is written on the left).
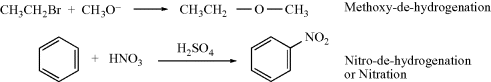
Multivalent substitutions are named by a modification of this system that includes suffixes (e.g., “bisubstitution” and “tersubstitution”).
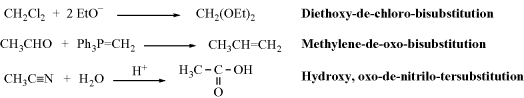
(Note: The nitrilo group is  N.)
N.)
II.A.ii. Additions
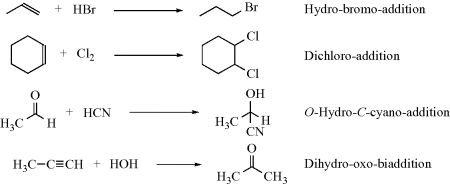
For simple 1,2-additions, the names of both addends are given followed by the suffix “addition”. The addends are named in order of priority in the Cahn–Ingold–Prelog system (Sec. 4.E.i), the lower-ranking addend coming first. Multivalent addition is indicated by “biaddition”, and so on,
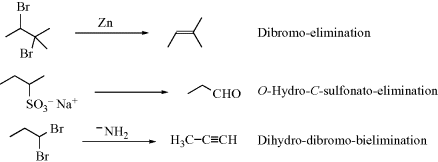
Eliminations are named the same way as additions, except that “elimination” is used instead of “addition”.
In the reaction sections of this book, IUPAC names are used for most transformations (these names will be printed in the same typeface used above), including examples of all eight types.3 As will become apparent, some transformations require more rules than we have given here.2 However, it is hoped that the simplicity of the system will also be apparent.
Two further notes: (1) Many transformations can be named using either of two reactants as the substrate. For example, the transformation methylene-de-oxo-bisubstitution above, can also be named ethylidene-de-triphenylphosphoranediyl-bisubstitution. In this book, unless otherwise noted, we will show only those names in which the substrate is considered to undergo the reactions indicated by the titles of the chapters. Thus the name we give to Reaction 11-11 (ArH + RCl → ArR) is alkyl-de-hydrogenation, not aryl-de-chlorination, although the latter name is also perfectly acceptable under the IUPAC system. (2) The IUPAC rules recognize that some transformations are too complex to be easily fitted into the system, so they also include a list of names for some complex transformations, which are IUPAC approved, but nonsystematic (for some examples, see Reactions 12–44, 18–34).
II.B. IUPAC System for Symbolic Representation of Mechanisms
In addition to providing a system for naming transformations, the IUPAC Commission on Physical Organic Chemistry has also produced one for representing mechanisms.4 As will be seen, many mechanisms (but by no means all) are commonly referred to by designations (e.g., SN2, AAC2, E1cB, and SRN1), many of them devised by C.K. Ingold and co-workers. While these designations have been useful (and we will continue to use them in this book), the sheer number of them can be confusing, especially since the symbols do not give a direct clue to what is happening. For example, there is no way to tell directly from the symbols how SN2′ is related to SN2 (see Sec. 10.A.i). The IUPAC system is based on a very simple description of bond changes.5 The letter A represents formation of a bond (association); D the breaking of a bond (dissociation). These are primitive changes. The basic description of a mechanism consists of these letters, with subscripts to indicate where the electrons are going. In any mechanism, the core atoms are defined as (1) the two atoms in a multiple bond that undergoes addition, or (2) the two atoms that will be in a multiple bond after elimination, or (3) the single atom at which substitution takes place.
As an example of the system, this is how an E1cB mechanism (Sec. 17.A.iii) would be represented:
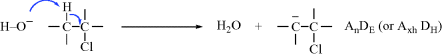
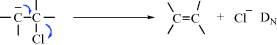
Overall designation: AnDE + DN (or AxhDH + DN). In this case, the overall reaction is

and the core atoms are the two carbons in boldface.
II.C. Organic Syntheses References
At the end of many numbered sections there is a list of Organic Syntheses references (abbreviated OS). With the exception of a few very common reactions (12-3, 12–23, 12–24, and 12–38), and to the extent that it is possible, the list includes all OS references for each reaction. The volumes of OS that have been covered are Collective Volumes I–XI. There are indices to OS.6 Organic Syntheses can now be accessed online.7 Certain ground rules were followed in assembling these lists. A reaction in which two parts of a molecule independently undergo simultaneous reaction is listed under both reactions. Similarly, if two reactions happen (or might happen) rapidly in succession without the isolation of an intermediate, the reactions are listed in both places. For example, at OS IV, 266 is
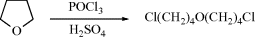
This reaction is treated as Reaction 10–49 followed by Reaction 10–12 and is listed in both places. However, certain reactions are not listed because they are trivial examples. An instance of this is the reaction found at OS III, 468:
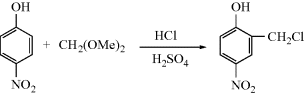
This is a chloromethylation reaction and is consequently listed in Reaction 11–14. However, in the course of the reaction formaldehyde is generated from the acetal. This reaction is not listed in Reaction 10-6 (hydrolysis of acetals), because it is not really a preparation of formaldehyde.
Notes
1. The classification of reactions into sections is, of course, to some degree arbitrary. Each individual reaction is different, and custom generally decides how we group them together. Individual preferences also play a part. No claim is made that the classification system used in this book is more valid than any other. For another way of classifying reactions, see Fujita, S. J. Chem. Soc., Perkin Trans. 2 1988, 597.
2. For a more complete set of rules, see Jones, R.A.Y.; Bunnett, J.F. Pure Appl. Chem. 1989, 61, 725.
3. For some examples, see: attachments (18-27, 19-29), detachments (19-72), simple rearrangements (18-7, 18-29), coupling (10-56, 19-34), uncoupling (19-9, 19-75), insertions (12-21, 18-9), extrusions (17-35, 17-38), ring opening (10-14, 10-35), ring closing (10-9, 15-60).
4. Guthrie, R.D. Pure Appl. Chem. 1989, 61, 23. For a briefer description, see Guthrie, R.D.; Jencks, W.P. Acc. Chem. Res. 1989, 22, 343.
5. There are actually two IUPAC systems. The one used in this book (Ref. 4) is intended for general use. A more detailed system, which describes every conceivable change happening in a system, and which is designed mostly for computer handling and storage, is given by Littler, J.S. Pure Appl. Chem. 1989, 61, 57. The two systems are compatible; the Littler system uses the same symbols as the Guthrie system, but has additional symbols.
6. Smith, M.B. Fieser and Fieser's Reagents For Organic Syntheses, Collective Index For Volumes 1–22, Wiley, New York, 2005; Smith, J.G.; Fieser, M. Fieser and Fieser's Reagents for Organic Synthesis: Collective Index for, Volumes 1–12, Wiley, New York, 1990; Liotta, D.C.; Volmer, M. Organic Syntheses Reaction Guide, Wiley: NY, 1991, which covers the series through Vol. 68. For an older index to Organic Syntheses (through Vol. 45), see Sugasawa, S.; Nakai, S. Reaction Index of Organic Syntheses, Wiley: NY, 1967.
7. Available at http://www.orgsyn.org/.