53.1 Introduction
Congenital heart defects (CHDs) are the most common birth defects and represent a substantial health care burden even in countries with advanced health care systems [1]. Approximately 70% of all CHD cases are idiopathic and a significant effort has been made in the last 10 years to identify the genetic basis of CHDs. However, there remains a significant gap in our understanding of the genetic heritability of CHDs. Splicing in the vertebrate heart has been shown to be dynamic and carefully regulated [2–5]. However, no direct link between alternative splicing and CHDs has been established. In the developing embryo, precise spatial and temporal signaling is required between the first heart field from which the left ventricle is derived and the second heart field (SHF) from which the right ventricle and the conotruncal outflow tract are derived [3, 6–11]. The regulatory networks that control heart morphogenesis are mediated by multiple master regulatory genes (e.g. NKX2.5, GATA4, MBNL1) that control key pathways, including the WNT and NOTCH pathways, all of which have alternatively spliced messages.
Nearly all protein-coding genes in eukaryotic genomes contain introns (94% in humans) which must be removed to produce a mature messenger RNA [12]. Additionally, recent estimates of alternative splicing suggest as many as 90% of human genes have alternative transcript isoforms [13, 14]. There is growing appreciation for the role that alternative splicing plays in normal development and pathogenesis [2, 15–19]. The spliceosome facilitates pre-mRNA processing (splicing) of almost all primary transcripts in eukaryotic genomes. The primary spliceosome, termed the U2 spliceosome, is a multi-megadalton ribonucleoprotein complex composed of numerous proteins and five small nuclear RNAs (snRNAs or spliceosomal RNAs, U1, U2, U4, U5 and U6). The conformation and composition of the spliceosome are highly dynamic and conserved between yeast and humans [20]. Elaborate RNA–RNA–protein interactions align the reactive subgroups and repeatedly rearrange as each intron is identified, intron-exon boundaries are located, and catalysis proceeds to remove each intron in every pre-mRNA.
Some exons are constitutive, that is, they are present in every mature message; however, there are a surprising number of alternatively spliced transcripts that dramatically increase the complexity of the transcriptome and thus the proteome. The significance of alternative splicing for vertebrate evolution was highlighted by two companion papers in the December 21, 2012, issue of Science [21, 22]. Alternative splicing is temporally and spatially controlled resulting in unique splice variants in different tissues and at different time points in the same tissue. Recently, the transition from a fetal to postnatal pattern of a conserved set of alternatively spliced isoforms was shown to regulate mouse heart development [23]. Clearly, mRNA splicing plays a significant role in mammalian cardiac development, but the potential contribution to human heart pathology remains unknown.
Only about 2% of the human genome is translated into protein, but it is now evident that as much as 80% of the genome is transcribed [24]. The importance of noncoding RNA (ncRNA) for heart development has recently been shown by the requirement for correct spatiotemporal expression of specific microRNAs to ensure proper heart development [25]. In addition, there are clear spatial and temporal transcript splicing transitions that are conserved in the vertebrate heart during fetal and postnatal development [23, 26]. It is likely that there are multiple checkpoints to ensure proper transcriptome content for correct heart development. If these checkpoints fail, it is likely that cardiac development will also fail. It is possible that some of the checkpoints may be encoded in ncRNA families yet to be investigated.
A significant class of evolutionarily conserved ncRNA is the small nucleolar RNAs (snoRNAs) with homologs in all eukaryotes. The snoRNAs primarily guide biochemical modifications of specific nucleotides (e.g., methylation and pseudouridylation) of ribosomal RNAs and small nuclear RNAs (snRNAs, also referred to as spliceosomal RNAs). Those snoRNAs that target spliceosomal RNAs are associated with Cajal bodies in the nucleus and are known as scaRNAs (small Cajal-body-specific RNAs). In most vertebrate species, snoRNAs reside in the introns of other genes and require splicing to initiate snoRNA maturation [27]. Interestingly, intron-encoded snoRNAs may have special promoters to drive transcription, suggesting tissue specificity [28]. While the biochemical targets of snoRNAs have been clearly elucidated over the last 20 years, there is a surprising paucity of information regarding the developmental significance of this abundant class of ncRNA.
Our initial studies focused on analyzing the transcriptome of cardiac tissues discarded after surgical correction of tetralogy of Fallot (TOF), a congenital heart disease which is phenotypically representative of disruption of normal conotruncal development. Our preliminary data demonstrated that scaRNAs and their target spliceosomal RNAs are reduced in TOF myocardium [29]. It is therefore possible that reduced levels of scaRNAs may impact stability or fidelity of the spliceosome, causing alterations in mRNA maturation that contributes to TOF. The accumulation of moderate reductions in scaRNA level may cause alterations in spliceosomal function, thus contributing noise to the communication between the first and second heart fields, and resulting in conotruncal misalignment. We hypothesize that tissue-specific control of the pattern of scaRNA expression could provide temporal and spatial specificity to the spliceosome, thus providing a ubiquitous mechanism for regulating splicing in the developing embryo.
While the epigenetic role that scaRNAs play in maintaining spliceosomal integrity and precision is currently unexplored in terms of human health, we have collected substantial evidence that scaRNAs and splicing patterns are tissue-specific and indeed play a critical role in vertebrate cardiac development. We have examined the impact on spliceosome function made by dysregulated scaRNAs using human primary cells derived from the right ventricle of infants with TOF and from normally developing infant heart tissue. In addition, using the well-established zebrafish model, we characterized the nature of scaRNA interaction and its impact on vertebrate heart development. These experiments allowed us to confirm that these epigenetic elements affect vertebrate heart development, which represents a paradigm shift in our understanding of human heart development.

ScaRNAs have reduced expression in fetal and TOF myocardium compared to normal myocardium
53.2 Subjects
Our subjects were children less than 1 year of age with CHDs: TOF; tranposition of the great arteries (TGA); or pulmonary atresia with intact ventricular septum (PA/IVS) requiring surgical reconstruction. Informed consent was obtained from a parent or legal guardian after reviewing the consent document and having their questions answered (IRB #11120627). Our original observations were based on analysis of tissue from 16 infants with idiopathic TOF (nonsyndromic, without 22q11.2 deletions, 11 males, 5 females), comparison tissues from eight normally developing infants (3 males, 5 females). In addition, we have now characterized scaRNA, spliceosomal RNAs and the splicing pattern of 6 key transcription factors from an additional 21 infants with idiopathic TOF. Our acquisition and characterization of control human infant heart tissues and human fetal heart tissue have been previously described [29, 32]. The fetal hearts were dissected by Dr. James O’Brien, a pediatric cardiac surgeon and co-investigator, who performed the reconstruction of the conotruncal defects to ensure the tissue analyzed was from a similar location as the tissues removed during surgery.
53.3 Results and Discussion
53.3.1 scaRNAs Target U2 and U6 snRNAs and Both snRNAs Are Significantly Reduced in TOF
We used the scaRNABase database (http://www-scarna.biotoul.fr/index.php) to identify the nucleotides targeted for modification by the 12 scaRNAs that are reduced in TOF. Only two snRNAs were predicted to be targeted: U2 and U6 snRNAs. Six of the scaRNAs targeted 10 nucleotides (of 23 nucleotides that are known to be modified by scaRNAs) in U2 and 6 scaRNAs targeted 5 nucleotides (of 8 total modified nucleotides) in U6. Interestingly, U2 and U6 had significantly reduced expression in our 16 TOF samples compared to the 8 controls (U2 was reduced 1.8-fold in TOF RV, p = 0.04, and U6 was reduced 3.2-fold in TOF RV p < 0.0001). This is consistent with reduced stability of U2 and U6 as a consequence of inefficient scaRNA biochemical modification.
53.3.2 Cardiac Regulatory Networks Are Enriched for Alternative Splice Isoforms in TOF

Alternative splicing (e.g., DICER and DAAM1) is similar in TOF and fetal myocardium
53.3.3 Reduced scaRNA Expression and Alternative Splicing Are Not a Consequence of Hypertrophy
Hypertrophy is known to reactivate the expression of some fetal genes. Thus we wanted to determine if the fetal expression patterns we observed could be a consequence of hypertrophy. As a comparison to TOF, we analyzed right ventricular samples from infants with TGA and PA/IVS. The embryological origins of TOF and TGA likely share some common features involving miscommunication between the first and second heart fields. However, PA/IVS is distinct and probably less complex in terms of origin of the regulatory defect. Nevertheless, TOF, TGA and PA/IVS share a common attribute: right ventricular hypertrophy. We observed only ten snoRNAs with differential expression in PA/IVS right ventricular tissue compared to control tissue. The ten snoRNAs that were reduced in PA/IVS were in common with TOF, and all target the 28S rRNA. In addition, U2 and U6 levels were not reduced in PA/IVS relative to the control tissue, and splice isoforms were essentially unchanged relative to the controls. On the other hand, RV from children with TGA bore a striking resemblance to the TOF pattern of scaRNA, spliceosomal RNA expression and fetal type splice isoforms. Therefore, the fetal type pattern of scaRNA and spliceosomal RNA expression, as well as fetal splice isoforms, does not appear to be simply a consequence of hypertrophy since they were not present in PA/IVS RV samples. These findings support our postulation that scaRNAs are of key importance in regulating heart development and not simply a consequence of hypertrophy.
53.3.4 Primary Cell Lines Derived from TOF Myocardium Retain the Same Relative Expression Patterns as the Tissue
We have derived primary cell lines from right ventricular myocardium obtained from 15 infants with TOF (TOF primary cells—TOFpc). These cells are most likely fibroblasts. The cell type is somewhat inconsequential since what we wish to examine is spliceosome response to changing levels of scaRNAs. We compared scaRNA levels, U2 and U6 levels, and splice isoform patterns between TOFpcs and primary myocytes derived from normally developing infant heart tissue. TOFpcs retained the same fetal type pattern of scaRNA, spliceosomal RNA expression and splicing isoforms of index genes relative to cells derived from normally developing neonatal cardiac tissue (data not shown). Splicing patterns also retained a TOF pattern in the TOFpcs relative to the normal cells. These changes were consistent through at least four passages of the cell lines.
53.3.5 Overexpression of ACA26 and SCARNA1 (ACA35) in TOF Primary Cells Was Associated with an Increase in U2 Levels and a Decrease in Fetal Splice Isoforms

Levels of fetal splice isoforms are reduced after overexpression of scaRNAs. Data are fold change, averaged from three different TOF primary cell lines (genotypes). ∗Significantly different from levels in sham transfected TOF primary cells
53.3.6 Knockdown of scaRNAs (scaRNA1 Targeting U2, or snord94 Targeting U6) Causes Heart Defects in Zebrafish
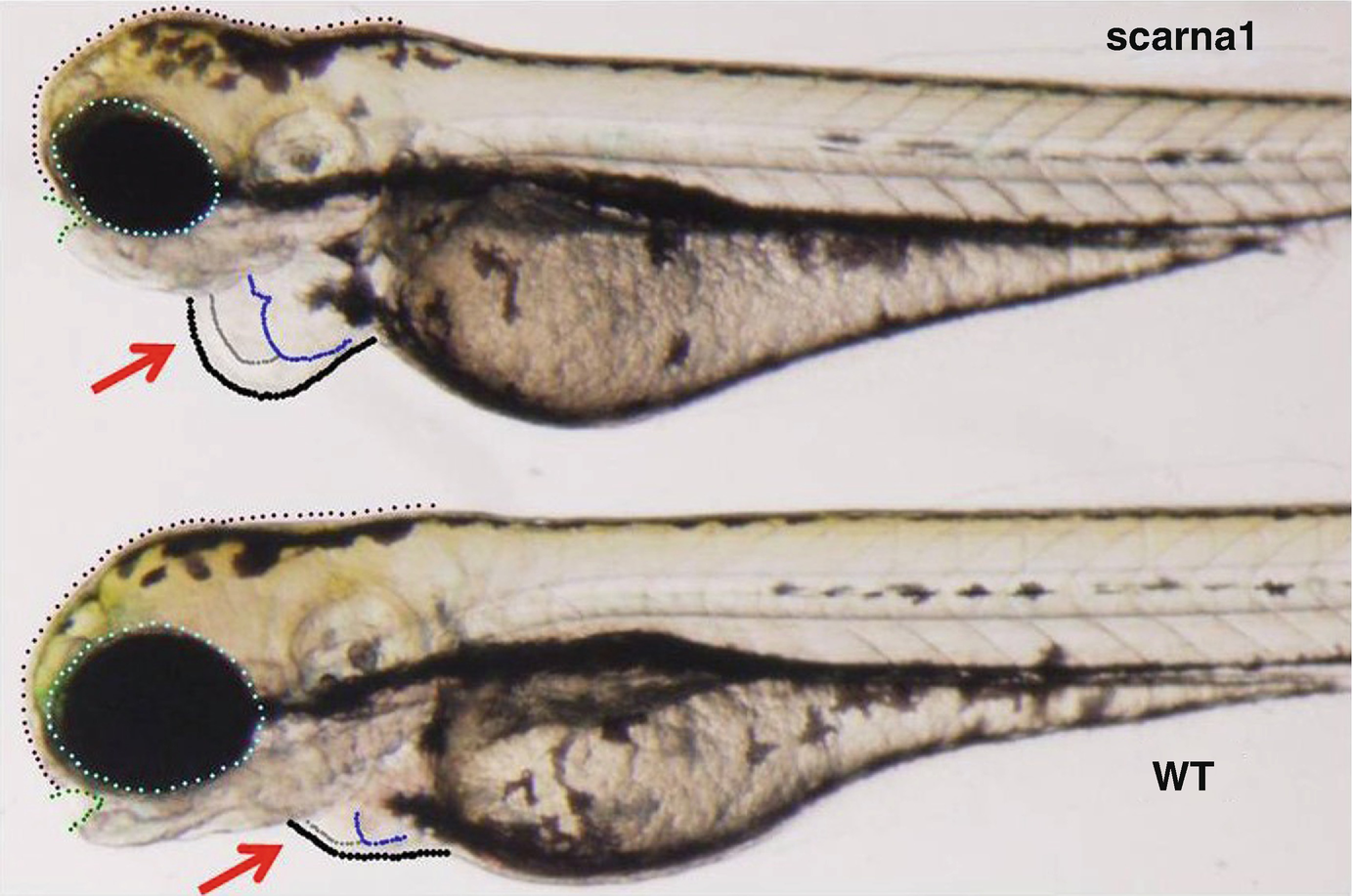
Representative example of zebrafish morphant at 72 hpf (hours post fertilization, knockdown targeted at scarna1). Arrows indicate the heart, blue and grey outlines indicate enlargement of both the atria and ventricle, respectively. Eye and head size are slightly changed but other organs, brain, notochord, otic vesicles, and fins appear essentially normal at this stage
53.3.7 Splice Isoforms Change During Development in Zebrafish and After Targeted Knockdown of scaRNAs

Splicing of WNT pathway genes is altered in zebrafish morphants. Treating zebrafish embryos with anti-scarna1 morpholino causes changes in exon retention of cardiac regulatory genes. Eleven of 39 members of the WNT family have changes in exon retention after treatment with antisense morpholino (assessed by RNA-Seq and qRT-PCR; values shown are from qRT-PCR data). The mismatch morpholino has no significant effect on splicing
53.4 Conclusions
We examined the noncoding transcriptome in myocardial tissue from children with tetralogy of Fallot (TOF) and observed changes in mRNA splice isoforms of genes that are critical for regulating heart development [29]. In parallel, we found a modest but significant reduction in the levels of 12 small Cajal body-specific RNAs (scaRNAs are a subset of snoRNAs and direct the biochemical processing of spliceosomal RNA) [29]. These patterns of scaRNA expression and splicing are similar to patterns we saw in the fetal myocardium. To explore the potential relevance of these findings, we manipulated the expression of two of these scaRNAs in cell cultures and zebrafish. We saw clear changes in splicing patterns and, importantly, developmental deficiencies including heart defects in zebrafish. Our findings support a direct role for scaRNAs in fine tuning the fidelity of the spliceosome in a manner that is critical to support splicing transitions that are essential for correct heart development. Our observations open the door on a new paradigm in developmental regulation and potentially may explain a substantial portion of the missing genetic heritability of CHDs. Importantly, by accurately characterizing the role of scaRNAs in disrupting genetic signaling, it is possible that this could lead to the identification of “targets” that may be amenable to change through intervention as is the case with microRNAs.

Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.