
Professor Colin Groves and me studying H. floresiensis at ARKENAS, Jakarta (2011). Image provided by Matt Tocheri.
It’s 2006 and I’m in a taxi, heading to Sydney airport to fly to Jakarta. I’m about to do something incredible: study the actual bones of H. floresiensis. This wonderful opportunity had come courtesy of Mike Morwood. He’d heard about the work that Colin Groves, Denise Donlon, Richard Wright and I produced that supported the theory of H. floresiensis as a new species, and had invited all of us to Jakarta. But due to other commitments, only I ended up flying out with Mike on a mission to meet H. floresiensis. I was beyond excited. This was a chance to understand more about just where the new species fitted into the family tree. I would be studying the bones in detail and working out which of the current theories about H. floresiensis’ origin might fit the evidence.
I had never been to Indonesia before, so I was glad Mike would be flying with me—his inside knowledge and his contacts would make life much smoother. But first I needed to get on the plane. Worryingly, there was no Mike at the check-in. Or at the boarding gate. I suddenly realised that I was not really prepared for this, and panic started to rise. But then that familiar akubra appeared above a sea of passengers: Mike running in, just as the gate was closing, something about a passport. And just like that, we were on our way to meet H. floresiensis!
The H. floresiensis fossils were being kept at ARKENAS. Mike and I met with the Director, Dr Tony Djubiantono, and after a pleasant chat with him in his spacious office, we were taken into the large study room. Dominating the room was the safe where the bones were kept. Rokus Awe Due, who’d been the first to recognise that the small skull of H. floresiensis was an adult of an unknown species, opened the safe to reveal the boxes of bones. Then I did what any professional, sensible, cool and relaxed researcher does on such occasions: a jig! Mike laughed as Rokus, unaware of this little display, turned from the safe and reverently placed the boxes on a desk.
I remember seeing the skull and two jaws of H. floresiensis and almost not being able to breathe. My notes captured something of what I felt: ‘I am in awe of the skull. I am so happy working on it … It has such a striking mix of characteristics—Australopithecine, habilis, modern.’
Before I departed for Jakarta, Colin Groves and I had compiled a list of characteristics we wanted to know more about. Now, carefully examining the skull and jaws, I located these characteristics and made notes on them. Over subsequent days, I recorded other characteristics and drew sketches of these. Taking a whole raft of measurements completed that part of my study; all of this information would be invaluable for my PhD study.
My ultimate objective was to work out where H. floresiensis fits on the human evolutionary, or phylogenetic, tree. A tree in this sense is a branching diagram used to portray relationships among species. Its structure is derived from the fact that species shared common ancestors at various points in time, with its form based on shared similarities—in our case, the fossils of hominins. On any tree, the most closely related species share the largest number of unique traits: that is, characteristics not shared with others on the tree. Such groupings are interpreted as having shared an immediate common ancestor.1 They are therefore more closely related to each other than they are to any other species in the analysis. In some ways, it is like anyone’s family tree, except of course we are dealing with species rather than mums, dads, grandparents, second cousins twice removed …
The process that sorts species into a phylogenetic tree is called cladistics. It’s as challenging and formidable as it sounds, even though we use a software program to perform the analyses. I recall learning about cladistics in the second year of my human evolutionary course. I found it really complex and at the end of that year I thought, ‘Well, I’ll never have to think about that again.’ Wrong. As I became more deeply involved in the study of human evolution, I began to see how useful the process was. Professor Colin Groves was an early adopter of the cladistics method and an expert in its use. Cladistics was a key technique I used in my PhD study.
Constructing the tree is a complex task. In the past it was laboriously done by hand and could take years to resolve. These days there are sophisticated programs that sort the species into relationships based on the data you provide. The tree is not considered ‘the answer’, though. Tests must be done to ensure the outcome is well supported. It is only then that a hypothesis of species relationships—a scientifically based idea or model—can be presented. Other researchers can test this model. And they usually do. That is how science progresses.
As I detailed in the previous chapter, we knew that H. floresiensis was not a modern human. So Colin Groves and I were particularly interested in examining the other main theories for H. floresiensis. One was that it evolved from a very early species in our genus,2 like the ones that lived around 2–2.3 million years ago in Africa. This was potentially an explosive theory because it challenged the strongly held idea that it was the large-bodied, larger-brained H. erectus, rather than a smaller-brained earlier species, that spread from Africa to Java. This concept is so embedded as a model of human evolution that it has a name: Out of Africa 1.3 The alternative idea was that H. floresiensis could have evolved from an unknown population of H. erectus that had arrived on Flores and become isolated from its mainland group. Without genetic exchange with that mainland group, H. erectus could have dwarfed into a new species. Peter Brown and colleagues had proposed these two ideas when they’d announced H. floresiensis back in 2004.4
As astonishing as the island-dwarfing idea seems, it did not come out of the blue. Dwarfing is a well-known evolutionary change that happens to some mammals that become isolated on islands. This is called the ‘island rule’. Within a year of Peter Brown and colleagues’ announcement of the new species, though, the H. floresiensis team had seriously rethought the dwarfing hypothesis, rejecting it when the discovery of more parts of the species showed just how archaic was H. floresiensis.5 The primitive form of the H. floresiensis jaws also convinced Mike Morwood and colleagues that something was going on in the evolution of H. floresiensis that had nothing to do with H. erectus.
For record-keeping purposes and later reference, I took photos of the skull and jaws from all angles, and also of the particular characteristics of note. This was for Colin’s and my use, so it did not matter to me that the images also included half my implements and my notes, or the background office furniture. Mike was thinking strategically, though. Picking up a black velvet cloth, he kindly taught me how to use this as a background to enable professional-looking photos, ones that he said would be good for publication. He had been dropping the words ‘publication’ and ‘runs on the board’ into our conversations for quite a while, and sure enough, at the end of my sojourn in Jakarta, Mike asked me if I would write a paper for an edition of the Journal of Human Evolution that would be dedicated to H. floresiensis.
Once I was back in Australia, and with Mike’s offer to publish in the Journal of Human Evolution foremost in my mind, I got straight to work. I prepared my data for a cladistics analysis; loaded it into the software program; ran my analyses; and tested, then re-tested, the results. The resultant evolutionary tree showed H. floresiensis and the earliest species in our genus, H. habilis and Homo rudolfensis, on the same branch. In a joint publication, Mike, Thomas Sutikna, Jatmiko, Wahyu Saptomo and myself proposed that H. floresiensis descended from an early but unknown species of Homo. H. erectus was on a different part of the tree: its closest relative was H. ergaster. We tested to see if H. floresiensis could possibly fit on the branch with H. erectus, but it could not. We therefore rejected the hypothesis that the small, enigmatic bones resulted from island dwarfing of H. erectus.6
After we’d published our results, I received an email from Professor Bill Jungers, who is an expert on the postcranial bones (those below the neck) of chimps and other non-human primates, the australopithecines, early Homo, modern humans and H. floresiensis. More and more information was emerging about the postcranial bones of H. floresiensis, so Bill suggested he, Colin and I do a joint analysis that covered the characteristics of the arms, legs and shoulders of various hominids. As it happened, Colin and I were already discussing this idea, so we jumped at Bill’s suggestion.

Professor Colin Groves and me studying H. floresiensis at ARKENAS, Jakarta (2011). Image provided by Matt Tocheri.
In August 2011, having acquired generous funding from the ARC, and after nearly a year of planning and organising research permits, Colin and I headed off to visit the institutions that held the fossils we wanted to study. We travelled to South Africa, Kenya, Ethiopia, Tanzania, the Netherlands, Germany and Indonesia; Bill joined us in Jakarta to study H. floresiensis postcranial bones and provide insights into the postcranial characteristics of other species. Along the way, we collected extensive data on the characteristics of each fossil we examined, no matter how fragmentary—we measured and photographed everything.
To see and handle real, actual fossils, well, there’s nothing like it for seeing nuances of form. Surprises can happen at any time. When we were studying the fossils of A. afarensis at the National Museum of Ethiopia, for example, we saw that the skulls had a fissure running along the length of the ear tube. Sure, it’s not the most dazzling characteristic, but we had been looking specifically for this fissure on all the fossil skulls we studied because H. floresiensis had it. When it showed up on the A. afarensis skulls, we knew we had discovered something new: yet another A. afarensis feature that H. floresiensis possessed.7
By late 2013, after a few false starts, we had submitted our paper about the place of H. floresiensis on the human evolutionary tree to the Journal of Human Evolution. It had taken around eighteen months to perform all the analyses and write up the results. The journal’s editors liked the way the paper was written, even asking us to do more work.
Enter Mike Lee. During 2013 I had accepted an invitation from Professor Gavin Prideaux (Flinders University) to give a talk on H. floresiensis at the Biennial Conference on Australasian Vertebrate Evolution, Palaeontology and Systematics (CAVEPS) in Adelaide in October 2013. I saw that there was something extra on offer at this conference. Being only too keen to hone my analytical skills, I signed up to a pre-conference workshop on how to use the Phylogenetic Analysis Using Parsimony (PAUP) software program for reconstructing evolutionary trees—Colin and I had been using PAUP to perform our analyses. Professor Mike Lee, whom I introduced in the previous chapter, and his workshop co-host, Associate Professor Matthew Phillips (Queensland University of Technology), specialise in these complex computer programs that work out where any given species sits on the evolutionary tree of life. Mike Lee is also an expert on reptiles and their evolution. Partway through the workshop, I had a revelation: the newer version of PAUP had far more potential than the one Colin and I had been using, so why not invite Mike to join our team. Mike ultimately took the lead in performing our analyses, and he was a co-author of the team’s paper on where H. floresiensis lies on the human tree.8
When Mike analysed the fruits of the fossil-studying trips that Colin and I had taken, two equally supported trees emerged. One of them showed H. floresiensis and H. habilis together on a branch separate from the other species in the analysis. The technical term for this arrangement is a clade. The species that form clades are often referred to as sister species, which are interpreted as sharing a common ancestor that is not shared with any other species in the analysis. This led us to propose that H. floresiensis and H. habilis shared a unique common ancestor, making the H. floresiensis lineage at least as old as the H. habilis lineage—H. habilis is known from 2.35 to 1.65 million years ago.
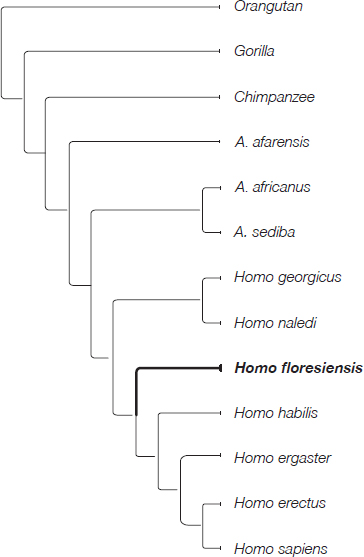
H. floresiensis at the base of the group comprising H. habilis, H. erectus, H. ergaster and H. sapiens.
The other tree showed a slightly different arrangement of species, with H. floresiensis on a separate branch of the tree. Its position suggested to us that the H. floresiensis lineage could be even older than the H. habilis lineage. Both the phylogenetic positions implied an Early Pleistocene appearance for the H. floresiensis lineage.
In both cases, H. floresiensis was quite separate from H. erectus. In no case did they form sister species, as would be expected had H. floresiensis evolved from H. erectus. In one analysis, H. erectus was a sister species to H. ergaster; in the other tree, it was a sister species to H. ergaster and H. sapiens. We did a number of tests for the scenario that H. floresiensis evolved from H. erectus, but they all verified our trees and showed us that H. erectus was unlikely to have given rise to H. floresiensis.
These results strongly suggested that a very early but unknown species of Homo, or H. floresiensis itself (unknown in Africa as yet), left Africa and that its descendants made it all the way to Flores. This in turn supported one of the original ideas presented by Peter Brown and colleagues.9
It’s an extraordinary idea: that something more archaic than H. erectus got out of Africa and made its way to Flores, where it held on for more than a million years after its closest relatives died out. It certainly challenges the Out of Africa 1 model of human evolution.
Ours wasn’t the only work going on in this space. Another team used a technique called Bayesian statistics. In essence, the Bayesian approach is a measure of probability of the strength of evidence in favour of one model over another.10 Using an extensive suite of characteristics of the skulls, jaws and teeth of twenty hominin species, the team led by Mana Dembo (Simon Fraser University, British Columbia) found that their best-supported tree was that in which H. floresiensis was on a branch leading to H. habilis and H. rudolfensis. This suggested that H. floresiensis was a descendant of pre-H. erectus small-bodied hominins, just as our team had found. The finding was pretty encouraging.
But then another team obtained a different result. Valéry Zeitoun (Université Paris-6, Sorbonne universités) and colleagues, using data from the calvarium—the part of the skull that encloses the brain—found that LB1 fitted on a branch of the evolutionary tree with H. erectus and H. ergaster.11 The team were quick to acknowledge that support for this outcome was weak.12 Nevertheless, they proposed that LB1 (the partial skeleton of H. floresiensis) was H. erectus, rather than a new species. How it became so small compared to other H. erectus individuals, they added, remained unanswered.13
Zeitoun and colleagues did not include information about the face, jaws, teeth or other body parts in their analyses, claiming that the australopithecine and early Homo characteristics in H. floresiensis’ jaw, hand, foot and shoulders merely showed that the partial skeleton was not a modern human.14 Other researchers, however, regarded the australopithecine and early Homo characteristics as very important evidence to consider when assessing the place of H. floresiensis on the human evolutionary tree.
So where did all that study leave us? Two cladistics analyses found that H. floresiensis likely stemmed from a very early hominin in our genus Homo, and a third proposed that the H. floresiensis individual was a small H. erectus and rejected H. floresiensis as a new species.
Beyond cladistics, we could get a sense of what H. floresiensis might be by comparing its skull shape with those of other hominin species. Essentially, we want to see if the H. floresiensis skull is similar to the skulls of any other species. We can then make a call as to which species it might be most closely related.
One of the ways to objectively assess skull shape similarities and differences is through measurements. About half of Colin’s and my time when studying A. afarensis, A. africanus and skulls of Homo, was spent taking copious measurements for just this purpose. You measure each skull of interest from specific landmark to specific landmark—landmarks, in this sense, are places on the skull (or jaw, tooth, leg bones and so on) that are anatomically recognisable places. The length of the skull, for example, is measured from a landmark between the eyebrows, called the glabella, to the area at the back of the skull that most protrudes. There are biological landmarks for many parts of the skull (the top, bottom, sides), for the face (eye orbits, nose opening, ear hole), you name it. Curvatures on the skull can also be captured. Once you’ve taken these measurements, you (patiently) load this data into a software program that will perform the required analyses. You can then find out which skulls are similar in shape and which ones differ significantly.
This is the kind of analysis I did for the ASHB conference I talked about in chapter 2, the one at which H. floresiensis was so hotly debated. Our team went on to do a comprehensive comparison of H. floresiensis with the fossils of Homo (including modern human microcephalics), Australopithecus and Paranthropus. I reported the results in the previous chapter, so I won’t repeat the details here, except to say that we found the H. floresiensis skull was more like H. habilis and a 1.8-million-year-old skull from the Koobi Fora research area in Kenya, called KNM-ER 3733, than it was to H. erectus.15 It is uncertain which species KNM-ER 3733 belongs to, although some researchers include it in H. ergaster.
A number of other research teams have also compared the shape of the H. floresiensis skull with other hominin skulls. Using a variety of analyses, Karen Baab and colleagues established that the shape of H. floresiensis’ skull was what would be expected for a very small specimen of early Homo.16 They found, as we did, that in some analyses H. floresiensis’ skull clustered with KNM-ER 3733. Adam Gordon (George Washington University, Washington, DC) and colleagues found that H. floresiensis was most similar to one of the skulls of H. georgicus,17 to KNM-ER 3733, and to a lesser extent to two H. habilis skulls. Because there was little similarity between H. floresiensis and H. erectus (Sangiran 17 from Java), Gordon and colleagues proposed that the ancestry of H. floresiensis did not include H. erectus.18
In contrast to these outcomes, a study by George Lyras (National and Kapodistrian University of Athens) and colleagues found that the skull shape of H. floresiensis was most similar to H. erectus and therefore suggested a close relationship between the two. Lyras and colleagues recognised that their results were different from the other skull shape studies, asserting that the disparity was due to the other teams using a dissimilar analytical technique. Unfortunately, they were unable to include some of the skulls of species that the other teams had found were closest in shape to the H. floresiensis skull, explaining that, in the case of H. georgicus,19 the Dmanisi material was unavailable to them.20
The results of the skull shape studies, then, were somewhat inconclusive. Three were in agreement that the H. floresiensis skull was not shaped like H. erectus. They each concluded that H. floresiensis was unlikely to have descended from that species, instead suggesting that we were looking at an early hominin lineage for H. floresiensis. Another study, though, suggested that H. floresiensis probably evolved from H. erectus.
Peter Brown and colleagues gave us good initial descriptions and measurement data regarding H. floresiensis’ skull to work with,21 but we were eagerly awaiting a full description of the skull so that we could learn about all of its characteristics. So it was a most welcome addition to our knowledge when, in 2011, Professor Yousuke Kaifu (National Museum of Nature and Science, Tokyo) and colleagues published a detailed description of H. floresiensis’ skull and face.22 Kaifu, as his friends and colleagues call him, is a human evolutionist, and one of the experts on H. erectus.
Now armed with so much more information than was available previously, Kaifu and colleagues went on to explore whether H. floresiensis’ skull and face could shed light on its ancestry. Assembling data from a comprehensive suite of fifty-four fossils that included H. habilis, H. ergaster, some later African hominins, H. georgicus, and H. erectus from Java and China, they tested whether H. floresiensis might have originated from H. habilis, H. georgicus or H. erectus.
Kaifu and colleagues identified twenty-one characteristics that are not seen in H. habilis or H. georgicus but occur in the H. ergaster/erectus group of skulls. They argued that, as these characteristics were absent from the earlier hominins and present in the later species, H. floresiensis must have inherited them from the later species. This would rule out the earlier species of hominins as ancestors of H. floresiensis. The small size of H. floresiensis could be explained, they said, by an unknown H. erectus or related form that arrived on Flores and dwarfed substantially in body and brain size in an isolated island setting. They left this question open to debate, however, noting that evidence from the skull alone could not solve the question of H. floresiensis.23 Turning to the H. floresiensis teeth, Kaifu and colleagues examined twenty-six characteristics, many of which they noted evolved after H. habilis had evolved. This, too, would argue against an early hominin such as H. habilis being ancestral to H. floresiensis.24
For more clues, let’s consider the jaws. When H. floresiensis was announced, we heard about some primitive traits in its jaws,25 as primitive as we see in the 3.7-million-year-old A. afarensis jaw LH 4.26 More details emerged when Peter Brown and Tomoko Maeda carried out a full study of the Liang Bua jaws.27 The two researchers found that these jaws shared a distinctive set of traits that placed them outside the range of variation seen in H. erectus. The front and sides of the jaws, the buttressing structure inside the jaw (see chapter 2), and the form of the bony part of the jaw that links it to the skull, were similar to Australopithecus and early Homo. All up, when they considered the jaws along with the form of H. floresiensis’ skull, its limb proportions, and its archaic wrists and shoulders, Brown and Maeda saw that H. floresiensis was in many respects closer to African early Homo or Australopithecus than to later Homo, including H. erectus.
This evidence suggested to them that the ancestors of H. floresiensis left Africa before the evolution of H. erectus as defined by H. georgicus. Brown and Maeda believed on the basis of their analyses that H. floresiensis, a distinctive, tool-making, small-brained, australopithecine-like, upright-walking species, arrived on Flores before the arrival of H. erectus and H. sapiens in the region.28
Brown and Maeda went further, however, predicting that H. floresiensis might possibly have left Africa even before the evolution of the genus Homo. This is a radical idea, very similar to one of the scenarios my colleagues and I presented when we suggested that H. floresiensis could have been in the process of evolving from Australopithecus to Homo when it made its way beyond Africa (see chapter 2). Brown and Maeda and our own team, then, independently came to a similar conclusion: that it is entirely possible that a small, archaic hominin left Africa before two million years ago,29 or 1.8 million years ago.30
Moving beyond the jaw, teeth contain a powerhouse of information. Readers will be familiar with how teeth and dental records are used in forensics. They are also one of the most informative components we can use in studying what happened in human evolution.31 Even better, they are very tough and can outlast other bones in the fossil record, so we have many of them.
Brown and Maeda showed that H. floresiensis had a mix of archaic and modern characteristics in its teeth, but one thing in particular stood out. The premolars had a crown shape only seen in apes that lived way back in the Miocene (from twenty-three million to five million years ago),32 and in the early australopithecines. By the time the later australopithecines and early Homo had evolved, this premolar shape had disappeared. The H. floresiensis premolars, then, have a very primitive form indeed, more primitive than H. erectus.33 Compare this to Kaifu and colleagues’ conclusions and we have two diametrically opposed views of the teeth of H. floresiensis.
We now move from the outside of the skull to the inside. Professor Dean Falk and colleagues looked at the issue of H. floresiensis’ ancestry from the perspective of its brain. As we have seen, an imprint of the brain may be preserved on the inside of some hominin skulls. Falk and colleagues produced an endocast of H. floresiensis’ brain by using 3D CT scanning. They then compared this to endocasts from the H. erectus skull from Java, four H. erectus skulls from China,34 and endocasts from an australopithecine, a Paranthropus and some modern humans and chimps. They established that H. floresiensis’ brain could not have been a miniaturised H. erectus brain, yet there were some similarities. So Falk and colleagues considered other physical aspects of H. floresiensis: its australopithecine-like pelvis, brain-tobody-size ratio, and characteristics of its upper leg bone, all of which are not expected in a miniaturised descendant of the larger bodied H. erectus.35 They thought it possible that H. floresiensis might have been an island dwarf form, but equally that both H. erectus and H. floresiensis shared a common ancestor that was an unknown, small-bodied and small-brained hominin.36
It’s now time to raise a principle that is basic to all science. The parsimony principle tells us to choose the simplest scientific explanation that fits the available evidence. That means regarding H. floresiensis that, all other things being equal, the best hypothesis is the one that requires the fewest evolutionary changes.
In two of the three cladistic analyses described above, H. floresiensis is on a branch with H. habilis, or with H. habilis and H. rudolfensis. Tests on those trees show that many more evolutionary changes would have had to take place had H. erectus evolved into H. floresiensis. As well, most of the analyses that compare skull shapes show that the skull of H. floresiensis is similar to that of H. habilis and two 1.5–1.8-million-year-old skulls from Africa, and quite dissimilar to H. erectus.
If H. erectus evolved into H. floresiensis, we have to accept that in doing so, a suite of archaic characteristics appeared, the likes of which had not been seen since the australopithecine and early Homo days. We call such characteristics ‘evolutionary reversals’; that is, a characteristic reverts to a more primitive form over time. I’ll explain what I mean by ‘evolutionary reversals’ by exploring some that are relevant to the H. floresiensis/erectus question.
Clearly, a massive shrinking of body and brain would have to take place.37 Changes in specific aspects of the jaw and premolars, and, I contend, the feet and pelvis, are other examples. The H. floresiensis jaws are australopithecinelike in structure, something not seen in H. erectus, H. ergaster or H. georgicus jaws, all of which are heading towards the modern form. Why such a reversal would be selected for as H. erectus (putatively) evolved into H. floresiensis, even under the conditions found on an island, I do not know. Likewise, H. floresiensis’ lower premolars are exceedingly and consistently primitive, similar to those of the australopithecines and early Homo.38
H. floresiensis has ape-like foot proportions, similar to the australopithecines: they lack arches on their feet, and they have a long forefoot and some long toes as well as an odd-shaped ankle bone.39 We do not have foot bones for H. erectus or H. ergaster but we have something else: footprints. Around 1.5 million years ago, some hominins walked across a silty, sandy area near Ileret in northern Kenya. The footprints are like our own: we can see that the big toe is aligned with the foot, the heel is rounded and the sole is arched. These footprints are our earliest evidence for essentially modern feet in the human fossil record. They show that by 1.5 million years ago, some hominins had evolved an essentially modern foot function and style of walking. The size of the footprints is consistent with individuals who had the body mass and stature of H. ergaster, and it is presumed that it is this species that was walking along the lake’s edge all those eons ago.40
Had H. floresiensis evolved from H. erectus, we would have to assume that H. erectus had the ape-like foot characteristics we see in H. floresiensis, or that, for some reason, the foot structure reverted to ape-like as H. erectus evolved into H. floresiensis. But we have never seen such an evolutionary reversal in human evolution. Why this would happen is puzzling when re-evolving long toes and a flat foot would impair walking performance.41 Also, as we saw in chapter 1, the form of H. floresiensis’ feet probably evolved before the characteristics for running appeared in our genus.
H. floresiensis’ pelvis is australopithecine-like. We do not have a pelvis for H. erectus. Again, had H. floresiensis evolved from H. erectus, we either assume that H. erectus also had an australopithecine-like pelvis, or, if we do not think this was the case, we would have to argue that the H. erectus pelvis reverted to australopithecine-like as it evolved into H. floresiensis.
The brain of LB1 is only 426 cubic centimetres. This is closer to the brain size of apes, the australopithecines and some Paranthropus species. H. floresiensis’ brain is smaller than any other species of Homo. It is even smaller than H. habilis, whose brain size ranges from 509 to 638 cubic centimetres. H. erectus brain sizes range between 813 and 1059 cubic centimetres (see Appendix A). An evolutionary reversal in H. erectus to LB1’s brain size would require us to accept that, in evolving to H. floresiensis, the H. erectus brain would have shrunk to half its size or even less than that.
It all comes down to whether we are comfortable accepting so many evolutionary reversals in one species when we have not seen this degree of reversal in the evolution of any other hominin species. As Bill Jungers has pointed out: ‘Some modern humans (pygmies) have reduced greatly in body size repeatedly and independently throughout the world, without any evidence of evolutionary reversals to such primitive morphologies and body mass.’42
Of course, while evolutionary reversals are not known to have occurred to such a significant extent during human evolution, that doesn’t mean they did not happen. They have certainly been a factor in the evolution of other species. When I asked Professor Mike Lee, who is an expert on the evolution of reptiles, especially snakes and lizards, if reversals have occurred in that domain, he replied:
Yes, at least in reptiles it happens a lot. For example there are many lizard species that have completely lost their legs during evolution. Why do we assume that those lizards have lost their legs, rather than primitively never had them? The reason is that there is a whole lot of anatomical and genetic evidence that places these legless reptiles deep within the lizard branch of the evolutionary tree—they are true lizards. So we know that they are descended from lizards with legs. For instance, many of the legless lizards in Australia are geckos that have lost their legs. They chirp like geckos, they always lay two eggs just like geckos, their ears are just like geckos.
We see many primitive traits of H. floresiensis, such as the jaw shape, short upper legs and so forth. Can we interpret these as reversals from a H. erectus condition? Well, to do that, we’d first need to find even more traits that put H. floresiensis high up the hominin tree, traits that link H. floresiensis and H. erectus—and there aren’t many compelling ones. Given that H. floresiensis mostly exhibits primitive traits that put it close to the base of Homo, you’d be inclined to accept the evidence at face value and say ‘That’s where it belongs.’
If you want to interpret those primitive traits as reversals and say H. floresiensis is descended from H. erectus, that’s only possible if you find a whole suite of other characteristics that put it up there with H. erectus. You need overwhelming evidence that H. floresiensis is related to H. erectus, and I just don’t see that.43
Mike here echoes the principle of parsimony, in which the most acceptable explanation for a phenomenon is the simplest. Scientists analyse and consider all possibilities and choose the solution that explains all the data, the solution that involves the fewest assumptions, and in our case, the solution that shows the fewest evolutionary changes. Cladistic analysis is based on this principle.
Another way to try to figure out the possible origins of H. floresiensis is to compare body and brain size differences among the likely ancestors. JAF Diniz-Filho (Universidade Federal de Goiás, Brazil) and colleagues assert that if H. floresiensis originated from an early Homo, such as H. habilis or H. rudolfensis, its small body and brain size does not need to be explained by the island rule and just reflects a deep ancestry.44 That is, H. floresiensis could be explained by a very early, small-sized (and small-brained) species of Homo emanating from Africa and arriving on Flores with little evolutionary change.
What concerns Diniz-Filho and colleagues about this scenario is that it runs contrary to the Out of Africa 1 model. Because earlier forms of Homo, including H. habilis and H. rudolfensis, have never been found outside Africa, and they are at least 1.5 million years older than H. floresiensis, Diniz-Filho and colleagues opt for H. erectus as the ancestor of H. floresiensis, explaining that ‘we feel it is not parsimonious to invoke an older and/or smaller-bodied African ancestor to explain H. floresiensis phenotype, especially if this implies revising the entire “Out of Africa I”’.45
But can we reject their first hypothesis, which proposes that a pre-H. erectus species diffused from Africa, simply because it does not agree with current dogma in human evolution—because it challenges the single Out of Africa 1 model of human evolution?
One of the roadblocks to the idea that the H. floresiensis lineage stems from a very early group of Homo is the lack of archaeological evidence for this outside Africa. The reasoning goes that, because there is no evidence for early hominins anywhere but Africa, then it is highly unlikely that any early, pre-H. erectus lineage would have left that continent. Interestingly, though, evidence has recently come to light that points to this scenario.
Assistant Professor Giancarlo Scardia (Universidade Estadual Paulista, Brazil) and colleagues discovered stone tools that date to between 2.5 and 2 million years ago in Jordan,46 a period well before that espoused by the Out of Africa 1 model of human evolution. Two-million-year-old tools are known from much further east, too, in Pakistan and China.47 These predate the recently discovered H. erectus in Africa by half a million years.48 They predate H. erectus in Java by a million years or so, and they predate H. georgicus by 700 000 years. The only species known to have existed at the time of the tools discovered in Jordan are P. boisei and H. habilis, and possibly A. africanus. The Scardia and colleagues’ finding might make it easier for critics to accept that something more primitive, more australopithecine-like, something like H. floresiensis, could have wandered out of Africa well before hominins arrived at Dmanisi.49
Still, we have no skeletal evidence for any early hominins anywhere between Africa and South-East Asia. And so, time and again, I hear this as an argument for rejecting H. floresiensis as an early hominin. It relies on the assumption that an absence of evidence can be a good reason for inferring that a species was never in a particular landscape. I find it perplexing that a case against an early hominin lineage spreading out of Africa is based on the lack of skeletal evidence anywhere between Africa and Indonesia, when we have no skeletal evidence for H. erectus between Africa and Indonesia either. Yet clearly H. erectus is on Java. Over thousands of years, H. erectus would have traversed the continents between Africa and South-East Asia. Logically, we’d expect the remains of at least some of these individuals to have been preserved as fossils. Yet no fossils of H. erectus have come to light across those regions. The absence of skeletal remains of either H. erectus or early hominins is more likely to be due to the fossils having not yet been discovered rather than anything else. It’s a case of that old truism: absence of evidence is not evidence of absence.
I find it just as perplexing that the presence of H. georgicus from Georgia is invoked as an example of H. erectus moving out of Africa. If anything, this should alert us to the fact that small-brained species could move out of Africa: two of the H. georgicus brains are within the range of H. habilis,50 and one is within the lower end of the range of H. ergaster.51
Now for some reflection. The implication of the work of my colleagues and I, and that of others, is that H. floresiensis is a remnant population of a lineage that emerged a long time ago in Africa. At some time, a group, or groups, of this lineage diffused from that continent to arrive at an unknown time on Flores. This dispersal represents a hitherto unknown movement of very early hominins out of Africa and challenges the Out of Africa 1 model of human evolution. Remember, too, that H. floresiensis survived on Flores until at least 53 000 years ago, more than a million years after its closest relative died out in Africa.
These are new concepts for us to grasp. They challenge us to rethink what we thought we knew about human evolution.
And there are other puzzles surrounding H. floresiensis. How did this species arrive on Flores, an island that emerged from the sea ten million years ago and that has never been joined to another landmass?52 Fossil hominin remains of very small individuals have been discovered at the Mata Menge dig on Flores. What are they? Another diminutive species, H. luzonensis, was recently discovered in Callao Cave on the island of Luzon in the Philippines. What can this tell us about human evolution in South-East Asia? We will delve into these mysteries in the following chapters.

There are strict rules for naming a new species of fauna or flora. This first came about in the eighteenth century when Swedish scientist Carl Linnaeus neatly streamlined the confusing botanical and zoological naming systems that had developed in an idiosyncratic way over many years. Under Linnaeus’ system, a two-part name was applied to each species: the first part of the name is its genus and the second part is the species name. Hence, we are in the genus ‘Homo’ and we belong to the species ‘sapiens’.
In assessing the characteristics of the newly discovered bones at Liang Bua cave, Peter Brown, Mike Morwood and colleagues found that the combination of features was unique, so much so that they proposed a new genus and species: Sundanthropus floresianus. However, before academic papers are published, experts in the same field of research extensively review them, and in this case the referees thought that the little Flores people, although unique in their combination of skeletal features, were not quite so different from everything else in the hominin family as to warrant a new genus.
The upshot of the peer review is that the new discovery is part of our genus Homo, but it is distinct from every other species in this genus. The authors proposed Homo floresianus. Luckily, one of the reviewers, who must have had a good knowledge of Latin, noted that ‘floresianus’ means ‘flowery anus’.1 Imagine the fun generations of students would have had with that name! It was quickly amended to the more satisfactory Homo floresiensis, named for the island on which the bones were discovered.