15
Assessment, Interpretation and Management of Impaired Respiratory Function in the Neurological Patient
INTRODUCTION
The nervous system regulates and controls respiration and so it is not uncommon that impairment in neurological function can affect the functioning of the respiratory system. Conversely, because the central nervous system (CNS) relies on a rich supply of oxygen, respiratory compromise can rapidly lead to CNS dysfunction. Either of these situations could be life threatening, so it is important that neuroscience nurses have the necessary knowledge and skill to assess accurately and act upon changes in respiratory function. This chapter presents an overview of respiratory physiology. The elements of a systematic respiratory assessment, the interpretation of findings, and the most common respiratory interventions that are employed to optimise and support respiratory function in the neurological patient will be discussed.
A sound understanding of the physiology of the respiratory system enables the nurse to interpret assessment findings and to understand the physiological basis of respiratory interventions and support systems.
PHYSIOLOGY
The primary function of the respiratory system is to supply tissues with the oxygen required for function, and to excrete the carbon dioxide produced from cell metabolism. The respiratory centre is located in the brain stem and is the main control of respiratory function.
Control of respiration
The medullary rhythmicity area is situated within the medulla oblongata and controls the basic rhythm of respiration. Normal inspiration lasts for two seconds; expiration lasts for three. Therefore, there is a basic inspiratory: expiratory cycle approximately every five seconds. Within the medullary rhythmicity area are two regions controlling the basic respiratory cycle: the inspiratory and expiratory areas.
Nerves originating in the inspiratory area determine the basic rhythm of respiration. At the beginning of expiration, the inspiratory area is inactive, but after three seconds it automatically becomes active due to the intrinsic excitability of the inspiratory nerves. The nerve impulses last for approximately two seconds and innervate the diaphragm and intercostal muscles via the phrenic and external intercostal nerves respectively. At the end of two seconds, the impulses stop and the cycle starts again.
Normally expiration is a passive process and therefore the expiratory area is inactive until a greater volume of carbon dioxide (CO2) removal is required, for example, during exercise or when air movement is impeded due to bronchospasm. At this time, impulses from the inspiratory area activate the expiratory area, which causes contraction of the internal intercostals and abdominal muscles. This action actively assists in reducing the size of the thoracic cavity which will result in greater volumes of CO2 being removed.
There are two additional centres situated within the pons that can modulate the output from the medullary rhythmicity area (Figure 15.1).
Figure 15.1 Locations of areas of the respiratory centre.
Sagittal section of brain stem.
Reproduced from Principles of Anatomy and Physiology 12e by Gerard Tortora and Bryan Derrickson. Copyright (2009, John Wiley & Sons). Reprinted with permission of John Wiley & Sons Inc.

The pneumotaxic area
The pneumotaxic area is situated in the upper pons. Its primary function is to limit inspiration. It transmits impulses to the inspiratory area, turning it ‘off’ before the lungs get too full. When impulses from this area increase, inspiratory time may be as short as 0.5 seconds, thus the volume of air entering the lungs is significantly reduced. When there is a decrease in the impulses leaving the pneumotaxic area, inspiration may last for as long as five seconds, filling the lungs with greater volumes. A strong pneumotaxic signal may increase the respiratory rate to 30–40 times/minute whereas a weak signal will significantly reduce respiratory rate.
The apneustic area
The apneustic area sends impulses to the inspiratory area to prolong inspiration, particularly during quiet breathing. The apneustic area can only influence inhalation when the pneumotaxic area is inactive. When the pneumotaxic area is active it overrides the apneustic area.
Regulation of the activity of the respiratory centre
There are several influences on the respiratory centre that can modulate and alter the basic pattern of breathing:
Cortical impulses
Cortical connections allow for voluntary control of respiration, such as holding your breath. However, the duration of voluntary control is limited by the rise in arterial partial pressure of carbon dioxide (PaCO2), which will stimulate the inspiratory area leading to involuntary inspiration (see chemical stimuli below).
Inflation reflex
Stretch receptors are in the walls of the bronchi and bronchioles. If they are ‘overstretched’, impulses are sent, via the vagus nerve, to the inspiratory and apneustic areas. This results in the inspiratory area becoming inhibited; the apneustic area is stopped from activating the inspiratory area and passive expiration occurs. This basic reflex is referred to as the Hering–Breuer reflex.
Chemical stimuli
Central chemoreceptors situated in the medulla are sensitive to changes in hydrogen ion concentration (pH) of the cerebrospinal fluid (CSF). Hydrogen is a product of CO2 carriage, thus if there is an increase in PaCO2, there will be a decrease in the pH. The increase in acidity stimulates the chemoreceptors which cause the inspiratory centre to become more active resulting in an increase in the rate and depth of respiration and an increased rate of CO2 removal.
In addition, peripheral chemoreceptors, also known as the carotid and aortic bodies, are sensitive to changes in PaCO2, pH and PaO2 (partial pressure of oxygen) of arterial blood.
Unlike the central chemoreceptors these are also sensitive to significant decreases in oxygen concentration. If the PaO2 drops by 40% (8 kPa), the peripheral chemoreceptors become stimulated and impulses are sent to the inspiratory area. However, as the PaO2 continues to drop to below 6–7 kPa, the cells of the inspiratory area will eventually become hypoxic, decreasing their response. This results in fewer impulses to the inspiratory muscles and leads to a reduction in the respiratory rate.
The respiratory centre is also affected by changes in blood pressure; an increase in blood pressure reduces respiratory rate and a decrease in blood pressure will increase the rate. The changes are detected by baroreceptors and conveyed to the inspiratory centre via the vagus and glosspharyngeal nerves.
Gas exchange
There are three main processes involved in gas exchange:
- Pulmonary ventilation
- External respiration
- Internal respiration
Pulmonary ventilation (‘breathing’)
During this process, respiratory gases are exchanged between the atmosphere and the lungs. This occurs when a pressure gradient exists between the lungs and the atmosphere.
Inspiration
Inspiration occurs when the pressure in the lungs is lower than atmospheric pressure. The pressure reduction happens when the volume of the lungs increases through contraction of the respiratory muscles. Boyles Law explains this phenomenon: if the volume of a closed container increases, the pressure inside the container reduces and vice versa. When the volume of the container becomes decreased the pressure in the container increases.
The diaphragm is the main respiratory muscle and is innervated by the phrenic nerve. Contraction of the diaphragm, from its resting dome shape to a more flattened one, increases the vertical diameter of the thoracic cavity (Figure 15.2). This action accounts for the movement of more than two thirds of the air that enters the lungs during inspiration. In conjunction with the diaphragm, the external intercostals muscles contract, lifting the ribs up and out, thereby increasing the anterior–posterior diameter of the thorax.
Figure 15.2 Pressure changes in pulmonary ventilation. During inhalation the diaphragm contracts, the chest expands, the lungs are pulled outward, and alveolar pressure decreases. During exhalation, the diaphragm relaxes, the lungs recoil inwards, and alveolar pressure increases, forcing air out of the lungs.
Reproduced from Principles of Anatomy and Physiology 12e by Gerard Tortora and Bryan Derrickson. Copyright (2009, John Wiley & Sons). Reprinted with permission of John Wiley & Sons Inc.

With contraction of the respiratory muscles, the pressure inside the lungs declines. The intra-alveolar pressure drops from 760 mmHg to 758 mmHg. As atmospheric pressure is 760 mmHg, a pressure gradient results, which allows air to enter the lungs and inspiration to occur. The entry of air continues until the pressures equalise.
During normal breathing, the intrapleural pressures are slightly sub-atmospheric, with a pressure of 756 mmHg prior to inspiration. During inspiration, this pressure drops to 754 mmHg, creating a vacuum effect, with the walls of the lungs being sucked outwards. This movement of the pleural membranes further aids expansion of the lungs.
Expiration
Expiration is also achieved through the creation of pressure gradients, but in this case the pressure inside the lungs becomes greater than atmospheric pressure. This results when respiratory muscles relax, the diaphragm resumes its dome shape and the intercostals relax, causing the rib cage to drop down and inwards. These actions are passive and lead to a reduction in the volume of the lungs, thus increasing the pressure inside them. Air moves out of the lungs and expiration occurs.
The alveoli have very elastic walls, and there is a risk of collapse as they recoil during expiration. The presence of surfactant within the alveoli reduces the surface tension of intra-alveolar water, thus reducing the inward pull of the surface tension and minimising the risk of collapse.
External respiration
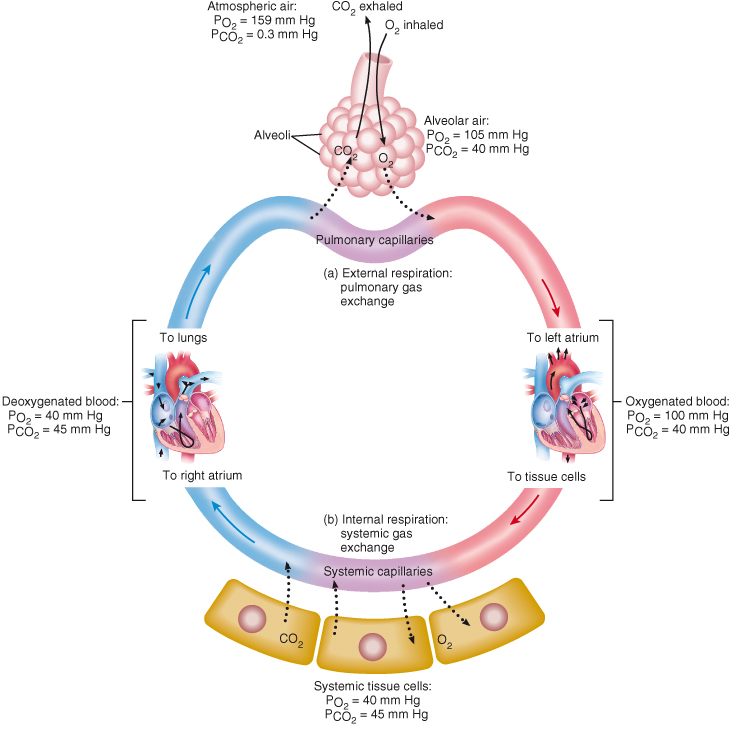
This refers to the diffusion of respiratory gases between the alveoli and the blood. During this process, deoxygenated blood within the pulmonary capillaries is reoxygenated. Blood, as it returns back to the heart from the systemic circulation has a PaO2 of 40 mmHg and a PaCO2 of 45 mmHg. However, in the alveoli, there is a PaO2 of 105 mmHg and a PaCO2 of 40 mmHg. Therefore, a concentration gradient exists between the alveoli and the blood. Oxygen diffuses into the blood and carbon dioxide diffuses into the alveoli until the concentration becomes the same in the alveoli and blood (Figure 15.3).
Figure 15.3 Changes in partial pressures of oxygen and carbon dioxide (in mm Hg) during external and internal respiration.
Reproduced from Principles of Anatomy and Physiology 12e by Gerard Tortora and Bryan Derrickson. Copyright (2009, John Wiley & Sons). Reprinted with permission of John Wiley & Sons Inc.
There are several features of the lungs that enhance external respiration. First, the alveoli-capillary membrane is only 0.5 µm thick. Secondly, there is a huge surface area across which diffusion can occur. The lungs contain around 300 million alveoli, which creates a 70 m2 surface area. Each alveolus is surrounded by a capillary network so, not only is there is a large surface area, but there is also a large blood supply to allow for effective gas exchange. Approximately 900 ml of blood participate in gas exchange at any one time. Furthermore, the pulmonary capillaries are so narrow that the red blood cells flow through them in single file. This allows for maximum perfusion to occur as each red blood cell can participate in gas exchange.
Internal respiration
This process refers to the exchange of gases between the blood and the cells. The left ventricle of the heart pumps blood through the arterial circulation and therefore into arterioles and capillaries. Oxygenated blood within the capillaries has a PaO2 of 100 mmHg and a PaCO2 of 40 mmHg, while cells have a PaO2 of 40 mmHg and a PaCO2 of 45 mmHg. Therefore these gases diffuse between the blood and the tissues, as a concentration gradient exists: oxygen diffuses into the tissues and carbon dioxide diffuses into the blood. The deoxygenated blood returning back to the heart has a PaO2 of 40 mmHg and a PaCO2 of 45 mmHg, where the cycle of external and internal respiration restarts (Figure 15.3).
Transport of gases
The lungs enable the oxygenation of blood and excretion of carbon dioxide; contraction of the heart and the properties of blood itself allow for the delivery and removal of the gases.
Oxygen
Oxygen does not dissolve easily in water and therefore the majority of oxygen (97%) is transported around the body in combination with the haem portion of haemoglobin. The remaining 3% of oxygen is dissolved in plasma. In resting conditions, 100 ml of blood contains about 20 ml of oxygen. Haemoglobin consists of a pigment portion called haem and a protein portion called globin. The haem portion contains four atoms of iron, each of which can combine with oxygen forming oxyhaemoglobin. This is an easily reversible reaction.
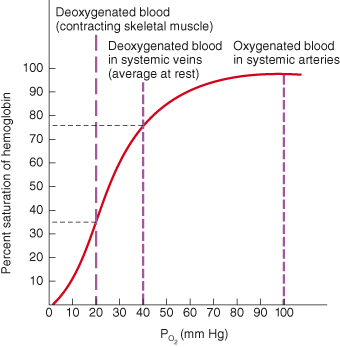
The PaO2 is the most important factor in determining how much oxygen combines with haemoglobin. If all the haemoglobin is converted to oxyhaemoglobin, then full saturation exists. If the haemoglobin consists of both haemoglobin and oxyhaemoglobin, then partial saturation is present. The oxygen dissociation curve illustrates the degree of saturation of haemoglobin as PaO2 changes (see Figure 15.4). From this, it is evident that when the PaO2 is high, the haemoglobin binds with lots of oxygen and is almost totally saturated. However, when the PaO2 is low, the haemoglobin is only partially saturated and oxygen is released for tissue use. Therefore in the pulmonary capillaries where the PaO2 is high, lots of oxygen binds with haemoglobin, whereas in the tissues where the PaO2 is lower, oxygen is released for diffusion into the tissues.
Figure 15.4 Oxygen–haemoglobin dissociation curve showing the relationship between haemoglobin saturation and PaO2 at normal body temperature.
Reproduced from Principles of Anatomy and Physiology 11e by Gerard Tortora and Bryan Derrickson. Copyright (2008, John Wiley & Sons). Reprinted with permission of John Wiley & Sons Inc.
The ability of oxygen to bind with haemoglobin and to be released for use by the tissues is influenced by pH, temperature and 2,3-diphosphoglycerate (DPG).
pH.
Tissues releasing lactic acid and/or having a high PaCO2 can cause a low pH (acidosis); haemoglobin gives up its oxygen readily in such an environment. This is known as the Bohr effect.
Temperature.
An increase in temperature will lead to more oxygen splitting from haemoglobin. In times of increased cellular activity, cells require more oxygen. The heat that is produced due to the increase in metabolic rate will increase the amount of oxygen released from haemoglobin.
2,3-diphosphoglycerate.
DPG is produced during glycolysis and can bind reversibly with haemoglobin altering its structure to release oxygen. The amount of DPG produced is greatest during times of decreased oxygen delivery to cells. Therefore, it enhances tissue oxygenation by helping to maintain the release of oxygen from haemoglobin.
Carbon dioxide
Carbon dioxide is transported around the body in several ways. About 7% is dissolved in plasma, around 23% combines with the globin portion of haemoglobin to form carbaminohaemoglobin whereas the remaining 70% is carried within plasma as bicarbonate. The formation of carbaminohaemoglobin is greatly influenced by the PaCO2. In tissues where PaCO2 is high, the formation of carbaminohaemoglobin is promoted whereas in the lungs where the PaCO2 is low, CO2 readily splits from haemoglobin and diffuses into the alveoli for excretion.
The majority of carbon dioxide is transported in the blood as bicarbonate. This comes about by CO2 diffusing into the red blood cells where it reacts with water. This requires the enzyme carbonic anydrase, and produces carbonic acid which dissociates into hydrogen and bicarbonate. The hydrogen combines with haemoglobin and the bicarbonate leaves the red blood cell and enters the plasma. To maintain the ionic balance of the red blood cell, chloride leaves the plasma and diffuses into the red blood cell, where it combines with potassium to form potassium chloride. This is known as the chloride shift. The bicarbonate that has diffused into the plasma then combines with sodium to form sodium bicarbonate in the blood (see Figure 15.5).
Figure 15.5 Carbon dioxide carriage.
CA – carbonic anhydrase; CO2 – carbon dioxide; H2O – water; H2CO3 – carbonic acid; H+ – hydrogen ion; HCO−3 – bicarbonate ion; HHb – hydrogen buffered with haemoglobin; NaCl – sodium chloride; Na+ – sodium ion; Cl− – chloride ion; NaHCO3 – sodium bicarbonate.

Deoxygenated blood therefore contains carbon dioxide that is transported in a variety of forms. When this blood enters the pulmonary capillaries, the above reactions and events are reversed which allows carbon dioxide to then diffuse into the alveoli.
The effect of respiratory gases on cerebral blood flow
Both CO2 and O2 can influence cerebral blood flow (CBF) and need to be controlled if the patient is demonstrating signs of raised intracranial pressure (ICP). Refer to Chapter 6 for a detailed description of how they affect CBF and to Chapter 7 for how CO2 is manipulated in the management of raised ICP.
Respiratory failure
Respiratory failure is a condition in which the lungs do not properly oxygenate the blood and/or fail to adequately clear carbon dioxide. Respiratory failure is not a specific disease but is rather a result of a number of conditions that impair ventilation, compromise gas exchange or disrupt blood flow through the lungs (Porth, 2004).
There are two types of respiratory failure:
Type 1
Type 1 respiratory failure is defined as hypoxaemia without retention of CO2 (i.e. PaO2 ≤8 kPa or 60 mmHg). It is typically caused by a ventilation/perfusion (V/Q) mismatch, i.e. there are areas of low ventilation relative to perfusion (low V/Q units). This is most commonly associated with any acute disease of the lungs (see Table 15.1) which involves fluid filling or collapse of the alveoli. Regardless of the cause, haemoglobin does not become fully saturated with oxygen prior to entering the systemic circulation. This leads to a reduction in the oxygen available to the tissues and organ dysfunction can ensue.
Table 15.1 Examples of causes of respiratory failure.

Type 2
Type 2 respiratory failure is defined as hypoxaemia with retention of carbon dioxide (i.e. PaCO2 > 6.5 kPa or 50 mmHg). This is due to ventilation failure which can occur as a result of:
- Depression of the respiratory centre
- Impairment of the muscles involved in respiration
- Degenerative lung disease
- Airway obstruction
Table 15.1 lists the specific causes of this type of respiratory failure.
With neurological illnesses, respiratory dysfunction could also be categorised in relation to whether it is due to a condition or disease of the central nervous system, or to peripheral nervous system dysfunction (see Table 15.1).
ASSESSMENT OF RESPIRATORY FUNCTION
Physical assessment skills, lung function tests and blood gas analysis are used to determine the patient’s respiratory function.
Airway
Assessment of the respiratory system commences with ascertaining whether the patient is able to maintain a patent airway or whether they require an airway adjunct. Neuroscience patients may have a decreased level of consciousness affecting their ability to maintain a patent airway. Patients with a Glasgow Coma Scale (GCS) below 8 will require intubation, however partial obstruction can occur with a much higher GCS.
A fast and easy way to ascertain airway patency is to talk to the patient. If the patient verbally responds their airway is clear. Patients with a reduced level of consciousness are at risk of partial airway obstruction because the tongue becomes flaccid and falls against the posterior pharyngeal wall. Noisy breathing or snoring are signs of partial airway obstruction.
Breathing
Colour
A central cyanosis occurs when large amounts of unsaturated haemoglobin are present and can be detected when the oxygen saturation of arterial blood drops to 85–90% (Lumb, 2000). Central cyanosis is detected by the presence of a bluish greyish colour of the mucus membranes and mouth. Peripheral cyanosis indicates poor circulation.
Respiratory rate
A normal resting respiratory rate in an adult is about 10–18 breaths per minute. Bradypnoea (less than 10 breaths per minute) could indicate: depression of the respiratory centre, hypothermia, opioid overdose or a late sign of raised ICP. Tachypnoea (>20 breaths per minute) may be a sign of pain, anxiety or hypotension.
Depth of breathing
The thorax should rise and fall gently, and there should be equal expansion of the lungs. Unequal expansion could indicate lung collapse, pneumothorax or chronic fibrotic respiratory disease. Shallow breaths (hypopnea) can lead to basal collapse of the lungs and is a potential problem for patients with a reduced conscious level. Deep and rapid breaths (hyperventilation) can be a sign of a metabolic acidosis, e.g. diabetic ketoacidosis or damage to the pons (see below: Rhythms of breathing).
Effort of breathing
Increased work of breathing is evident by the use of additional respiratory muscles. Recruitment of the accessory muscles, i.e. the scalene, the sternomastoids and the external intercostals, can assist the diaphragm in increasing inspiratory effort. Expiration becomes active when the abdominal muscles and internal intercostal muscles are recruited. When the abdominal muscles contract they drive intra-abdominal pressure up, pushing up the diaphragm in the process, which raises pleural pressure, and subsequently alveolar pressure, which results in more air being expelled from the lungs.
Patients with a neuromuscular disorder or a spinal injury may not be able to increase their work of breathing and therefore these signs may be absent.
Rhythm of breathing
The following abnormal breathing patterns maybe observed in neurological patients in a coma:
Cheyne–Stokes breathing:
is an irregular breathing pattern that alternates between hyperventilation and hypoventilation sometimes to the point of apnoea. It usually results from bilateral dysfunction of the deep cerebral or diencephalic structures.
Central neurogenic hyperventilation:
is regular rapid deep breathing. Patients can breathe 40 to 70 times per minute. It usually results from central lesions of the pons, anterior to the cerebral aqueduct or the fourth ventricle, which result in the loss of the inhibitory influence of the apneustic and pneumotaxic areas on the medullary rhythmicity centre. Possible metabolic and pulmonary causes need to be excluded.
Apneustic breathing:
is characterised by prolonged inspiration followed by a pause prior to expiration. This pattern of breathing is caused by lesions of the dorsolateral lower half of the pons, leading to loss of modulation of respiration by the pneumotaxic area.
Cluster breathing:
is observed as periodic breathing with irregular frequency and amplitude, with variable pauses between the clusters of breaths. It results from high medullary damage.
Ataxic breathing,
or Biot’s breathing, is irregular in relation to rate, depth and rhythm. This pattern is caused by medullary lesions, and usually is a preterminal respiratory pattern (Malik and Hess, 2002).
Pain
The location, type, severity and degree of pain present on inspiration/expiration should also be assessed. Pleuritic pain is usually described as severe sharp stretching pain which is worse on inspiration. It is caused by inflammation of the parietal pleura. Musculoskeletal pain originates from the muscles or nerves of the thoracic cage. Tracheitis is described as a constant burning pain in the centre of the chest. It is most commonly caused by a staphylococcus aureus infection.
Auscultation
Auscultation involves listening to the sounds generated by breathing. A lung area on one side of the thorax should be compared to the same area of the opposite lung, i.e. apex of the right compared to apex of the left lung, right base compared to left base.
Breath sounds need to be interpreted in relation to their location, intensity, pitch and duration during inspiration and expiration.
Normal breath sounds
Normal breath sounds are:
Vesicular
These are soft and low pitched. They are heard through inspiration, continue into expiration and gently fade about a third of the way through expiration. They are heard throughout the lung fields.
Broncho-vesicular
These are louder and with a higher pitch than vesicular breath sounds. The inspiratory and expiratory sounds are about equal. These are heard centrally around the 1st and 2nd intercostal spaces.
Bronchial
These sounds are loud and have a high pitch. Expiratory sounds may last slightly longer than the inspiratory sounds. Bronchial sounds are usually heard over the trachea.
If broncho-vesicular or bronchial breath sounds are heard in locations distant from where they should be heard (usually referred to as bronchial breathing), then it is likely that the sound has been transmitted through consolidated or fluid filled lung tissue.
Adventitious sounds
Adventitious sounds are breath sounds that are heard in addition to normal breath sounds. The common adventitious sounds are:
Crackles
Crackles are intermittent, non-musical and brief sounds heard during inspiration. They can either be fine (soft, high pitched and brief) or coarse (louder, lower in pitch and heard for longer). Fine crackles are heard in diseases affecting the lower airways as the alveoli reopen, or when there is fluid in the alveoli and lower airways, e.g. pulmonary oedema. Coarse crackles occur when bronchioles open and are usually caused by copious secretions in the large airways.
Wheeze
Wheeze is a musical sound that is more commonly heard on expiration. Wheezes are high pitched and have a hissing or shrill quality. They are produced by airflow vibrating through narrowed and compressed airways as in asthma or bronchospasm.
Pleural rub
This is a rubbing sound which occurs when the pleural surfaces are roughened by inflammation, neoplasms or infection. They can be heard during inspiration and expiration, and sound like walking on snow.
Stridor
Stridor is an inspiratory musical wheeze which is loudest over the trachea. It is indicative of an obstructed trachea or larynx and requires immediate medical attention.
Breath sounds can also be absent or decreased, e.g. in atelectasis, pneumothorax, pleural effusion.
Transmitted voice sounds
If abnormally located broncho-vesicular or bronchial breath sounds are heard, it is useful to assess transmitted voice sounds. This can be performed by listening with a stethoscope over the chest wall as:
- the patient says ‘ninety-nine’. Normally sounds are muffled and indistinct but if there is an area of consolidation, the sound will be louder and clearer. This test is known as bronchophony.
- the patient says ‘ee’. Normally this will sound like a muffled long E sound, however over an area of consolidation it will be heard as ‘ay’. This is known as egophony.
- the patient whispers ‘ninety-nine’. Over an area of consolidation, this will sound louder and clearer. This is known as whispered pectoriloquy.
MONITORING AND INVESTIGATION OF RESPIRATORY FUNCTION
Sputum
Sputum should be examined for colour, consistency and quantity. A sample should be sent for microscopy, culture and sensitivity (MC&S) if infection is suspected.
Pulse oximetry
The non-invasive assessment of the arterial oxygen saturation of haemoglobin is an adjunct to the physical respiratory assessment outlined above. There are however some limitations to its use which include:
Poor peripheral circulation/hypotension
Pulse oximetry relies on a pulsatile pressure therefore the sensor may not detect a sufficiently strong signal to give an accurate reading in patients with poor peripheral circulation.
Anaemia
Haemoglobin that is fully saturated, i.e. SaO2 98%, will not always imply adequate oxygenation. A low haemoglobin, although fully saturated, may in fact have insufficient oxygen content for adequate tissue oxygenation.
Dysheamoglobinaemias
Pulse oximetry cannot distinguish between carboxyhaemoglin (COHb) and HbO2. Therefore in situations where COHb may be elevated, e.g. a patient suffering from smoke inhalation or carbon monoxide (CO) poisoning, the readings will be falsely high.
Pigments
Dark fingernail polish can give false readings of up to 3–5%. Dark skin pigmentation can also give inaccurate readings.
Hypercarbia
Although pulse oximetry can detect episodes of hypoxaemia it does not provide any information about levels of carbon dioxide in arterial blood.
Lung function tests
Lung function tests assist in the diagnosis of respiratory disease, but are also essential for the assessment of deterioration of ventilatory function in conditions such as Guillain–Barré syndrome and myasthenia gravis, and following acute spinal cord injury.
The lung function test that is most commonly used in the neurological setting is forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) for which a spirometer is used (spirometry).
Vital capacity (VC) is the maximum volume of air that can be exhaled after a maximal inspiration; it is equivalent to the inspiratory reserve volume plus the tidal volume plus the expiratory reserve volume (Figure 15.6). To measure VC the patient is required to breathe out as hard and fast as possible after maximal inspiration (forced vital capacity, FVC). Most hand held spirometers measure FVC and FEV1. FEV1 measures the volume of air expired in the first second of the FVC. To undertake the test the patient is required to purse their lips tightly around the tube after maximal intake of breath and then to exhale until no more air can be forced out. Patients with facial weakness will find it difficult to create a seal around the mouthpiece, in such cases a face mask can be attached to the spirometer. The patient is asked to repeat the test three times, and the best of the three results is recorded.
Figure 15.6 Spirogram of lung volumes and capacities.

Forced vital capacity is normally approximately 65 ml/kg, and FEV1 is usually 85% of the FVC. A reduction in FVC to 30 ml/kg is associated with a poor forced cough and the patient may need supplementary oxygen. Elective intubation and mechanical ventilation is indicated if the FVC is less than 20 ml/kg (Ropper et al., 2003). Other respiratory data must be used in conjunction with the readings to make informed clinical judgements.
Arterial blood gases
In addition to these assessments, arterial blood gases provide useful information about the adequacy of pulmonary gas exchange and the acid base balance. Normal blood gas ranges are given in Table 15.2.
Table 15.2 Normal blood gas values.
| Measurement | Normal blood gas range |
| pH | 7.35–7.45 |
| PaO2 | 10.0–13.3 kPa |
| PaCO2 | 4.6–6.0 kPa |
| HCO3 | 22–26 mmol/l |
| Base excess | −2 to +2 |
| O2 saturation | >95% |
Adapted from Adam and Osbourne, 2005.
ACID–BASE BALANCE
Hydrogen ions and pH
The body’s cells can only function effectively within a narrow range of pH. Each day the body produces hydrogen ions (H+) (acidic) in abundance; these ions must be effectively eliminated from the body to maintain pH within normal range. The more hydrogen ions that are present, the lower the pH becomes. A lower pH indicates an increased relative acidity. If the blood pH falls below 7.35 it is referred to as acidaemia; the physiological change that occurs as a result of the acidaemia is called acidosis. A reduction in hydrogen ion concentration means that the blood becomes more alkaline and the pH will increase; a blood pH >7.45 is referred to as an alkalaemia, and the physiological state is called alkalosis.
As cells only function optimally within a narrow range of pH, the body requires systems to regulate and maintain pH within this range.
Body systems to regulate pH
Buffer
Buffering mechanisms allow for the binding or release of hydrogen ions thereby reducing or increasing the number of free hydrogen ions in solution. When the H+ is buffered it becomes a much weaker acid and therefore has less effect on the pH than it would if it were unbuffered.
The degree to which the buffers are working to maintain the pH of the blood is reflected within the base excess recording of the blood gas. So in a situation where there is excess H+, e.g. type II respiratory failure, the buffers respond by buffering the excess H+ and the number of available buffers will subsequently be reduced. This will be reflected in the base excess reading as a negative reading (otherwise referred to a base deficit). Conversely in a situation where H+ concentration is decreasing, e.g. excessive vomiting, buffers will release hydrogen ions in an effort to return the pH to normal. The base excess reading, in this situation, is positive indicating that there are buffers available for hydrogen should the need arise.
Buffers include proteins, phosphates and the carbonic acid–bicarbonate system. The carbonic acid–bicarbonate system only will be briefly explained as this is essential to enable a better understanding of blood gas results. A buffer consists of a buffer pair: a weak acid, i.e. carbonic acid (H2CO3), and weak base, i.e. bicarbonate (HCO3−). Hydrogen ions produced by cells will react with the HCO3− (base) to produce H2CO3 (weak acid) which can subsequently be removed from the body by the lungs as CO2. The H2CO3 is a weak acid and has much less effect on the pH than if the H+ ions remained unbuffered. Thus when the blood becomes more acidic, the HCO3− level will decrease as it is used up in the buffering of the excess acids. When there is a reduction in the concentration of hydrogen ions then H2CO3 can release H+ to maintain pH, and the HCO3− will subsequently increase.

Respiratory regulation of pH
If cells produce too much H+ (metabolic acids) the excess can be converted into carbon dioxide (as outlined above) and eliminated from the body by the respiratory system. The fall in pH stimulates the respiratory centre to increase the rate and depth of breathing (see: Physiology/Chemical stimuli) which results in more CO2 being removed by the lungs, returning the pH towards normal. When pH increases the reverse will occur, i.e. a reduced rate and depth of breathing.
Renal regulation of pH (2–3 days)
The kidneys play an essential role in maintaining the pH by excreting large amounts of hydrogen ions in the urine each day. When disturbances in pH occur the kidneys can respond by changing the rates of hydrogen and bicarbonate ion excretion. Therefore if the blood becomes more acidic (fall in pH) the kidneys respond by excreting more hydrogen and reabsorbing more bicarbonate ions. When alkalaemia develops (increase in pH) the rate of hydrogen ion excretion declines and the rate of bicarbonate ion reabsorption also declines.
There are four common disturbances of acid base balance. Disturbances can be due to a respiratory or a metabolic cause and will result in either an acidosis or alkalosis.
DISTURBANCES OF ACID–BASE BALANCE
Respiratory disturbances
If the pH disturbance is due to a respiratory cause the primary change will be in the carbon dioxide level. Carbon dioxide is an acid when it combines with water. Therefore, an increase in carbon dioxide will result in a fall in pH (PaCO2 will be high and pH will be low); this disturbance is called a respiratory acidosis. The reverse will happen if there is a decrease in the carbon dioxide (PaCO2 will be low and pH will be high); this is called respiratory alkalosis.
The primary cause of a respiratory acidosis is type II respiratory failure (see: Respiratory failure). Respiratory alkalosis is much less common and it is caused by hyperventilation. In neurological patients this can be caused by lesions in the region of the pons (see: Central neurogenic hyperventilation).
Metabolic disturbances
When there is a metabolic cause for changes in pH, the primary change will be the bicarbonate level. A metabolic acidosis can result from the body producing too many acids, e.g. poor perfusion of tissues resulting in a build up of lactic acid; renal failure resulting in a failure to eliminate excess hydrogen ion and, less commonly, due to excess loss of alkaline from the body, e.g. profuse diarrhoea. Regardless of the cause, the metabolic acidosis will result in an excess of hydrogen ions which will be buffered by the bicarbonate ions. There will therefore be a reduction in the bicarbonate level (HCO3− will be low, pH will be low and the base excess reading will be negative).
A decrease in the concentration levels of hydrogen ions or an increase in bicarbonate levels results in a metabolic alkalosis, but this is relatively uncommon. It can occur due to excessive losses of acid either through the renal or gastrointestinal system, i.e. diuretics or profuse vomiting. The bicarbonate will be raised, the pH will be raised and the base excess will be positive.
Compensatory mechanisms
In certain situations, when there has been long-term derangement of either metabolic or respiratory function, compensation of the pH disturbance occurs. Blood gas analysis would reveal a normal pH, despite either the HCO3− or PaCO2 being outside ‘normal’ limits. In compensation, the primary (or chronic) alteration will be compensated for by the ‘opposite’ system. For example, with chronic carbon dioxide retention, the high levels of carbon dioxide are compensated for by bicarbonate retention by the kidneys. Therefore, there is metabolic compensation for the respiratory problem. In metabolic acidosis, the compensation seen will be an increase in respiratory rate with a subsequent reduction in carbon dioxide. Therefore there is removal of carbon dioxide to compensate for the metabolic acidosis and thus the pH is helped to return to within a normal range.
NURSING CARE/MANAGEMENT
Airway management
Emergency airway equipment should be easily accessible by the bedside of all neurological patients. Suction equipment should be checked at least once per shift to ensure that it is in working order and that the necessary sized suction catheters are available (see below: Suctioning), in addition to a yankuer sucker to clear the mouth and orophaynx of blood, vomitus and excessive secretions. Flow meters for piped oxygen should have the appropriate nipple nozzle to enable quick attachment of oxygen tubing or bag-valve mask if required. Airway adjuncts of various sizes should also be available.
A compromised airway is managed in the first instance by head tilt and chin lift. The severity of airway compromise will determine subsequent management. Patients with a reduced level of consciousness may not be able to maintain a patent airway, therefore, an airway adjunct may be required to maintain an open, patent airway. Partial airway obstruction can lead to hypoxia and hypercapnia which can further compromise neuronal function, so early identification and appropriate management is essential.
Positioning the patient in the recovery position may suffice for patients who are drowsy, but continuous assessment is required to ensure that the patient is able to maintain optimal oxygenation and ventilation. Oropharyngeal airways should not be used in patients who are conscious as they will trigger the gag reflex. A nasopharyngeal airway will not trigger the gag reflex and is better tolerated in the conscious patient. Chest secretions can be suctioned via a nasopharyngeal airway. Their use is however contraindicated in patients with suspected basal skull fracture, so they should not be used following head injury until skull integrity has been confirmed on x-ray. Other contraindications include patients with nasal polyps, and, because of the high risk of causing haemorrhage during insertion, they should be used cautiously in patients who are receiving anticoagulants or who have coagulopathies.
An oropharyngeal airway can be used as a short term measure for airway maintenance if a patient is unconscious; however, this does not protect against aspiration of oral secretions such as saliva or gastric contents should the patient vomit/regurgitate. They should only be inserted by practitioners who are competent to do so, as incorrect insertion can displace the tongue into the pharynx, causing airway obstruction. If a patient has a GCS < 8, they should be assessed by an anaesthetist for intubation. An endotracheal tube maintains a patent airway and protects against aspiration of oral/gastric secretions. If a patient is intubated they will require admission to an intensive care unit for ongoing care and support.
If a patient has a longer term reduction in the level of consciousness or bulbar dysfunction resulting in an inability to maintain a patent airway for the foreseeable future, a tracheostomy may be required. A tracheostomy may also be performed if a patient is likely to require mechanical ventilation for more than seven days.
Tracheostomy management
Tracheostomy formation can either be a surgical procedure which requires a horizontal incision between the second and third tracheal ring, or a percutaneous approach may be used during which a dilator system is used to insert the tracheostomy tube; the latter is more common. There is evidence to suggest that there is less perioperative bleeding and post-operative complications associated with the percutaneous approach (Freeman et al., 2000), but the decision as to which approach is used will be influenced by available resources as well as the patient’s anatomy and medical requirements. Following insertion, the tube should remain in place for five to seven days to allow for the skin to trachea tract to form (Marino, 1998). Following this, the tube can be changed as required.
Tracheostomy tubes
There are a variety of different tubes available. The type of tube used will depend on a number of factors, e.g. the length of the time it is anticipated that the patient will require the tracheostomy, and the purpose of the tube, e.g. for clearance of secretions only or for ongoing ventilation.
Single and double lumen tubes
Single lumen tubes are typically placed when a tracheostomy is newly formed, although it is becoming more common to use double lumen tubes from the outset (Figure 15.7). After five to seven days, a single lumen tube is usually changed to a double lumen tube. The inner tube acts as a removable liner for the more permanent, outer tube. The advantage of the inner tube or cannula is that it can be withdrawn for brief periods to be cleaned, which helps to prevent blockage. If the tube did become blocked the inner cannula can be temporarily removed to leave a patent airway.
Figure 15.7 A double lumen cuffed tracheostomy tube.
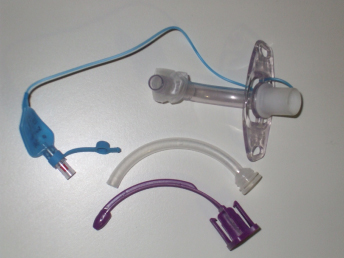
Cuffed and uncuffed tubes
A cuff is a soft balloon around the distal end of the tube that can be inflated to create a complete seal with the walls of the trachea (see Figure 15.7). The cuff provides protection of the airway and reduces the risk of aspirate reaching the lungs or, in ventilated patients, ensures that gases reach the lungs. When the cuff is inflated, the patient is unable to phonate as air is prevented from passing over the vocal cords. An uncuffed tube is often used if a patient has a weak cough, and therefore cannot clear their secretions effectively, but has a safe swallow and is breathing spontaneously.
Fenestrated or unfenestrated
Some double lumen tubes have a fenestration (window or hole) within the outer tube. Fenestrated tubes are very useful for weaning patients off a tracheostomy. The fenestration enables air to pass through the patient’s oral/nasal pharynx as well as the tracheal stoma, which helps patients to return to normal breathing. The fenestration also permits speech when the cuff is deflated, and a speaking valve is placed on the opening of the tracheostomy because exhaled air can then pass over the vocal cords.
Cuff-related complications of a tracheostomy
The cuff prevents leakage of respiratory gases through the mouth and helps to reduce the risk of aspiration of oral secretions. The cuff is designed to allow the trachea to be sealed with minimum risk of pressure-induced injury to the tracheal mucosa. The cuff pressure should be routinely checked, e.g. once a shift, and should be less than capillary closure pressure of 24–30 cm H2O (St John, 1999). It is recommended that cuff pressures should be <25 cmH2O. The main cuff related problems are:
Aspiration
Despite the presence of an inflated cuff, aspiration of mouth secretions and enteral nutrition can still occur. This risk can be reduced if 30° to 45° semi-recumbent positioning is used, oral secretions are regularly suctioned and cuff pressures are regularly checked.
Cuff leaks
If a leak is present respiratory gases can pass around the cuff and move out of the lungs, and oral secretions can pass down into the lungs. When this occurs in a mechanically ventilated patient, ventilation may become compromised. Cuff leaks are usually detected by sounds generated as the air passes over the vocal cords. However, cuff leaks are rarely caused by disruption of the cuff itself. It is more likely that a cuff leak results from non-uniform contact between the cuff and the trachea wall. A leak can also be caused by faulty function of the one way valve at the air injection inlet that normally keeps the cuff inflated (see Figure 15.7). If these valves leak, air can escape from the cuff. Although this is not a common problem, if the valve fails, the tracheostomy tube will need to be replaced.
Following the formation of a tracheostomy, the trachea may be inflamed and oedematous. As healing occurs, the inflammation will subside increasing the lumen of the trachea. This could lead to a cuff leak and is especially likely if the patient is receiving active treatment for inflammation, for example with steroids (Moore and Woodrow, 2004).
If a cuff leak is present, the cuff should be inflated until the sounds of a leak (e.g. gurgling, audible words or the sound of air through the patient’s mouth) disappear and then the cuff pressure should be rechecked. If it is more than 25 cmH2O, then the tracheal tube should be replaced as tracheal ulceration may develop (Marino, 1998).
Ulceration
Inflated cuffs place continuous pressure on the tracheal epithelium and therefore ulceration of the trachea can develop. Maintaining the cuff pressures at the lowest possible pressure to prevent a cuff leak will help to reduce the risk of ulcer development while maintaining a seal.
Loss of normal airway function
Normally, inspired air passes through the upper airways which warm, humidify and filter it. A tracheostomy by-passes the upper airway and therefore the air needs to be warmed and humidified through artificial means. Ideally a heated humidifier system should be used as this delivers 44 mg H2O/l at 37°C with a relative humidity of 100%. This is essentially the same humidification that occurs during normal breathing. A cold water humidifier only has a relative humidity of 50%. A heat moisture exchanger (HME) filter generally delivers about 25 mg H2O/l, so there is considerably less humidification of the air when using this device.
Tracheal stenosis
Stenosis of the trachea is a late complication that can appear days to weeks following tracheal decannulation. The clinical features include dyspnoea, wheezing and in severe cases stridor. It is the result of cuff pressure and infection causing ischaemic death of tracheal cartilage and scarring. Low-pressure cuffs markedly reduce the occurrence of cuff injury.
Specific nursing care of the patient with a tracheostomy
Safety priorities
All patients who have a tracheostomy must have tracheal dilators and two spare tracheostomy tubes at their bedside at all times, for situations of accidental extubation. The replacement tubes should be the same size and one size smaller than the tube in place. A smaller tube is often required if the tracheostomy is newly formed and will be easier to insert in an emergency. Suction, oxygen, airway adjuncts, and a bag valve mask (BVM) should be available and ready to use by the patient’s bedside.
Care of the stoma/prevention of site infection
The newly formed stoma is a surgical wound and therefore needs to be routinely aseptically cleaned and dressed to prevent infection. Dressings should be renewed daily (more often if indicated) and the site inspected for signs of inflammation, infection or bleeding. Normal saline (NaCl 0.9%) is sufficient to clean the site and a dry dressing such as lyofoam® is used to protect the site.
Changing the dressing always requires two people in order to prevent accidental extubation. One person undertakes the dressing whilst the second person holds the tube firmly in place, but taking care not to move the tube inwards, which will cause the patient to have explosive coughing. Following the dressing change, the ties/tube holder can be changed. This should be tight enough to allow for two fingers to slide beneath the ties so that they are not uncomfortably tight for the patient.
Inner tube management
If a double lumen tube is used, the inner tube should be used at all times and, when it is removed for cleaning, a spare inner tube should be inserted. There is no consensus on how frequently the inner tube should be cleaned. The need should be assessed on an individual patient basis as it largely depends on the amount and consistency of secretions. Inner tubes can be fenestrated or unfenestrated, but it is important to ensure that an unfenestrated inner tube is inserted prior to suctioning as the suction catheter can pass through the fenestration and damage the lining of the trachea.
Communication
A tracheostomy tube is inserted below the vocal cords so it is usual for patients who have a cuffed tube in situ not to be able to make sounds. Loss of speech is isolating and creates difficulties in expressing needs or concerns. The nurse should explain to the patient that this is a temporary loss and once the tracheostomy is removed, normal speech should be regained. However, information such as this must be appropriate to the individual patient; a patient who has expressive dysphasia or has dysarthria and also requires a tracheostomy may not ever regain normal speech due to the neurological dysfunction. Communication aides in the form of letter boards, pen and paper, keyboards or signs will reduce isolation and their use should be encouraged. The patient should also be encouraged to mouth words and family and friends supported to lip read.
Speaking valves can also be used. Speaking valves are one way valves which are placed on the tracheostomy opening: air enters through the tube on inspiration but closes during expiration. This allows air to pass over the vocal cords and therefore speech can be facilitated. To be able to use a speaking valve, the cuff must be deflated and therefore the patient must be able to swallow safely and have a cough reflex. This will require formal assessment by the speech and language therapist (SLT) (see Chapter 12).
Weaning and removal of the tracheostomy tube (decannulation)
Most patients with a tracheostomy will recover sufficiently to have the tracheostomy removed. Removing the tube increases work of breathing by a third (Chadda et al., 2002), so the patient does need to be carefully assessed to ensure that they will be able to cope with the additional effort required and will be able to cough effectively enough to clear secretions. Assessment of the patient prior to decannulation ensures that:
- There is no ongoing requirement for mechanical ventilation that cannot be met through non-invasive methods
- There is an adequate cough (peak expiratory cough flows > 200 l/min)
- There is no physiologically significant upper airway lesion (such as overgranulation or tracheal stenosis) or swelling
(Bourjeily et al., 2002)
The weaning process should be systematic and involve the multidisciplinary team (Hunt and McGowan 2005). Tracheostomy management is often undertaken by a specific tracheostomy team consisting of a SLT, physiotherapist, nurses and medical staff who will review patients with tracheostomies across the hospital. If this is not available, then the team should include at least one member of staff who is confident and competent in tracheostomy management.
The weaning process should be stepped with a flexible approach that is reflective of the patient’s ability and individual needs. Braine and Sweby (2006) advocate a six stepped approach, which allows the patient to progress to decannulation safely. This may require changing the tube to a fenestrated tube or perhaps using a tube that is a smaller size.
It is advocated that prior to decannulation, the patient should have had the following procedures:
- Swallow assessed by a SLT
- Cuff deflated for at least 24 hours without an indication of aspiration or increase in chest infection
- Tracheostomy capped off for 24 hours
(Serra, 2000)
Following removal of the tube, the stoma should be covered with an occlusive dressing forming a complete seal. The stoma should close up within a few days.
RESPIRATORY SUPPORT
Depending on the underlying condition of the patient, their presentation and assessment findings, respiratory support may be required. In type 1 respiratory failure when hypoxaemia is the primary respiratory problem, efforts must be made to improve tissue oxygenation. This may be achieved through the administration of higher concentrations of oxygen and/or the use of positive end expiratory pressure (PEEP) (see below: Continuous positive airway pressure (CPAP)). In type 2 respiratory failure, there is retention of carbon dioxide so the mechanics of ventilation need to be improved. To reduce the carbon dioxide, the patient’s minute volume needs to be increased; this can be achieved by either increasing the tidal volumes of the patient, or increasing the respiratory rate or, indeed, doing both. Therefore ventilatory support is needed which can be either invasive or non-invasive.
Oxygen therapy and delivery devices
Neurological patients often require oxygen therapy to optimise respiratory and neurological function. There are a number of oxygen delivery devices which generally can be categorized into those that can deliver a specific oxygen concentration (fixed performance delivery systems) and those that do not (variable performance delivery systems). The condition of the patient will determine which device is used.
Variable performance devices
Variable performance devices include nasal cannulae and simple face masks and deliver a variable concentration of oxygen. Because they deliver oxygen at flow rates below the normal patient inspiratory flow rate, the oxygen concentration that is delivered will depend on the patient’s rate and depth of breathing. For example a patient breathing rapidly and deeply will entrain large volumes of room air which will dilute the supplemental oxygen being delivered. As the exact percentage being delivered cannot be determined, the prescription for oxygen should be written in litres/min and not as a percentage. The variable performance delivery systems should not be used in patients where hypoxia is a concern.
Fixed performance devices
A fixed performance device, such as the Venturi system, delivers a prescribed concentration of oxygen as stated on the valve, irrespective of the patient’s breathing pattern. It delivers oxygen at rates above the normal patient inspiratory flow rate, which is made possible by the Venturi valve which accelerates the oxygen flow and mixes it with air in a precise ratio. Table 15.3 provides a summary of oxygen delivery devices, their indications for use and specific management.
Table 15.3 Oxygen delivery devices.
| Oxygen delivery device | Indications for use and specific management |
| Nasal cannula (NC)
(Variable flow) |
|
| Simple face mask, e.g. Hudson
(Variable flow) |
|
| Humidified oxygen, e.g. Aquapak |
|
| Venturi mask
(Fixed flow) |
|
| Nonrebreathe mask
(Variable flow) |
|
NON-INVASIVE RESPIRATORY SUPPORT
Non-invasive respiratory support avoids the use of invasive strategies. The two forms of non-invasive strategies which are used are continuous positive airway pressure (CPAP) and non-invasive ventilation (NIV).
Continuous positive airway pressure (CPAP)
CPAP is primarily used in situations of type 1 respiratory failure when higher concentrations of supplementary oxygen via a fixed delivery system (such as the Venturi system) or humidified circuit are not resolving the patient’s hypoxaemia. It is also used as a way of weaning from mechanical support. Positive pressure is applied throughout the respiratory breath cycle which increases the functional residual capacity, thereby increasing the opportunity for gas exchange to take place (Keen, 2000). In addition, work of breathing is reduced as lung compliance is improved (Kannan, 1999).
The CPAP system consists of a high flow generator that is capable of delivering the required pressure throughout the breath cycle, a tightly fitting mask or T piece for use with a tracheostomy tube, and a flow resistor on the distal portion to the circuit (CPAP valve) through which the patient exhales (see Figure 15.8). The pressure from the flow generator needs to be sufficient to keep the valve within the flow resistor open throughout the breath cycle. It is usual to have an oxygen analyser within the circuit so that the prescribed oxygen concentration can be delivered. It is essential to have a second CPAP valve within the circuit (a pressure relief valve) which requires a pressure of at least 5 cm H2O above the prescribed CPAP level to open it: this acts as a safety valve should the CPAP valve through which the patient is exhaling become occluded or stuck (MHRA, 2000).
Figure 15.8 Continuous positive airway pressure (CPAP) circuit.
Reproduced with permission from Vital Signs Limited.
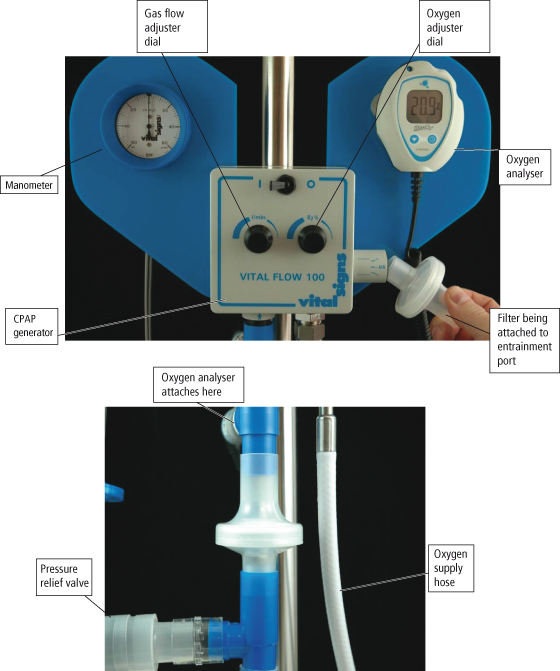
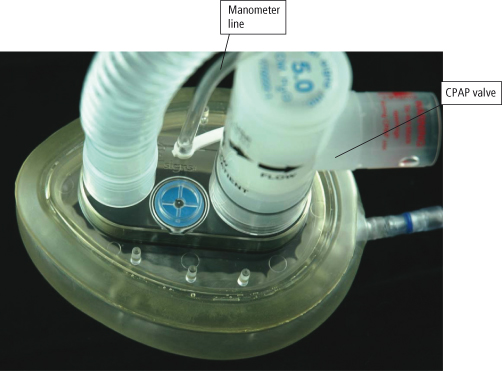
The CPAP mask needs to be tightly fitting to prevent air leaks and loss of pressure which patients may find difficult to tolerate. Pressure damage particularly on bony prominences may develop and the patient may experience claustrophobia. Air leaks could cause corneal dryness and abrasions. Therefore, CPAP via a face mask is seen to be a temporary measure to avoid or postpone intubation (Marino, 1998).
More recently CPAP helmets are being used in the critical care setting. These avoid some of the complications of using a CPAP mask and are generally less claustrophobic and well tolerated (Antonelli et al., 2005). Patroniti et al. (2003) found that there was little difference between mask and helmet in the delivery of CPAP, but to avoid re-breathing of CO2, the helmet required higher flow rates. The transparent helmet allows for better patient communication and interaction with their surroundings. Patient compliance with the mask and CPAP is enhanced when the patients is well educated, prior to the application of the CPAP, about its purpose, and by continued support from the nurse caring for the patient.
CPAP via a mask is contraindicated in patients who have a reduced level of consciousness, as there is a risk of aspiration if they vomit, and in patients with facial trauma. The tight mask and straps could reduce cerebral venous return and therefore could increase ICP, so the potential risk/benefit for patients with suspected raised ICP should be assessed on an individual basis. Refer to Table 15.4 for how to troubleshoot common problems of CPAP and the section below on the adverse effects of positive airway pressure support.
Table 15.4 CPAP/NIV nursing considerations.
| Patient problem | Nursing actions |
| Mask related problems | |
| Tolerance
Claustrophobia Eye irritation– corneal drying and abrasion Tissue damage | Consider use of CPAP helmet. Offer psychological support to assist patient tolerance
Ensure mask correctly sized and fitted Avoid air leaks Consider use of foam backed dressings (e.g. Granuflex®) on bridge of nose/ears |
| Difficulties of receiving CPAP/NIV | |
| Communication
Eating and drinking difficult Gastric distension | Use of communication aides
If possible, ensure patient has breaks from using CPAP/NIV for nutrition and hydration. NB: Closely observe SpO2 throughout and recommence CPAP/NIV as clinically indicated Consider use of nasogastric tube to decompress stomach |
CPAP – continuous positive airway pressure; NIV – non-invasive ventilation.
Non-invasive ventilation (NIV)
The British Thoracic Society (2002) recommends that NIV is indicated for:
- Chronic obstructive pulmonary disease (COPD) with a respiratory acidosis
- Hypercapnic respiratory failure secondary to neuromuscular disease, e.g. Guillain–Barré syndrome, myasthenia gravis, or spinal injuries, or chest wall deformity, e.g. scoliosis
NIV can also be used to improve quality of life for patients with motor neurone disease (Bourke et al., 2006) (see Chapter 27), and to treat central sleep apnoea (Garner and Amin, 2006).
NIV is also referred to as Bilevel NIV and often the brand name of whatever system is used (such as NIPPY®, BiPAP®). As these machines were initially developed for domiciliary ventilation, they are often easy to use but offer relatively few options compared to the positive pressure ventilators used for invasive ventilation within the intensive care setting.
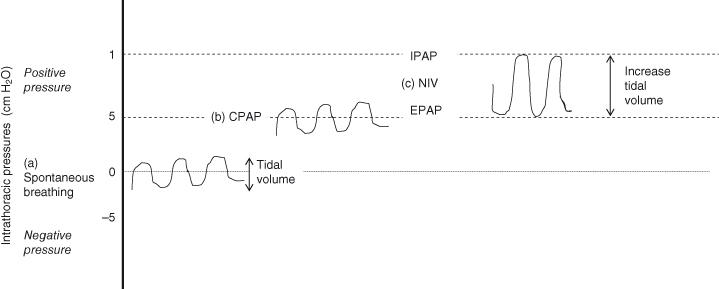
The principle of NIV is to reduce carbon dioxide by increasing the patient’s spontaneous minute volume. There is alternation between two pressure levels during the breath cycle: a higher level on inspiration and a lower one on expiration. The inspiratory positive airway pressure (IPAP) provides support on inspiration so that a larger inspiratory volume is taken, which reduces the work of breathing and increases alveolar ventilation (Tully, 2002). The higher the IPAP the more air is exchanged and the more CO2 is removed. The expiratory positive airway pressure (EPAP) creates less resistance and discomfort on expiration, prevents alveolar collapse and increases functional residual capacity (Figure 15.9). Increasing the difference between the IPAP and the EPAP increases the volume of each breath. This augmentation of the tidal volume will enhance carbon dioxide clearance.
Figure 15.9 Effect of CPAP and NIV on spontaneous breathing. (a) Spontaneous breathing – the intrathoracic pressures alternate between positive and negative. (b) Once CPAP is applied – the same pattern of breathing occurs as is seen in spontaneous breathing but the intrathoracic pressures remain positive throughout. (c) If NIV is applied, the tidal volumes of spontaneous breathing are increased due to the IPAP that is applied to inspiration. The pressures are positive throughout the breath cycle.
CPAP – Continuous positive airway pressure; EPAP – expiratory positive airway pressure; IPAP – inspiratory positive airway pressure; NIV – non-invasive ventilation.
As with CPAP, NIV is usually delivered via a tight fitting mask therefore the same complications of the mask apply. If used for domiciliary ventilation, a nasal mask can be used rather than a facemask (see Table 15.4 and Chapter 27 for nursing care considerations).
Adverse effects of positive end expiratory pressure (PEEP)
In normal breathing, i.e. without respiratory support the total volume of inhaled air with each breath (tidal volume) is exhaled completely. The normal airway pressure at the end of expiration and before inspiration is therefore equivalent to atmospheric pressure. With CPAP and NIV there is a pressure greater than that of atmospheric pressure remaining within the alveoli at the end of expiration (PEEP) therefore the distal airways are less likely to collapse, preventing atelectasis and improving oxygenation. Additionally, PEEP can reopen collapsed alveoli, improving gas exchange through alveolar recruitment.
However, there are adverse effects of PEEP that need to be considered, particularly in patients with raised ICP. The application of PEEP will increase central venous pressure which may in turn induce an increase in intracranial pressure through impeding cerebral venous return. The increase in central venous pressure (CVP) can also result in reduced cardiac filling pressures with a subsequent reduction of cardiac output and blood pressure, which could produce reductions in cerebral perfusion pressure (CPP) and may lead to cerebral ischaemia. However, this is more likely in patients who are haemodynamically unstable and who have impaired cerebral autoregulation (Muench et al., 2005). Low levels of PEEP (≤5 cm H2O) are usually well tolerated without haemodynamic effects (Myburgh, 2003). Higher levels of PEEP in patients with raised ICP should be used with caution and with close monitoring of the patient’s haemodynamic status and ICP. The risk/benefit of the application of higher PEEP is assessed on an individual patient basis. The benefits of adequate oxygenation may be assessed as being more important than the possible risk of increasing ICP in some patients.
Intermittent positive pressure breathing (IPPB)
Intermittent positive pressure breathing is the delivery of positive airway pressure throughout inspiration in the spontaneously breathing patient. It is most commonly used as an adjunct to physiotherapy. The most commonly used machine is called the Bird 7 ventilator (more commonly referred to as the BIRD). IPPB augments tidal volume which helps in the clearance of bronchial secretions and in improving gas exchange. It is typically used for short sessions to optimise respiratory function. In the neurological setting it is commonly used in patients with neuromuscular disease affecting the respiratory muscles, e.g. Guillain–Barré syndrome and myasthenia gravis, and following acute spinal cord injury when the respiratory muscles are affected.
If the Bird is being used by the physiotherapist to treat a patient it should form part of the patient’s regular respiratory management and nurses should therefore be competent in using the equipment. By working collaboratively with the physiotherapist optimal respiratory care can be given. The Bird is fairly straightforward to use. There are three controls that are usually set by the physiotherapist. The pressure control is usually set between 12 and 15 cmH2O. When the patient takes a breath either through a mouthpiece or mask the airflow will continue until the set pressure is reached. The ease with which a breath is triggered and the rate at which the gas flows are determined by the sensitivity and flow rate controls respectively. Patients are encouraged to take slow breaths through the mouth until the pressure is reached. A nebuliser can be incorporated into the circuit during treatment. When patients have difficulty using the mouth piece due to neuromuscular facial weakness a facemask can be used.
The Bird is often used by several patients within the same clinical area. In such cases it is imperative to prevent cross infection. Each patient’s disposable breathing circuit (which attaches directly to the Bird) should be kept by the patient’s bedside in an appropriate container and dated. The Bird should be cleaned as per local protocol between patients.
The management of patients requiring invasive respiratory support is beyond the scope of this book. Readers should consult a comprehensive critical care nursing text such as Adam and Osbourne (2005).
SUCTIONING
Clearing chest secretions by suctioning through either an endotracheal tube, tracheostomy tube or nasopharyngeal airway is an essential part of nursing care. There are clear indications when suctioning is required and ‘routine’ suctioning, for example, every 4 hours, should be avoided. The indications include:
- Coarse breath sounds on auscultation
- Spontaneous coughing
- Audible/visible secretions in tracheostomy tube
- Increased work of breathing
- Deteriorating blood gases/SpO2 (saturation of haemoglobin with oxygen as measured by pulse oximetry)
There are inherent risks to suctioning which could be detrimental to the neurological patient. These include hypoxia and cardiac instability such as bradycardia, arrhythmias or hypotension. In patients with a spinal cord injury, there is an increased risk of these cardiovascular complications due to possible unopposed vagal stimulation (see: Neurogenic shock in Chapter 14).
Prior to suctioning, the patient should be informed of the procedure. Suctioning can be uncomfortable and adequate preparation of the patient can avoid undue anxiety and distress. Suctioning can increase ICP, so it is important to ensure that those patients who are at risk of deleterious rises in ICP are adequately sedated prior to suction. In ventilated patients Kerr and colleagues (1997) found that short duration, controlled hyperventilation with 30 breaths per minute reduced PaCO2, which resulted in a short lived reduction in cerebral blood flow. This subsequently led to fewer rises in ICP during suctioning and cerebral perfusion was maintained.
Suctioning can reduce SaO2 so preoxygenation is recommended to avoid hypoxaemia and cardiovascular compromise (Thompson et al., 2000; Wong, 2000). If atelectasis is present, several hyperinflation breaths may be useful, however its use is not without risk and should only be performed by suitably trained staff. Barotrauma, ventilator induced lung injury and patient discomfort may result (Robson, 1998). There is also an increased risk of cardiovascular instability, so suctioning should be avoided in patients with serious cardiac pathology.
A suction pressure of 100–150 mmHg or 15–20 kPa should be used during suctioning. Mucosal trauma, hypoxia and negative pressure atelectasis can occur if too high a suction pressure is used (Wood, 1998). If secretions are thick, the suction pressure should not be increased: the secretions should be made looser through humidification and saline nebulisers.
The instillation of saline does not have any proven benefit (O’Neal et al., 2001). In fact, it has been shown that:
- The instillation of saline increases risk of infection (Glass and Grap, 1995)
- As saline and secretions do not mix, the secretions are not thinned (Ackerman, 1993)
- Instilled saline does not disperse beyond the main bronchi, therefore it does not affect lung periphery secretions (Hanley et al., 1978)
- To elicit a cough, suction catheter stimulation is as effective as saline (Gray et al., 1990)
During the suction procedure, suction should not be applied on insertion to avoid trauma, atelectasis and hypoxaemia. On withdrawing the catheter, continuous suction should be applied as this technique prevents mucus plug loss and reduces direct suction on the tracheal wall. The application of suction should be limited to a maximum of 15 seconds duration to avoid suctioning complications (McKelvie, 1998) and to a maximum of two suction passes per procedure in patients with raised ICP (Wainwright and Gould, 1996). The likelihood of these complications occurring also increases with multiple suctioning attempts.
The suction catheter should be the correct size in relation to the inner lumen of the endotrachel tube (ETT) or tracheostomy:
| • ETT/tracheostomy size 7.5 | size 10 catheter |
| • ETT/tracheostomy size 8 | size 12 catheter |
| • ETT/tracheostomy size 8.5 | size 12 catheter |
| • ETT/tracheostomy size 9 | size 14 catheter |
If the catheter selected is too big for the lumen of the ETT, the catheter blocks the airway during suctioning causing alveolar collapse (Dean, 1997) and the flow of air down the tube also becomes impeded (McKelvie, 1998). Suctioning is an invasive procedure so asepsis should be maintained throughout the procedure.
Closed suction units should be used when high concentrations of oxygen and PEEP are required to maintain oxygenation as they reduce the risk of arterial desaturation during suctioning (Carlton et al., 1987). They also reduce the risk of environmental contamination when active chest infections are present.
ASSISTED COUGH
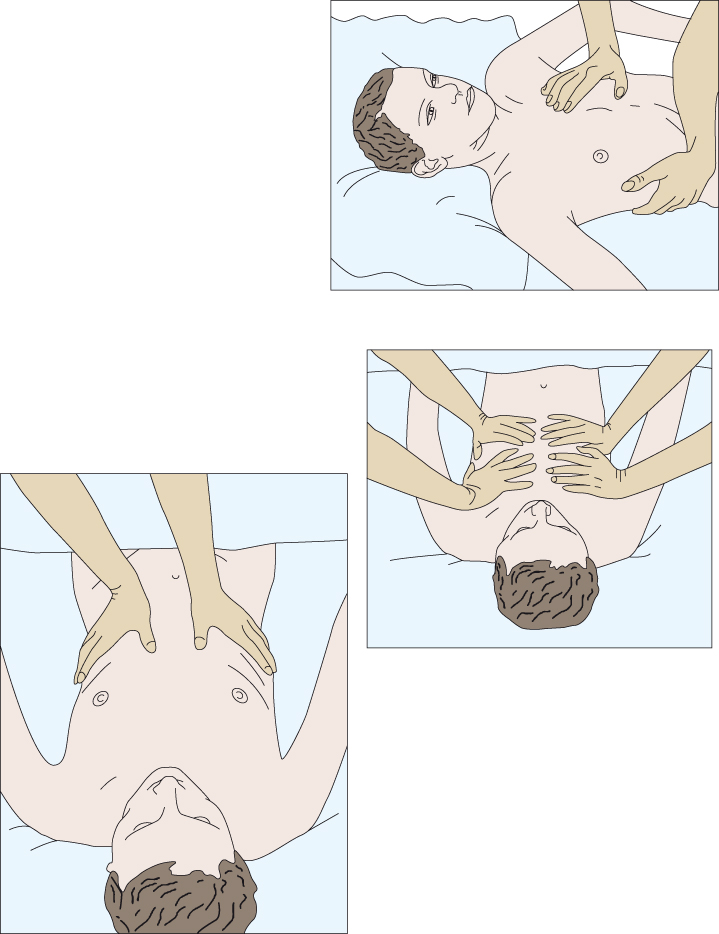
Patients with weakness or paralysis of the abdominal muscles will be unable to cough effectively, putting them at risk of atelectasis and chest infection. An assisted cough replaces the function of the affected muscles by manually creating increased pressure under the diaphragm. The technique should only be performed by practitioners who have been deemed competent to do so. Assisted cough is necessary for patients with neuromuscular disease affecting the respiratory muscles, e.g. Guillain–Barré syndrome and myasthenia gravis, and following acute spinal cord injury when the respiratory muscles are affected. There are three methods to give an assisted cough which require either one or two persons (Figure 15.10).
Figure 15.10 Different hand positions to give an assisted cough.
SUMMARY
Frequent, thorough respiratory assessments are essential for early detection of respiratory compromise. The nurse has a key role in monitoring the patient’s respiratory status and acting promptly on the assessment findings. Nurses should be working collaboratively with physiotherapists to plan and implement individualised respiratory treatment so as to optimise the respiratory care being delivered. Neurological patients often require non-invasive and invasive respiratory support and frequent respiratory interventions which require neurological nurses to have additional knowledge, skills and competency to manage the patient safely and competently.
REFERENCES
Ackerman MH (1993) The effects of saline lavage prior to suctioning. American Journal of Critical Care 2 (4) 236–230.
Adam S, Osbourne S (2005) Critical Care Nursing: Science and practice. (2nd edition). Oxford: Oxford University Press.
Antonelli M, Pennisi MA, Montini L (2005) Clinical review: Noninvasive ventilation in the clinical setting – experience from the past 10 years. Critical Care 9:98–103.
Bourjeily G, Habr F, Supinski G (2002) Review of tracheostomy usage: complications and decannulation procedures Part II. Clinical Pulmonary Medicine 9(5):273–278.
Bourke SC, Tomlinson M, Williams et al. (2006) Effects of non invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurology 5(2):140–147.
Braine ME, Sweby S (2006) A systematic approach to weaning and decannulation of tracheostomy tubes. British Journal of Neuroscience Nursing 2(3):124–132.
British Thoracic Society (2002) Non-invasive ventilation in acute respiratory failure. Thorax 57(3)192–211.
Carlton GC, Fox SJ, Akerman RN (1987) Evaluation of a closed-tracheal suction system. Critical Care Medicine 15(5):522–555.
Chadda K, Louis B, Benaissa L et al. (2002) Physiological effects of decannulation in tracheostomized patients. Intensive Care Medicine 18(12):1761–1767.
Dean B (1997) Evidence- based suction management in accident and emergency: a vital component of airway care. Accident and Emergency Nursing 5(2):92–98.
Freeman BD, Isabella K, Lin N, Buchman TG (2000) A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest 118(5):1412–1418.
Garner A, Amin Y (2006) The management of neuromuscular respiratory failure: A review. British Journal of Neuroscience Nursing 2(8):394–398.
Glass CA, Grap MJ (1995) Ten tips for safer suctioning. American Journal of Nursing 95(5):51–53.
Gray JE, MacIntyre NR, Kronenberger MA (1990) The effects of bolus normal saline installation in conjunction with endotracheal suctioning. Respiratory Care 35(8):785–790.
Hanley MV, Rudd T, Butler J (1978) What happens to intratracheal instillations? American Review of Respiratory Disease 117(Supp): 124.
Hunt K, McGowan S (2005) Tracheostomy management in the neurosciences: A systematic multidisciplinary approach. British Journal of Neuroscience Nursing 1(3):122–125.
Kannan S (1999) Practical issues in non-invasive positive pressure ventilation. Care of the Critically Ill 15(3):76-79.
Keen A (2000) Continuous positive airway pressure (CPAP) in the intensive care unit – uses and implications for nursing management. Nursing in Critical Care 5(3):137–141.
Kerr M, Rudy E, Weber B et al. (1997) Effects of short duration hyperventilation during endotracheal suctioning on intracranial pressure in severe head injured adults. Nursing Research 46(4):195–201.
Lumb A (2000) Nunn’s Applied Respiratory Physiology (5th edition). Oxford: Butterworth-Heinemann.
Malik K, Hess DC (2002) Evaluating the comatose patient: rapid neurological assessment is key to appropriate management. Postgraduate Medicine 111(2): 38–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11868313 Accessed July 2010.
Marino PL (1998) The ICU Book (2nd edition) London: Lippincott, Wiilliams and Wilkins.
McKelvie S (1998) Endotracheal suctioning Nursing in Critical Care 3(5):244–248.
Medicines and Healthcare products Regulatory Agency (2000) Continuous positive airway pressure circuits: risk of misassembly. Available from: http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/Safetynotices/CON008853 Accessed July 2010.
Moore T, Woodrow P (2004) High Dependency Nursing Care: Observation, intervention and support. London: Routledge.
Muench E, Bauhuf C, Roth H et al. (2005) Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Critical Care Medicine 33(10):2367–2372.
Myburgh JA (2003) Severe head injury. In: Bernsten, A and Soni, N (eds) Oh’s Intensive Care Manual (5th edition). Oxford: Butterworth Heinemann.
O’Neal P, Grap M, Thompson C et al. (2001) Level of dyspnoea experienced in mechanically ventilated adults with or without saline instillation. Intensive and Critical Care 17:356–363.
Patroniti N, Foti G, Mangio A et al. (2003) Head helmet versus face mask for non-invasive continuous positive airway pressure: physiological study. Intensive Care Medicine 29:1680–1687.
Porth C (2004) Pathophysiology: Concepts of altered health states (7th edition). London: Lippincott, Williams and Wilkins.
Robson WP (1998) To bag or not to bag? Manual hyperinflation in intensive care. Intensive and Critical Care Nursing 14(5):239–243.
Ropper A, Gress DR, Diringer MN et al. (2003) Neurological and Neurosurgical Intensive Care. (4th edition). Philadelphia: Lippincott, Williams and Wilkins.
Serra A (2000) Tracheostomy Care. Nursing Standard 14(42):45–55.
St John RE (1999) Protocols for practice: applying research at the bedside. Critical Care Nurse 19(4):79–83.
Thompson L, Morton R, Cuthbertson S et al. (2000) Tracheal suctioning of adults with an artificial airway. Best Practice 4(4):1–6.
Tully V (2002) Non-invasive ventilation: a guide for nursing staff. Nursing in Critical Care 7(6):296–299.
Wainwright SP, Gould D (1996) Endotracheal suctioning in adults with severe head injury. Literature review. Intensive Critical Care Nurse 12:303–308.
Wong F (2000) Prevention of secondary brain injury. Critical Care Nurse 20(5):18–27.
Wood CJ (1998) Endotracheal suctioning: a literature review. Intensive and Critical Care Nursing 14(3):124–136.