The basic emotions are natural kinds that have specifiable neural substrates within the mammalian brain. If we do not come to terms with such foundation principles, we will have impoverished views of psychological and cultural complexities that ultimately arise from emotional learning.
—Jaak Panksepp, “Emotions as Natural Kinds Within the Mammalian Brain”
BRAIN IMAGING HAS BECOME a popular tool in the neurosciences. The brain imaging procedure that has become the most common and easiest to administer is functional magnetic resonance imaging (fMRI). Like computed tomography (CT), MRI is able to generate cross-sectional images of human anatomy without using X-rays. However, fMRI combines MRI technology with highly specialized statistical analyses to measure localized brain activity. Typically, fMRI uses the blood-oxygen-level dependent signal contrast to measure blood flow in a brain region. The assumption is that when brain activity increases, blood flows to that region and hence oxygen levels increase as well. While this assumption generally holds when measuring the activity of cortical regions of the brain, with their rapidly firing neurons, subcortical neurons tend to fire at much lower rates than cortical neurons creating a detection bias against finding statistically significant estimates of brain activity increases in subcortical regions. In addition, functionally relevant subcortical regions are generally smaller in size, with many nearby, overlapping functional circuits, making them even more difficult to detect.
PET ALTERNATIVE: THE DAMASIO GROUP
An alternate brain scanning procedure, positron emission tomography (PET), is more invasive but substantially more relevant for imaging emotional feelings and particularly so for the smaller, slower-firing subcortical regions of the brain. With PET, not only can general brain activity measures be monitored, typically, by measuring glucose utilization (because the main fuel for brain activity is glucose) but there is also the possibility of measuring various neurochemicals from traditional transmitters—biogenic amines such as dopamine, serotonin, and norepinephrine to more specific functional controls such as brain opioids, as long as positron emitting forms of these molecules have been synthesized.
A landmark study published in the prestigious scientific journal Nature by Antonio Damasio’s research group, which included his wife Hanna who is a specialist in brain anatomy (Damasio et al. 2000), used PET technology to image the whole brain while their subjects actually experienced emotionally powerful states evoked by reminiscences of their own lives. In other words, their goal was to identify neuroanatomical regions whose activity correlated with the experiencing of specific personal memories of past emotional feelings. To ensure optimal results, they first screened their subjects for their ability to self-induce emotions through recalling autobiographical memories of emotionally powerful personal experiences, as well as a neutral episode recalling normal daily events.
For this project, the Damasio group investigated four target emotions: sadness, happiness, anger, and fear. Because of the emotionally taxing reenactment of these powerful experiences, for example, the death of a relative or close friend for the sadness emotion, subjects were assigned to recall only two of the four emotions, based on their prescreening demonstrations, as well as a neutral experience serving as the experimental control. Importantly, the PET brain data were collected (i.e., the radioisotope was intravenously infused) only after the subject reported actually feeling the emotion.
As might be expected, a whole-brain image analysis during the generation of different emotions would produce complex results. However, many of the remarkable correlational findings using their forty-one carefully selected human subjects were consistent with the experimental animal research using deep brain stimulation and related procedures to study primary emotions. These investigators also collected physiological measures of bodily arousals, such as skin conductance and heart rate, that had previously been used to monitor emotional states in humans.
One of the main findings from this Damasio study was the consistent activation of brainstem regions such as the periaqueductal gray (PAG) when these emotions were aroused, regions that are often cited in animal studies of primary emotions. This finding was especially interesting because up to this time “The brainstem has not been noted to be active in other human studies of emotion” (Damasio et al., 2000, p. 1052). Indeed, most previous brain imaging research studying human emotions had focused on cortical brain regions. For example, a meta-analysis (Phan, Wager, Taylor, & Liberzon, 2002) found that, of fourteen studies imaging sadness, a cortical region known as the subgenual anterior cingulate cortex (also known as Brodmann area 25, an evolutionarily older cortical midline structure located just below the anterior portion of the corpus callosum and just posterior to the prefrontal cortical region known as Brodmann area 11) was the most consistently activated brain region, which, although evolutionarily older than neocortex, was not a subcortical brain region. Thus, Damasio et al. (2000) were among the first to confirm previous primary emotion research in animals by using human subjects to illuminate the activation of subcortical brain regions during their experience of personally relevant emotional arousals, in stark contrast with the dominant picture emerging from other human brain imaging studies attempting to discern which cortical brain regions correlated with the generation of specific human emotions. For an easily interpretable picture summary of Damasio et al.’s (2000) PET imaging results, see Panksepp, 2011a, which is readily available.
Another of Damasio et al.’s (2000) findings that contrasted with the opinions of many psychologists, who believed that emotional feelings arise from neocortex, was that during the experience of strong human emotions, many neocortical brain areas were deactivated rather than activated. In accord with previous affective neuroscience research using animals, not only were subcortical areas consistently activated when humans experienced strong emotions but neocortical regions were, if anything, commonly deactivated, which was consistent with the animal evidence that cortical regions were not necessary for the experience of emotions. Animals as well as humans are able to express and experience a full range of primary emotions even if the neocortex had been removed at birth or, in the case of humans, when they were born without neocortex (Panksepp, Normansell, Cox, & Siviy, 1994; Merker, 2007; Solms & Panksepp, 2012).
The activation of ancient subcortical brain regions such as the PAG and the deactivation of more recently evolved neocortical brain regions, such as the dorsolateral prefrontal cortex, during the experience of primary emotions is also consistent with the idea that these physically as well as evolutionarily separate brain regions exhibit a kind of reciprocal “seesaw” interaction as the human brain contends with events that trigger or inhibit the expression of primary emotions (Liotti & Panksepp, 2004). With psychopathology, this seesaw relationship may become imbalanced as the emotional regulation processes become impaired, leading to consistent and persistent dysfunctional biases in the interpretation of socioenvironmental events, which may lead to less-regulated emotional experiences.
As the subcortical brain perceives various survival challenges and we experience intense emotional feelings, the subcortically based primal emotional systems may impose “states of mind” over many regions of the cerebral cortex, thereby altering the “color”, “tone”, and “interpretation” of experiences without changing the neocortical processing of specific cognitive contents (Mesulam, 2000, p. 79). While the connections from the cerebral cortex to the subcortical-limbic networks may be less extensive even in the human brain than the reciprocal subcortical to cortical connections, the recovery from emotional arousals likely involves the activation of diverse cortical-cognitive regulatory processes and thereby the reciprocal deactivation of emotional arousals as the subcortical emotional substrates are downregulated (Liotti et al., 2000). Indeed, Frank et al. (2014) have summarized the field of emotional regulation and provided consistent evidence of prefrontal cortex (PFC) regions becoming activated in service of downregulating negative emotions.
Related to the previous two findings, a third result from this Damasio study was another big surprise. One of the major areas focused on in studies of conditioned fear in animals has been the amygdala (LeDoux, 2012b). However, arousal of the amygdala in human fear experiences was not prominent in the Damasio results. Indeed, the authors noted: “There was no significant activation of the amygdala on either side [of the brain] for any of the emotion/feeling states” (Damasio et al., 2000, p. 1050). While it is clear that the amygdala plays a role in the expression and learning of fear and anger, it is also true that the amygdala is not essential for the experience and expression of fear or of anger, although its participation is more extensive for the learning of specific fear responses (Panksepp, 1998a).
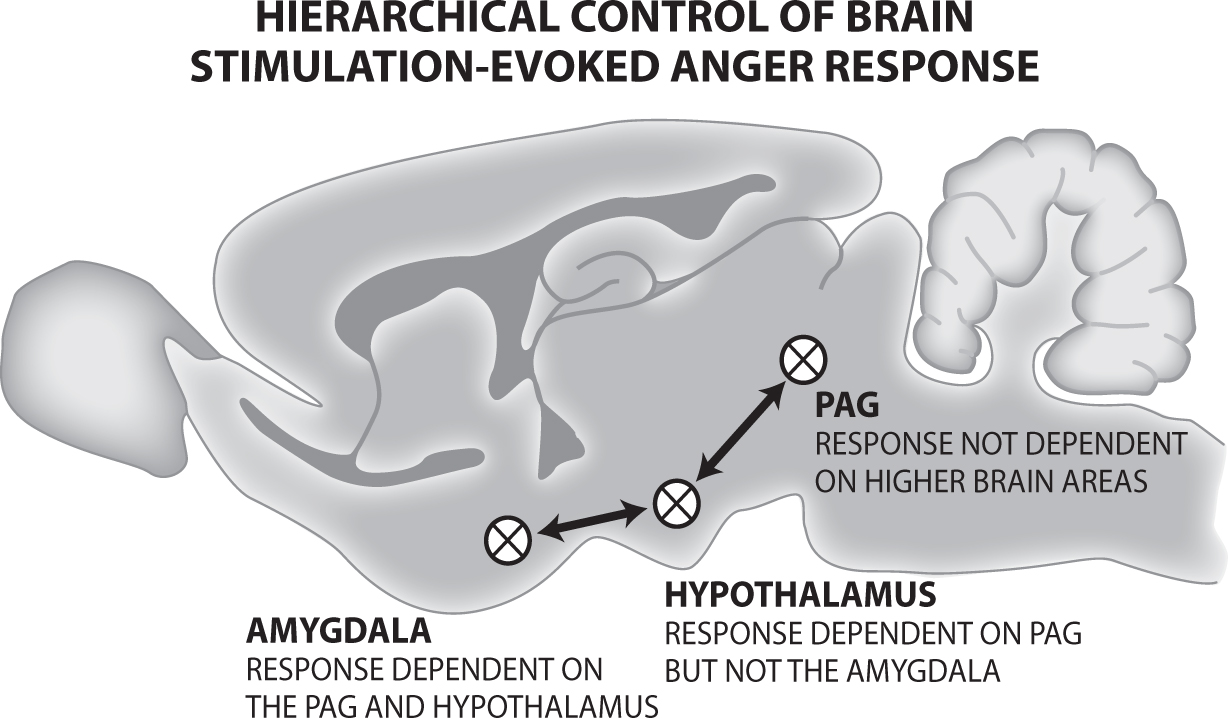
Earlier animal research (preceding brain imaging technology) by A. Fernandez De Molina and Robert W. Hunsperger at the University of Zurich (building on the work of their colleague, the Nobel Laureate Walter Hess introduced in Chapter 7) strongly supports the Damasio group’s third finding. These Swiss brain studies were the first to demonstrate that what we call the basic mammalian RAGE/Anger system runs from the PAG in the midbrain up to the medial hypothalamus and further up to the medial amygdala. De Molina and Hunsperger (1962) showed that the rage responses of cats can be evoked using electric brain stimulation from all three sites. However, the system is hierarchically organized, in levels of progressively increasing importance, such that aggressive responses evoked from the amygdala were abolished by lesions at the hypothalamic or PAG levels, aggressive responses elicited from the hypothalamus were dependent on the PAG but not on the amygdala, and aggression triggered from the PAG was not dependent on either of the other two “higher” brain regions. But, De Molina and Hunsperger were not satisfied by laboratory demonstrations. Cats receiving the small lesion of what they called the “hissing zone” of the PAG, “when confronted with a dog, no longer hissed or attacked” (p. 201). For a hierarchical illustration of this system, see Figure 16.1.
While the amygdala likely integrates psychological learning into the RAGE/Anger system and the hypothalamus blends in physiological influences, the PAG seems to be the primal source of RAGE/Anger responses, with damage to the PAG dramatically reducing rage evoked from the other two regions (Panksepp, 1998a). An additional corroborating demonstration in humans from the Mobbs group will be covered but first a brief summary.
In sum, the Damasio group’s 2000 PET study yielded three remarkable results, which still need to be more fully integrated into psychological and psychiatric thinking about human emotions: the emphasis on subcortical activation, bilateral neocortical deactivations, and the lack of amygdala activation during strong emotional arousal. The Damasio et al. paper, along with the supporting animal findings, also provides compelling evidence for the affective neuroscience interpretation of where raw emotional feelings arise in the brain (Panksepp, 1998a). Indeed, the subcortical regions of the brain, which are homologously shared by all mammals (and many other vertebrates), provide more than just the evolutionary foundations of human emotional experience. In other words, their functional role in the generation of primary human emotional experiences remains as “primary” for humans as it is for other mammals.

Figure 16.1. Hierarchical control of RAGE in the brain. Lesions of higher areas do not diminish responses from lower areas, while damage of lower areas compromises the functions of higher ones.
WHEN FEAR IS NEAR: MOBBS’S VIRTUAL PREDATOR RESEARCH
Dean Mobbs of the University College London and colleagues, took a very evolutionary approach to studying fear that largely confirmed the Damasio group’s work described above, as well as De Molina and Hunsperger’s work emphasizing the emotional importance of the PAG. Mobbs’s group used high-resolution fMRI, rather than PET, to acquire participants’ brain images during a computer-simulated survival challenge called the virtual predator and prey paradigm (Mobbs et al., 2007, p. 1080), or what they sometimes referred to as an active escape-from-pain task.
This task required volunteer subjects to escape from the virtual predator to avoid receiving a painful electric shock intended to simulate the predator’s bite. It involved displaying the positions of the predator and the participant (who was the prey) on a two-dimensional maze grid that appeared on a screen, which the subject could see while in the scanner. In an initial neutral “pre-encounter” phase, the symbol depicting the inactive predator was displayed in the lower left corner of the grid or wandering about the maze but posing no immediate danger, and the symbol for the subject appeared in the upper right corner of the grid. Next, in a “postencounter” cue phase, the threat was detected: the subject saw that the “flashing” predator was active and also learned whether being captured by the predator would result in one shock (for the low level of pain) or three shocks (for the high level of pain). Then, when the predator symbol ceased flashing, the “chase” phase was on, and the participant could now attempt to move his or her symbol to escape the predator. If captured by the predator, the subject received the amount of painful shock previously indicated. Importantly, Mobbs et al.’s (2007) results showed that their subjects were motivated to escape the shocks, especially the higher level.
When the threat was first detected but not yet imminent, their analysis showed enhanced activity in frontal cortical areas such as the medial orbitofrontal cortex (mOFC; just above the eyes—orbit is another term for eye socket), the ventromedial prefrontal cortex (vmPFC) (just above the mOFC), and the anterior cingulate cortex (ACC), an adjacent older cortical area known to be involved in pain processing. During the chase phase, increased activity was observed in subcortical areas such as the PAG. As the threat became more imminent (regardless of whether subjects were caught and received the shock, or managed to escape from the virtual predator and received no shock), PAG and amygdala activity were evident, with the highest PAG activity occurring when subjects were facing the highest imminent shock level. These researchers also asked their subjects to rate the levels of “dread” of being chased and “confidence” of escaping capture that they had experienced. Increased dread and lower confidence were also associated with increased PAG activity, whereas diminished dread and higher confidence of escaping were associated with stronger prefrontal cortical activity.
To summarize, when the threat is more distant, cortical activity is more prominent, and when threat is near with increased dread and decreased confidence of escape, PAG activity is more prominent. “From an evolutionary viewpoint, higher cortical systems control behavior when the degree of threat is appraised as not-life-endangering. . . . At extreme levels of threat, the PAG may in turn inhibit more complex control processes when a fast and indeed obligatory response is required” (Mobbs et al., 2007, p. 1082). So, consistent with cross-species affective neuroscience research, and continuing the theme of this chapter, low levels of fear are accompanied by cortical arousal and corresponding cognitive threat assessment, but as the level of threat mounts, the PAG in combination with other subcortical regions inhibit cortical activity, exert increasing influence over the brain, and provides rapid reactions based on evolutionary affective memories, along with one’s personal higher autobiographical history, to prepare and guide the body for survival.
Mobbs et al. (2009) repeated their simulated predator and human prey experiment and largely replicated their results. In this study, they also measured subjects’ skin conductance levels (a physiological measure of anxiety) during the experiment. After subjects emerged from the scanner, researchers also asked them to rate how much anxiety they felt during the various phases of the simulation, as well as how much panic they felt during the predator encounters. These measures allowed researchers to verify that anxiety onset occurred when an encounter with the predator was signaled and that skin conductance levels, anxiety, and panic were all at their highest levels when actually being chased by the predator in the high-danger condition, when midbrain PAG activity also peaked, rather than in the low danger condition. It is relevant to note that we would prefer to reserve the term panic for use with the PANIC/Sadness brain system rather than an extreme expression of the FEAR system. This clearly highlights the need for a different lexicon for primary-process emotions, which we try to achieve with the convention of full-capitalization of primal emotional terms). In any case, we will continue to use Mobb’s terminology.
In this replication, researchers also measured the number of button-press errors (e.g., accidentally guiding their computer icon into the wall of the maze), which likewise peaked during the high-danger chase and correlated with self-rated panic levels. Indeed, they “found that midbrain [PAG] activity increased with the amount of panic-related locomotor errors,” which was consistent with “chemical stimulation of the rodent dorsolateral PAG eliciting uncoordinated panic-like behaviors” (Mobbs et al., 2009, p. 12,241). (Beside FEAR, the separation-distress PANIC response is also well represented in the PAG.) Further, the increase of “all thumbs” uncoordinated fine motor responding that Mobbs and colleagues observed is consistent with the idea that the frontal cortex motor planning functions are inhibited (or disrupted) during high levels of threat that require faster, more ancient, evolutionarily conserved escape responses, while on the other end of the affective seesaw, the prefrontal cortex can exert regulatory inhibition on subcortical regions when lower threat levels allow for more carefully planned and cognitively coordinated escape strategies.
BRIDGING THE HUMAN AND ANIMAL PAG RESEARCH
Studies such as those by Mobbs and colleagues have encouraged other researchers to use fMRI procedures to focus more on the role of the subcortical PAG in the experience of emotions. Indeed, Buhle et al. (2013) took on the challenge of investigating whether they could replicate animal research and elicit comparable PAG activation in human subjects using negative emotional responses to pain or negative emotional responses while viewing aversive photographs (taken from the International Affective Picture System of Lang, Bradley, & Cuthbert, 2008).
All participants were subjected to high and low thermal pain as well as aversive or neutral cognitive images, and then were asked to rate how negatively they felt about the stimulus. The participants consistently reported that they experienced greater negative affect from high heat than from low heat, and from the aversive images than from the neutral images. Furthermore, the ratings for the painful heat and the aversive images did not significantly differ. Importantly, Buhle et al. noted that “Whole brain contrasts of both high vs. low pain and negative vs. neutral image viewing revealed activity in the PAG” and that “the activity did not reliably differ between the conditions [pain or aversive image]” (2013, p. 611).
As an additional check on their results, Buhle et al. (2013) identified eight independent human studies, four examining responses to high or low pain and four studying responses to negative versus neutral images. In each of the eight independent data sets, whole brain analyses identified activity in the PAG. They concluded that, combined with their own results, “these results support the hypothesis that PAG plays an important role in human negative affect, in line with previous evidence from research in animals” (p. 612). In sum, beneath our “crowning glory” of neocortex, we are like other mammals below our “thinking caps.” In this context, it is important to recognize that practically all mammalian cortical functions (e.g., including vision) are probably learned (Sur & Rubinstein, 2005) rather than tightly programmed by brain evolution.
SHARPER FOCUS ON THE HUMAN PAG
Apart from examining the PAG as a whole, animal research has shown that there are distinct subregions of the PAG involving specific affective processes (Bandler & Shipley, 1994). Indeed, Sapute et al. (2013) set out to determine whether PAG subregions could be identified in humans, thus further supporting the homologies between human and animal emotions. Yet, the PAG is difficult to accurately isolate, let alone subdivide, using typical brain scanning procedures. This is in part due to the small size of the PAG—about 10 mm long (three-eighths of an inch), with a diameter of about 6 mm (less than a quarter inch) in humans. Further adding to difficulties imaging the PAG, its structure is shaped like a sleeve or hollow cylinder surrounding the cerebral aqueduct, such that the inner half of the PAG’s diameter is the cerebral aqueduct, a part of the brain that, as its name implies, ensures the flow of cerebrospinal fluid, mainly downward, from the rest of the brain. During fMRI scanning, strong signals from the fluid in the cerebral aqueduct can interfere with and mask signals from the PAG. Sapute et al. (2013) addressed these problems by using an fMRI procedure incorporating an exceptionally strong 7-tesla magnet, which could provide higher scanning resolution—down to 0.75 mm—than typical fMRI equipment.
Sapute’s group elicited emotions in their eleven scanning participants by showing them either neutral or highly aversive images that were related to threat, harm, and loss (again, taken from the International Affective Picture System). After having their brains scanned while viewing a set of images, participants were asked to report their emotional response to the images with five separate emotional labels: “Activated” (for arousal), “Angry,” “Disgusted,” “Sad,” and “Scared” (for fear). The emotional labels were always presented in random order and were rated on a five-point, low-to-high scale.
An initial analysis showed that overall activity in the PAG was greater when subjects were viewing highly aversive compared to neutral images. However, an exploratory factor analysis of the high-resolution scanning results from their 7-tesla fMRI, along with subjects’ self-rated emotional experiences when viewing the aversive versus neutral images, yielded three factors representing three different PAG subregions, with each of the subregions corresponding with a different emotional experience: They reported not only having “observed definitive activation in the human PAG” but also that “segmenting the PAG into both radial and longitudinal subregions illustrated that activity during negative affect was not diffuse but was concentrated along a spiral pattern from ventrolateral caudal PAG to lateral and dorsomedial rostral PAG. This [spiral-like] pattern mirrors functional and structural observations in nonhuman animals” (Sapute et al., 2013, p. 17,104). Further, spiraling around the central aqueduct from caudal to rostral (tail to head), the three PAG subregions generally corresponded to (1) disgust, arousal, and fear; (2) anger; and (3) sadness.
In short, Sapute and colleagues provided robust evidence in support of evolutionarily conserved mammalian brain homologies—from mice to men, so to speak; they had demonstrated that ultra-high-resolution fMRI procedures could be used to explore the functional architecture of the PAG, an approach that could perhaps be extended to other midbrain regions, which have so far been largely ignored in brain imaging studies of human emotion. Still, it is gratifying to see such clear functional continuities across all mammalian species that have been studied. It reinforces the conclusion that we all share a variety of evolutionarily conserved basic emotions.
REVIEWS OF NEUROTICISM AND HUMAN BRAIN IMAGING: META-ANALYSIS
Meta-analyses allow researchers to statistically combine the data from many published scientific studies and obtain a collective result that may be more valid than any of the individual studies alone. The underlying idea is that, while a single study might not report valid results because of various procedural problems or sampling errors, when the data of multiple studies are pooled something closer to a true picture is likely to emerge.
One such meta-analysis reported by Servaas et al. (2013) identified eighteen studies published from 2001 to 2011 that provided fMRI brain imaging data and self-report measures of neuroticism (also sometimes called negative emotionality or low emotional stability) using psychologically healthy subjects as participants. Their concern was that individual studies attempting to use neuroimaging to identify neurobiological correlates of neuroticism had yielded inconsistent findings. They hoped that merging these generally similar studies into a single meta-analysis would reveal more consistent data patterns and thus better validate the different roles of various brain regions than the individual studies.
Remarkably, none of the brain regions the Servaas group identified as being positively correlated with neuroticism were subcortical. Indeed, there was no mention of periaqueductal gray (PAG) activity. In search for the clearest associations with neuroticism, they identified three general brain regions that positively correlated with self-report measures of neuroticism. The first was the left parahippocampal gyrus, along with closely adjacent areas, which were primarily associated with fear-conditioning studies. The second and third areas were the left superior frontal gyrus and the dorsal and ventral regions of the right middle cingulate gyrus. These latter two areas were associated with general emotional processing, such as viewing negative emotional facial expressions, categorizing emotional words, and choice tasks resulting in the relative loss or gain of small amounts of money ($4 or less). Why the disjunction with studies monitoring immediate emotional feelings?
We would suggest that personality is ultimately an acquired result of how past primary emotional arousals have helped construct diverse brain action systems that mediate emotional activity. In the Servaas meta-analysis, each of the three general personality-relevant areas had previously been associated with cognitive emotional processing. The parahippocampal gyrus had been shown to interact with the amygdala during the encoding of negative film clips (Kilpatrick & Cahill, 2004) and in the recall of negative words (Thomaes et al., 2009). The superior frontal gyrus had been shown to be involved with maintaining human self-awareness (Goldberg, Harel, & Malach, 2006) and had been associated with general cognitive control and perhaps especially modulating the current emotional state (Frank et al., 2014). The dorsal and ventral regions of the cingulate gyrus seemed to be important in regulating the balance between external and internal attentional factors (Leech & Sharp, 2014).
Another reason that Servaas et al. (2013) did not identify any subcortical associations with neuroticism is possibly that the NEO PI-R Neuroticism scale was used to measure neuroticism in twelve of the studies they reviewed, a scale that mainly deals with a tertiary cortical cognitive appraisal of negative emotion. Items like “I have fewer fears than most people” and “Frightening thoughts sometimes come into my head” may require more cognitive reflection than a direct assessment of how the self-rater feels at the moment. Also, items like “I feel I am capable of coping with most of my problems” and “I can handle myself pretty well in a crisis” not only entail cognitive reflection but also are very general and may not tap into the patterns of specific primary emotional tendencies that have guided personality development.
A review by Montag et al. (2013) has suggested that a brain-based personality assessment such as the Affective Neuroscience Personality Scales (ANPS) might be better suited for parsing emotion-related brain regions. The ANPS was designed to address the primary-process negative emotions, namely, RAGE/Anger, FEAR, and PANIC/Sadness, and uses items that more directly tap into a self-rater’s affective experience rather than relying on more general affective judgments. It is no coincidence that the ANPS negative emotionality scales target the three primary emotions Sapute et al. (2013) linked to specific regions of the periaqueductal gray using fMRI imaging: fear, anger, and sadness.
Yet another possibility is that the tasks used in the eighteen studies reviewed by Servaas et al. (2013), such as viewing negative emotional facial expressions and categorizing emotional words, are more cognitively oriented and may not strongly engage strong emotional feelings. Those tasks may be more similar to the early stage of the predator task used in the fMRI study by Mobbs et al. (2009), when there was no imminent danger and the participant had not yet encountered the predator.
In any event, the overall meta-analytic results by Servaas et al. (2013) seem more reflective of a cognitive neuroscience approach to emotions, which is inclined to look for sources of emotional brain activity in the human cortex than in subcortical regions such as the PAG originally identified in animal research, which is only recently becoming accepted as a key region for the experience of human emotion. Thus, we need to see human personality in part as an “emergent process” of how basic emotional arousals have guided the life trajectories of individual human beings. Personality may reflect how the ancient primal affective tools for living guide how one has learned to be a specific type of person in a specific environment.
EXTENDING FINDINGS WITH MORE META-ANALYSIS
Another meta-analysis by Adina Mincic (2015) took advantage of a very active new research field, examining fifty-seven studies relating negative emotionality to brain activity and brain structure sizes, with most studies reporting cortical gray matter differences. A predominant finding was that higher negative emotionality correlated with reduced gray matter volume in the left orbitofrontal cortex (OFC)—sometimes included in the ventromedial prefrontal cortex—a region positioned just above and behind the eye sockets in humans.
Another prominent finding was greater gray matter volume in the left amygdala for participants with higher negative emotionality. Mincic (2015) also found evidence for increased volume in the hippocampus and the parahippocampal gyrus associated with higher negative emotionality scores. She only found a few studies focusing on the cingulate cortex—an evolutionarily older cortical region lying immediately above the corpus callosum—but focused on the anterior cingulate cortex ACC rather than the posterior cingulate cortex, with the reduced OFC volume extending into the rostral (very front portion) of ACC, which lies adjacent to and immediately behind the OFC. In addition, Mincic (2015) found neuroanatomical evidence for decreased volume in the left uncinate fasciculus, a Latin name for a nerve tract connecting frontal areas such as the OFC to the amygdala/hippocampal regions. All of this “supports the idea that the diminished grey matter in OFC/ACC and white matter integrity of the uncinate fasciculus may represent a structural phenotype of the NE [Negative Emotionality]-related personality traits” (p. 110). Such a conclusion highlights the top-down regulation of emotional arousal in maintaining emotional balance and well-being. However, these studies are still missing the critical midbrain emotional foundational areas, which are so apparent in the work of Damasio, Mobbs, and Sapute, as already highlighted.
Another relevant concern is the heterogeneity of results in the literature covered by these two meta-analyses. For starters, the only result the two had in common was a positive correlation in the parahippocampal region with negative emotionality. Mincic (2015) pointed out that Servaas et al. (2013) had found increased activity in the parahippocampal gyrus during negative emotional processing but, surprisingly, not in the amygdala. However, regarding Mincic’s most consistent finding of reduced OFC volume associated with higher negative emotionality, she pointed out that of thirty studies investigating this particular relationship, twelve did not find this association, with two actually reporting opposite results. Similar disparities were evident for each of her conclusions.
Why is there such inconsistency in the brain imaging literature? Why did these two meta-analyses report such inconsistent results, in stark contrast to the animal studies? There are likely many reasons, including variations in the size and anatomy of human brains, the frequent use of low-resolution scanners, difficulty in the use of fMRI to identify activity in midbrain regions such as the PAG where neurons fire quite slowly relative to higher brain regions, and the fact that many studies do not even target midbrain regions. However, we may need to probe deeper into the reason for these disparities: The midbrain regions and structures like the hypothalamus have strongly inherited genetic/anatomical foundations, while the cortical regions are not as tightly genetically programmed but, rather, acquire their functions much more through individual learning and development, providing another source of cortical anatomical heterogeneity.
A theme throughout this book has been that the neocortex is not essential for the experience of emotions. The neocortex has an amazing capacity to re-represent and refine input from the more genetically defined parts of the nervous system, such as the subcortical emotional and motivational networks that guide diverse affective survival responses and to allow the rest of the brain to integrate those “survival values” with our various sensory-perceptual organs that provide information primarily about our external (rather than internal) worlds. Because cortical capacities are acquired—developmentally programmed, if you will—by our basic affective (sensory, homeostatic, and emotional) direct survival systems, there are bound to be more individual differences in cortical brain regions than the more ancient intrinsic subcortical functions. This helps explain the dramatic cortical flexibility demonstrated in Sur’s animal brain research showing how temporal cortex can assume a visual function rather than its more typical auditory function (Sur & Rubenstein, 2005), as well as the dramatic cortical plasticity being demonstrated in subjects who recover from relatively minor cortical brain damage (Nudo, 2013).
Moreover, there is also convincing evidence for the recruitment of visual cortex for somatosensory processing—exercising well-developed tactile skills for reading braille or identifying objects by finger tracing—in individuals who have been blind from an early age (Cohen et al., 1997). Indeed, Sadato et al. (1998) further compared PET imaging of blind and sighted subjects and found imaging results during braille and nonbraille tasks, suggesting that tactile input usually processed in the somatosensory cortex (closer to the top of the skull) of sighted individuals was rerouted in subjects blinded early in life to occipital cortical regions that typically specialize for visual processing. Further, Weeks et al. (2000) used PET to investigate which cortical areas were activated in blind versus sighted subjects when performing auditory localization tasks. Indeed, congruent with the aforementioned work, these investigators observed auditory to visual plasticity: while sighted and blind participants both showed activated posterior parietal cortex, blind participants also showed activated right occipital cortex, a region typically identified with vision. Thus, the superior touch and hearing skills of blind individuals may be due to occipital regions (normally involved in the visual processing of sighted individuals) being “programmed” to participate in the processing of tactile and auditory information, thus providing additional cortical resources enabling expanded tactile and auditory abilities.
Perhaps one day new brain analysis tools will provide a means to show how much of the variability in cortical brain function researchers are currently observing is due to the variability in how neocortical regions are differentially “programmed” in individuals beginning life with distinct genetically promoted endophenotypes and then growing up and maturing under the influence of different environmental circumstances. One might anticipate a clearer appreciation of developmental plasticity in the cortical processing and regulation of emotions.
FIRST-ORDER EMERGENCE OF EMOTIONS: THE DAMASIO GROUP
It is fitting to close this chapter using another contribution from the Damasio group (Damasio, Damasio, & Tranel, 2013), which like the Panksepp group has challenged “the traditional view that mental states are subserved mainly or exclusively by the cerebral cortex” (p. 833). Many have proposed cortical structures as the basis for emotional experience, but A. D. “Bud” Craig (2009, 2011) has asserted that the insula, an interior region of cortex inside the cortical temporal lobe, holds the keys to the kingdom, so to speak, and is the primary neural platform for feelings of emotion.
In response to this argument, Damasio et al. (2013) reviewed subcortical evidence for the first-order emergence of emotions, but more important for this chapter, they put the insula hypothesis to the test by publishing brain images and corresponding emotional data from a person that had sustained extensive brain damage, which included bilateral insula destruction. In their 2013 paper, Damasio’s group described patient B, who as a result of herpes simplex encephalitis had lost not only his insula but also his amygdala, hippocampus, and other brain cortices, including much of his orbitofrontal cortex, the temporal pole, the parahippocampal cortex, and the anterior cingulate cortex. Importantly, there was no insular cortex remaining in either hemisphere of his brain, which was clearly evident in the brain images presented by Damasio et al. (2013). They further stated that, perhaps surprising to some, “unwarned strangers interacting with him for the first time had no inkling that he had major neurological damage, the fact only becoming apparent once his dense amnesia was exposed. To put it plainly, patient-B was a whole human being suffering from a very poor episodic memory” (p. 834). Patient B’s spouse completed a structured questionnaire comparing his behavior before and after his disease, with twenty-five of the questions dealing explicitly with emotions. On eighteen of the twenty-five items, his spouse rated him the same before and after he lost significant portions of his cerebral cortex. On three items she rated him up a point and on four down a point. In addition, patient B frequently and routinely expressed his pleasure or displeasure with life events, including lab procedures such as taking tape off his arm that pulled on his hairs. In short, the questionnaire data, the observations of strangers, and the observations of the research team, including psychological evaluations, all indicated patient B retained a full range of appropriate emotions after his brain disease.
The data from patient B describing his full emotional life makes it difficult to support the idea that the insula is necessary for subjective affective experiences. Indeed, this case provides compelling evidence that structures such as the amygdala, the hippocampus, the parahippocampal gyrus, the temporal pole, the orbitofrontal cortex, and the anterior cingulate cortex are not essential for the expression and experience of emotion. Thus, we agree with Damasio’s group and argue that the structures essential for the generation of emotions are largely subcortical brain regions, especially the PAG and hypothalamus. Clearly, cortical regions play a big role in the full sophistication of human emotional life, but that role is largely a secondary-level one that is acquired—“programmed”—through learning resulting from life experiences, with perhaps early life experiences being especially influential. It isn’t that cortical areas do not play an important emotional role—especially with humans. Many regions of cortex elaborate emotional learning. However, that function is similar to the one provided for all our sensory and motor modalities: Learning provides nuanced refinements that not only enrich and complexify our affective experiences but also offer the potential for diverse learned regulations and elaborations of our emotions—both augmenting and inhibiting (Frank et al., 2014). Without our subcortical affective systems we lose cognitive consciousness. Without our neocortices, at least if lost near birth, affective consciousness survives, without the developmentally added cognitive complexities such as the capacity for spoken language that patient B was fortunately spared.
As neuroscientists become more sophisticated in the use of brain imaging and as new techniques emerge, such as diffusion tensor imaging, which has enabled Coenen et al. (2012) to better understand subcortical deep brain stimulation options for the treatment of psychiatric disorders (Coenen et al., 2012; Panksepp et al., 2014; Panksepp, 2016), we will better understand the origins of subcortical affective imbalances and the role cortical regions contribute to both affective equilibrium and disequilibrium (Panksepp, 2015, 2016).
An important theme running throughout this book is the necessity of first understanding our subcortical primary-process emotional action-affect systems before we can hope to illuminate the subtleties of the human mind and the heights and depths it can traverse through learning and culture. At the core of our mental lives there are profound positive and negative affects, which find their origins in ancient regions of our brain that we share homologously with all the other mammals. The massive “mushrooming” of our neocortex expands our subcortical capacities and allows us complex thoughts and unique creative endeavors of our own making. With the addition of abundant neocortex instantiated and energized by our lower mind, our upper mind can reach creatively, indeed uniquely, into the depths of each human life, as it attempts to optimize its present moment as well as imagined mindscape trajectories into the future.