22
Management of Patients with Stroke and Transient Ischaemic Attack
INTRODUCTION
Stroke is a preventable and treatable disease, and has only recently been perceived to be a high priority within the NHS (RCP, 2008). The publication of the National Stroke Strategy (DH, 2007) has led to a national drive for the prevention of stroke, and major improvements in both acute and long-term stroke care in the United Kingdom (UK). This strategy is to be implemented over the next ten years to address health inequalities and secure stroke service improvements in line with evidence-based quality markers and the recently published clinical guidelines (RCP, 2008; NICE, 2008).
DEFINITIONS
Stroke is defined as:
…a clinical syndrome of rapidly developing clinical signs of focal (or global in case of coma) disturbance of cerebral function, with symptoms lasting twenty-four hours or longer or leading to death with no apparent cause other than of vascular origin.
World Health Organisation (1988)
A transient ischaemic attack (TIA) is defined as signs and symptoms of stroke which resolve within 24 hours, although TIA symptoms normally resolve in a few minutes or hours. If neurological symptoms persist for longer it should be assumed that the person has had a stroke (RCP, 2008). The term brain attack has also been used to describe a neurovascular event, which emphasises the level of urgency with which stroke and TIA should be treated (NAO, 2005; RCP, 2008).
EPIDEMIOLOGY
Stroke is the third most common cause of death worldwide after coronary heart disease and all types of cancer combined (Warlow et al., 2008). It also causes a greater range of disabilities than any other condition and is the leading cause of long-term disability in the developed world (Adamson et al., 2004). Two-thirds of stroke deaths occur in developing countries and, as a result of an increase in the proportion of older people and the predicted effects of current smoking patterns in developing countries, it is expected that stroke mortality will have almost doubled by 2020 (Warlow et al., 2008).
Incidence
Each year approximately 130,000 people in England and Wales will have a stroke of which 87,700 are first strokes and 53,700 recurrent strokes (Office of National Statistics, 2001). This is roughly the equivalent of someone having a stroke every five minutes. Nearly three quarters of cases occur in people over the age of 65 and half in those over the age of 75. If they live to their 85th year, one in four men and one in five women can expect to have a stroke (Sudlow and Warlow, 1997). The incidence of TIAs is more difficult to establish, since they may not be reported due to their sometimes fleeting nature. The number of TIAs occurring each year in England alone is estimated to be 20,000 (NAO, 2005).
Mortality
Stroke causes over 60,000 deaths each year in the UK and in 2004 stroke caused 8% of deaths in men and 12% of deaths in women (Allender et al., 2006). Approximately two-thirds of deaths occur within the first week after a stroke, most commonly due to neurological consequences, e.g. raised ICP and acute obstructive hydrocephalus. For older patients about half of all deaths are due to the complications of immobility, e.g. pneumonia or pulmonary embolism, usually after the first week (Bamford et al., 1990).
In recent years stroke mortality and incidence has declined at varying rates across Japan, North America and Western Europe (Thorvaldsen et al., 1999), but the proportion of deaths from stroke has remained constant. Reasons for this international fall in stroke mortality rate remain unclear although they could include advances in detection by neuro-imaging or improvements in management (Thorvaldsen et al., 1999). The Oxfordshire Community Stroke Project (OCSP, 1981–1986) (Bamford et al., 1990) indicated a higher mortality rate for primary intracerebral haemorrhage (PICH). The overall 30-day case fatality rate was 19% (10% infarction: 50% PICH); the 1-year case fatality rate was 31% (23% infarction: 62% PICH).
Burden of stroke
At one year after stroke approximately 30% of patients will be dead, and 40% of survivors will be dependent on others (Warlow et al., 2008). In the UK it has been estimated that one in five acute hospital beds and a quarter of places in residential or long-term care are occupied by patients with stroke. The costs of direct care are £2.8 billion a year; £1.8 billion is lost from reduced productivity and disability, while informal care costs £2.4 billion – a total of £7 billion a year (DH, 2007). Patients who survive a stroke or transient ischaemic attack (TIA) are at particularly high risk of subsequent cardiovascular events, including recurrent stroke, myocardial infarction (MI) and death from vascular causes (Hankam and Spence, 2007); survivors are also more likely to be left with a major disability (Rothwell, 2007).
AETIOLOGY
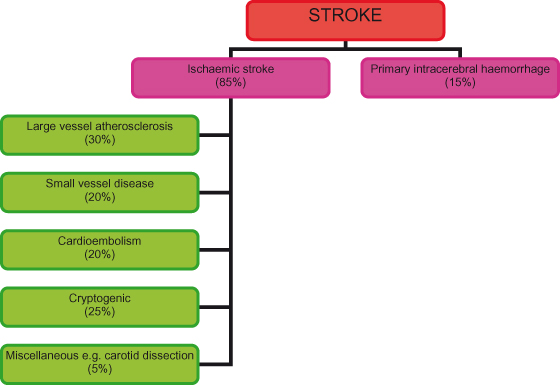
About 85% of strokes result from ischaemia and infarction of brain tissue caused by diminished blood flow due to thrombotic or embolic complications of atheroma. Risk factors for ischaemic stroke are detailed in Table 22.1. The remaining 15% of strokes are caused by a primary intracerebral haemorrhage (PICH) (Figure 22.1).
Table 22.1 Risk factors for ischaemic stroke.
| Risk factors | Relative risk |
| Modifiable | |
| Hypertension
Diabetes mellitus Atrial fibrillation Smoking Alcohol (> 30 units/week) Physical inactivity Obesity Hyperhomocysteinaemia Life events (malnutrition) Serum albumin <42 g/l | 2.3
2–3 5 2 2.5–4 0.3–0.5 1–2 5–7 2 0.6 |
| Non-modifiable | |
| Age (per decade)
Male sex Ischaemic heart disease Social class Peripheral vascular disease Previous stroke Previous TIA Ischaemic heart disease Heart failure Family history Ethnicity | 2.2
1.4 2.5 1.6–3.5 2 9–15 7 2.5 2.5–4.4 1.4–2 (see text) |
Adapted from MacWalter and Shirley, 2003.
Figure 22.1 Stroke aetiology.
Ischaemia
Ischaemia is a term used to refer to a reduction in blood supply, whereby there is a mismatch between blood flow and the requirements of the cerebral tissue for substrates to maintain normal cellular functions. Infarction refers to irreversible damage and death of tissue (necrosis) caused by ischaemia.
Atherosclerosis
Atherosclerosis is the most common cause of ischaemic stroke. It is believed that atheroma begins to develop as a result of an inflammatory response, leading to the gradual deposit of lipid compounds within the arterial wall. These may fibrose leading to the formation of plaque. This process is accelerated by factors such as hypertension, diabetes, cigarette smoking and hyperlipidaemia and the arterial walls may become necrotic, ulcerated or calcified. Atherothrombotic plaque may build up within the arterial lumen causing a critical stenosis. The development of atheromatous plaque may also excite platelet activity thus causing thrombus to develop within the arterial wall. Atherothrombotic material may then fragment (embolise) leading to arterial occlusion within the brain. The most common sites for build up of plaque affecting the cerebral circulation are at places of arterial branching, e.g. the carotid bifurcation and the vertebral arteries. Extracranially, the aortic arch and carotid arteries are the most common sites.
Cardio-embolic stroke
Cardiogenic emboli are the second most common cause of ischaemic stroke. Single or multiple emboli may arise from the heart, most commonly as a result of atrial fibrillation (AF) or damage to the myocardium (e.g. post MI). About 20% of all ischaemic strokes are embolic (Warlow et al., 2008). Symptoms vary depending on the site (or sites) of occlusion; blockage of a major vessel such as the internal carotid artery may cause a total anterior circulation infarct whereas microscopic particles may cause a TIA. Most commonly the route of the embolus is via the extracranial carotid arteries but emboli may also occlude branches of either the vertebral or basilar arteries. The clinical priority is to find the possible source of the embolism, for example: recent myocardial infarction, valvular disease (or prosthetic valves), infective endocarditis, or patent foramen ovale (PFO).
Intracranial small vessel disease (SVD)
This is a condition caused by widespread changes in the small arteries and arterioles of the brain predominantly in older age groups and usually in the presence of diabetes and hypertension; changes occur within the vessel walls, which thicken and become less elastic. Lacunar infarcts make up about 20% of ischaemic strokes and are thought to be caused by SVD although debate continues (Warlow et al., 2008). Vasculitis and cerebral amyloid angiopathy are rare causes of SVD. Other rare causes of ischaemic stroke are detailed in Box 22.1.
Box 22.1 Rare causes of ischaemic stroke
- Head injury (see Chapter 32) and neck trauma
- Arterial dissection
- Connective tissue or inflammatory disorders, e.g. systemic lupus erythematosus (SLE), vasculitis
- Migraine associated vasospasm
- Haematological disorders, e.g. polycythaemia, sickle cell disease, antiphospholipid syndrome
- Infection, e.g. tuberculosis, syphilis, human immunodeficiency virus (HIV)
- Damage to intra or extracranial arteries following cancer, irradiation and chemotherapy
- Substance abuse, e.g. cocaine, ecstasy
- Perioperative stroke particularly during cardiac or vascular procedures
- Rare genetic conditions, e.g. Fabry’s disease, homocystinuria, and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)
- Oral contraceptives, hormone replacement therapy (HRT), pregnancy
RISK FACTORS
Age
Although stroke can occur in children or young adults, it is predominantly a disease of older people. The Framingham study (Wolf et al., 1992) indicated that the incidence of stroke increases dramatically with age, approximately doubling in successive decades.
Ethnicity
Although it is widely accepted that the rates of cardiovascular disease and stroke mortality and morbidity are higher in black and minority ethnic groups, the causes of this are not fully understood and these groups have been under-represented in research (Lip et al., 2007). Socio-economic factors may exert an influence.
Hypertension
Chronic hypertension is the greatest modifiable risk factor for both ischaemic and haemorrhagic stroke independent of age or gender (Warlow et al., 2008). Risk increases with the degree of hypertension and risk factors may also be inter-related, e.g. blood pressure is affected by diet and alcohol. Traditionally greater significance has been attributed to the diastolic rather than the systolic blood pressure, but the opposite is probably true (Wolf, 2003). Prolonged hypertension causes structural changes in the vessel walls such as arteriolar thickening, fibroid necrosis, formation of aneurysms and the build up of atheroma in the large vessel walls (Poulter et al., 2000).
Cholesterol
Recently the Stroke Prevention with Aggressive Reduction of Cholesterol Levels (SPARCL) trial has demonstrated the relationship between serum cholesterol levels and stroke and has shown that statins reduce risk of stroke and overall cardiovascular risk (Amarenco et al., 2006).
Causes of primary intracerebral haemorrhage (PICH)
The main cause of PICH is hypertension. Aneurysms and arteriovenous malformations are less common causes and are presented in Chapter 23. Table 22.2 lists other causes of PICH.
Table 22.2 Causes of primary intracerebral haemorrhage.

PATHOPHYSIOLOGY
Ischaemic stroke
The critical level for ischaemia to develop is when cerebral blood flow falls below 18 ml/100 g/min; lethal levels are below 10 ml/100 g/min (Dirnagl and Pulsinelli, 1990). When CBF falls below 15 ml/100 g per minute, electrical activity of the affected neurones ceases due to insufficient energy substrates being delivered to fuel the sodium–potassium adenosine triphophate (Na+/K+) pumps (see Chapter 1) but structural integrity is retained. If reperfusion occurs quickly, it is possible for the cells in the affected zone to recover (Brodal, 2004). This zone has been termed the ischaemic penumbra (Touzani et al., 2001), and is derived from the Latin paene ‘almost’ + umbra ‘shadow’. The penumbra region may recover completely if good cerebral blood flow is re-established, e.g. following thrombolysis. This highlights the importance of rapid recognition and treatment’’ (NAO, 2005). It remains unclear how long the window for therapeutic intervention may be; it may be as little as three hours. If reperfusion does not occur the affected area will infarct.
In addition to vascular occlusion (thrombotic or embolic) ischaemia can be caused by prolonged or significant hypotension. The elderly are particularly vulnerable as with increasing age autoregulation becomes less effective. The resultant reduction in cerebral blood flow usually causes hypoperfusion in areas between two major cerebral arteries and these events are termed ‘watershed infarcts’.
Symptoms of neurological dysfunction arise from the specific area of the brain which has been affected. The most widely accepted method of stroke classification is the Oxford Stroke Classification, also known as the Bamford classification (Bamford et al., 1991). With this system strokes are divided into four categories (Box 22.2). This system of classification is relatively straightforward and uses clinical information to determine underlying pathology. Examples of clinical signs for each classification are shown in Table 22.3
Box 22.2 Classification of stroke
TAC – Total anterior circulation stroke
LAC – Lacunar stroke
PAC – Partial anterior circulation stroke
POC – Posterior circulation stroke
An additional code is added as the last letter:
(S) – Syndrome: uncertain pathogenesis prior to imaging
(I) – Infarct (e.g. TACI)
(H) – Haemorrhage (e.g. TACH)
Table 22.3 Clinical signs for stroke classification.
| Stroke classification | Clinical signs | |
| TACI (Total anterior circulation stroke – infarct) | All of these symptoms:
| |
| PACI (Partial anterior circulation stroke – infarct) | Either: Two out of three signs as TACI:
Or: Cortical dysfunction alone Or: Mild motor/sensory deficit | |
| POCI (Posterior circulation stroke – infarct) | Any of the following features:
| |
| LACI (Lacunar stroke – infarct) | Any one of the following:
| None of these:
|
Haemorrhagic stroke
The small penetrating end-vessels (perforators) that supply the deep grey and white matter of the basal ganglia, internal capsule, thalamus, cerebellum and brain stem are most affected by hypertension. Chronically raised blood pressure causes degenerative changes in these vessels which may eventually rupture causing subsequent haemorrhage. Bleeding into the brain tissue results in a mass of blood being formed which raises intracranial pressure and can cause significant brain damage. Intracranial pressure is discussed in Chapter 7.
Venous stroke
Venous stroke is a rare disorder resulting from thrombosis of the venous sinuses or intracerebral veins. The most common location for thrombosis is the superior sagittal sinus (70–80% of cases). Venous stroke accounts for only 0.5% of all strokes; it can affect any age group and has often been associated with poor outcomes. Morbidity is estimated at 15–25%, with mortality at 10–50% (Baker et al., 2001). The severity of the symptoms at outset determines the outcome; the more severe cases are nearly always fatal. The most common presenting symptoms are headache, nausea, focal neurological deficits, seizures, altered consciousness or papilloedema.
The patient must be monitored closely for signs of rising intracranial pressure (see Chapter 7). Diagnosis is confirmed using MRI with MR venography or CT with CT venography. Imaging may reveal a variety of lesions including haemorrhages, oedema and infarctions. Medical management may appear contradictory since the presence of haemorrhage does not preclude treatment with anticoagulation therapy; in fact evidence supports the use of early anticoagulant therapy, with a reduction in death and dependency (RCP, 2008).
CLINICAL SIGNS AND SYMPTOMS OF STROKE
The clinical features of stroke (Box 22.3) depend on the location and extent of brain damage; no two patients will display identical symptoms. Differentiation between haemorrhagic and ischaemic stroke on clinical signs alone is not straightforward. Urgent CT scanning is necessary to determine a management plan, particularly if thrombolysis is to be considered, but also with a view to the prescription of anti-platelet medication where there may be risk of further haemorrhage.
Box 22.3 Signs and symptoms of stroke
- Motor deficits: facial weakness, focal limb weakness, loss of fine finger movement
- Altered sensation: numbness, tingling
- Disturbance of conscious level
- Acute memory impairment
- Altered higher cerebral function: orientation
- Disorders of speech and language
- Visuo-spatial dysfunction: neglect, inattention
- Apraxia (loss of the ability to perform learned movements)
- Visual disturbance: diplopia, homonymous hemianopia, loss of vision
- Disturbance of hearing
- Loss of balance, vertigo
- Ataxia: poor co-ordination
- Nausea, vomiting
- Headache
DIAGNOSIS
Immediate clinical assessment and treatment are vital to optimise outcomes. Outside hospital, the use of a simple reminder of the symptoms of stroke such as the FAST test (Facial weakness, Arm weakness, Speech disturbance, Time to call 999) is advocated as a method of rapidly screening patients for stroke or TIA (DH, 2007). For people who are admitted to accident and emergency it is recommended that diagnosis is made using a detailed validated tool such as Recognition of Stroke in the Emergency Room (ROSIER) (RCP, 2008). However it should be noted that some strokes (e.g. posterior circulation) may not be picked up using these tools. Patients suspected of having had a stroke should be assessed by either a neurologist or a physician with a special interest in stroke to establish an accurate clinical diagnosis and the best course of treatment as rapidly as possible (RCP, 2008). Other differential diagnoses that may present with focal cerebral symptoms of sudden onset (Box 22.4) must be excluded.
Box 22.4 Conditions which may mimic stroke
- Migraine
- Epilepsy
- Structural intracranial lesions, e.g. brain tumour
- Central nervous system infections
- Multiple sclerosis
- Metabolic and toxic disorders
- Transient global amnesia
- Benign postural vertigo and other labyrinthine disorders
- Psychological disorders
- Neuromuscular disorders
Diagnostic tests
Computerised tomography (CT) scan
All patients displaying symptoms of a stroke should be scanned as soon as possible within 24 hours of initial onset of the event. When clinically indicated, urgent scans must be available within one hour of admission, e.g. indications for thrombolysis, severe headache, or patients on anticoagulation therapy (RCP, 2008). CT is the most accurate method of demonstrating cerebral haemorrhage within the first week and it is identifiable almost immediately. It is possible to detect early ischaemia on CT within two hours of stroke, although usually signs of infarction develop over the first one to seven days (Figure 22.2). A normal scan does not mean that the patient has not had a stroke. CT may also be used to detect subarachnoid blood (see Chapter 23) and to identify other neurological disease which may be confused with stroke.
Figure 22.2 Unenhanced CT brain scan 4 h after stroke onset (a,b). There is hypoattenuation of the right caudate and lentiform nuclei, insular and temporal cortex (arrowheads) and a hyperattenuated (blocked) sylvian branch of the right middle cerebral artery (MCA) (arrow). (c) Follow-up scan at 48 h shows an extensive, hypoattenuated, and swollen right hemisphere infarct involving most of the MCA territory and also the territory of the right anterior cerebral artery (arrow) (ACA). The ACA involvement is probably because of compression of the ACA by the infarct mass effect.
Reproduced from Charles P. Warlow et al., Stroke: Practical Management 3e, Wiley-Blackwell, with permission.

Magnetic resonance imaging (MRI)
About 50% of infarcts never become visible on CT, therefore a scan using diffusion weighted magnetic resonance imaging may be indicated to detect small infarcts, particularly lesions occurring in the brain stem. Recent guidelines (NICE, 2008) recommend MRI for all patients following TIA.
Electrocardiogram (ECG)
This should be carried out for all patients following a stroke. Cardiac arrhythmias are a cause of cardio-embolic stroke and should be treated; ventricular hypertrophy or cardiomyopathy may indicate previously undiagnosed chronic hypertension. Acute myocardial infarction (with or without pain: the so-called ‘silent’ infarct) may occur at onset of stroke and remain otherwise undetected.
Echocardiography is used to screen for cardiogenic causes of emboli, e.g. thrombus, vegetation or patent foramen ovale (PFO).
Ultrasound studies
Doppler or duplex sonography of the major extracranial arteries (particularly the carotid arteries) may be used to identify stenosis or occlusion which may require surgical intervention, e.g. carotid endarterectomy.
Laboratory tests
Blood is screened for haematological and clotting abnormalities, inflammatory and infection markers and electrolytes; renal and hepatic chemistry and vitamin deficiency will be checked. Most underlying haematological disorders will be identified through routine investigations, but other more rare conditions may require specific screening, e.g. anti-phospholipid syndrome (APS) (Woodward, 2007).Younger female patients with symptoms of stroke or TIA should be screened for APS by checking for the presence of anticardiolipin antibodies and lupus anticoagulant, particularly if there is a history of recurrent miscarriages or migraine.
Transient ischaemic attack (TIA)
Early identification and diagnosis of TIA is imperative since effective intervention may dramatically reduce the risk of completed stroke (RCP, 2008). Risk of subsequent stroke should be assessed using a validated scoring system such as the ABCD2 score (Johnston et al., 2007) (Box 22.5). People with crescendo TIAs (two or more in a week) should be treated as high risk whatever their ABCD2 score.
Box 22.5 ABCD2 score: presenting within 7 days of symptoms
| Score | |
| Age ≥60 years
Blood pressure systolic > 140 or diastolic ≥ 90 Unilateral weakness Speech disturbance (no weakness) Duration ≥60 minutes Duration 10–59 minutes Diabetes | 1
1 2 1 2 1 1 |
Patients with a score ≥4 must be assessed and investigated within 24 hours of symptom onset. Those at lower risk, with an ABCD2 score ≤3, should receive specialist assessment and treatment within seven days.
Source: Royal College of Physicians, 2008.
MEDICAL MANAGEMENT OF STROKE AND TIA
The aims of medical treatment following stroke and TIA are to optimise survival, prevent recurrent stroke or other serious vascular events and to prevent complications, such as aspiration pneumonia.
In addition to the management of neurological symptoms, the medical management of patients following stroke includes cardiac and respiratory care, metabolic and fluid management and blood pressure control.
Thrombolysis and hyperacute management
Thrombolysis with alteplase is the recommended treatment for acute ischaemic stroke (NICE, 2008). It has been shown to significantly improve outcomes in patients meeting the criteria for administration within three hours of onset of symptoms (Wardlaw et al., 2003). However the national guidelines (RCP, 2008) recommend that it should only be administered in centres which have sufficient infrastructure to provide a well-organised stroke service, namely: staff specifically trained in the delivery of thrombolysis and in monitoring for associated complications, nursing staff with specialist training in stroke and thrombolysis, immediate access to imaging and staff trained to provide accurate interpretation of images, and access to neurosurgery for the treatment of raised ICP.
Secondary prevention of stroke and TIA
Patients who have suffered a stroke remain at a 30–40% increased risk of a further stroke within five years. Measures for secondary prevention should be commenced as soon as a diagnosis of stroke or TIA is confirmed. Patients found to have causal factors such as carotid stenosis may benefit from early interventions such as carotid endarterectomy and those found to have a cardiac cause may require anticoagulation to reduce their risk of having a large completed stroke.
Antiplatelet medications
The national guidelines (RCP, 2008) recommend the introduction of aspirin 300 mg daily for the first two weeks after an ischaemic stroke unless contra-indicated. Discussion of individual risk factors should be incorporated into the plan of care. Patients who suffer from aspirin intolerance or dyspepsia may be prescribed an alternative antiplatelet agent such as clopidogrel 75 mg or a proton pump inhibitor such as omeprazole in addition to aspirin. Aspirin is not effective in preventing stroke or TIA in those who have no history of such events.
Anticoagulation
The national guidelines (RCP, 2008) recommend delaying the consideration of anticoagulation for patients following disabling ischaemic stroke in the context of atrial fibrillation for up to two weeks due to the risk of haemorrhagic transformation.
Management of hypertension
Numerous treatment trials have demonstrated that the risk of stroke is substantially reduced when blood pressure is lowered (RCP, 2008). Following a TIA or stroke, hypertension should be tightly controlled using anti-hypertensive medications such as diuretics, ACE inhibitors and calcium channel blockers. Blood pressure targets should be set at 140/80 mmHg or less in this patient population (Williams et al., 2004).
Statin treatment
Recommended targets for cholesterol levels in this patient population are <3.5 mmol (RCP, 2004). Simvastatin, 40 mg, is the initial drug of choice, however recent evidence suggests that statin treatment may increase the risk of haemorrhagic extension or transformation in the acute phase and therefore should not be prescribed either in the hyperacute stage or for haemorrhagic stroke (RCP, 2008).
Lifestyle modification
Evidence suggests that for every 43 patients provided with advice on smoking cessation, one stroke per year would be prevented (Hankey and Warlow, 1999). Reducing alcohol intake to less than two units per day is recommended since excess alcohol contributes to hypertension (Williams et al., 2004). Health promoting behaviours such as exercise, weight loss and the introduction of a healthy diet (five portions of fruit and vegetables per day) should also be encouraged. Patient’s compliance with changes to their lifestyle will require ongoing advice, monitoring, encouragement and reinforcement (Birns and Fitzpatrick, 2005).
Diabetes mellitus
Diabetes is an independent risk factor for stroke or TIA, and hyperglycaemia is also a risk factor for death and increased disability. No hard evidence has been found from limited studies in this area but the consensus of opinion is that the recommended treatment target for blood glucose concentration for patients following acute stroke is between 4.0 and 11 mmol/l (RCP, 2008).
SURGICAL MANAGEMENT OF STROKE AND TIA
Carotid endarterectomy
Carotid endarterectomy (CEA) is a surgical procedure used to clear plaque from a blocked artery and is usually carried out under local anaesthetic. CEA is the treatment of choice for patients with symptomatic carotid stenosis of 70–99% according to the European Carotid Surgery Trial (ECST) criteria (Rothwell et al., 2003) or 50–99% according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (Rothwell et al., 2004). Carotid angioplasty or stenting is sometimes used as an alternative to CEA, although there is limited research to compare the efficacy and safety of stenting to CEA (RCP, 2008).
Decompressive craniectomy
Malignant middle cerebral artery infarction is a rare, life-threatening complication of stroke where space occupying brain oedema presents within two to five days of stroke onset. This usually occurs in younger people without brain atrophy. It is essential that this is treated rapidly before damage becomes irreversible; patients should be referred within 24 hours of onset and treated within a maximum of 48 hours (RCP, 2008). Decompressive craniectomy is discussed in Chapter 20 and Chapter 7.
Surgical treatment of acute intracerebral haemorrhage
There is little evidence to establish the efficacy of surgical evacuation of haematoma at the acute stage. Patients should be monitored for the development of symptomatic hydrocephalus in specialist neuroscience or stroke units. The current recommendations state that previously fit patients should be considered for neurosurgical intervention if they have hydrocephalus (RCP, 2008).
EVIDENCE-BASED NURSING MANAGEMENT
A holistic and multiprofessional approach
The National Service Framework for Older People (DH, 2001 p65) stipulated that all patients should be treated by ‘specialist stroke teams within designated stroke units’. This was reinforced by the National Clinical Guidelines for Stroke (RCP, 2008). A collaborative multidisciplinary or interdisciplinary team approach to stroke care is considered essential, as no single profession is able to manage the care of the patient following a stroke. A collaborative approach facilitates an individualised treatment programme, with members of the team working closely together to assess and identify the needs of patients and their families throughout the stroke illness trajectory.
Nurses are integral to the stroke multidisciplinary team (Figure 22.3) and the range of knowledge, skills and competencies required for effective stroke nursing is continually expanding (McMahon et al., 2003). Health care policy and professional developments in stroke care have added impetus to the growth in specialist stroke nursing roles. Indeed, the increasingly specialised nature of stroke nursing has been recognised in the foundation of the National Stroke Nursing Forum (NSNF); one of its aims being to identify and develop the knowledge and skills required by nurses working in stroke services (McMahon et al., 2003; Perry et al., 2004).
Figure 22.3 The model of care or rehabilitation that can be adopted on a geographically defined stroke unit, in which each member of the multidisciplinary team influences the nursing input to the patients and carers, as well as having direct interaction with them.
Reproduced from Charles P. Warlow et al., Stroke: Practical Management 3e, Wiley-Blackwell, with permission.
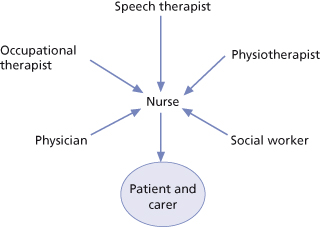
A key attribute of stroke nursing is its holistic approach and many nursing interventions are informed by other members of the multiprofessional stroke team, such as physiotherapists, occupational therapists and speech and language therapists. This is evident in the aspects of stroke nursing which have received most research attention and which represent crucial elements of stroke management that require a skilled, consistent approach across the multiprofessional team; for example, patient positioning and mobilising, swallowing assessment and nutritional management, and an awareness of cognitive and perceptual problems. Stroke nurses are increasingly involved in identifying stroke and TIA, undertaking acute assessments of eligibility for thrombolysis on admission to hospital and fast-tracking patients into an acute stroke bed. By initiating investigations and treatment without having to wait for medical staff, nurses can positively influence patient outcomes. Other key elements of the stroke nurse’s role include physiological monitoring, pain management, facilitating rehabilitation through emotional support, motivation and education, and the support of families. In addition, end of life care has been recognised as a new and challenging aspect of stroke care. Each of these elements is addressed here, with reference to the current evidence base.
Monitoring the acutely ill stroke patient
Physiological monitoring of the acutely ill stroke patient is vital in order to reduce the risk of death and disability (Jones et al., 2007). As most deaths following a stroke arise from complications associated with the clinical symptoms, continuous physiological monitoring is a priority in at least the first 24 hours. Nurse-led physiological monitoring can ensure the early detection of complications, resulting in faster interventions and improved stroke survival (Jones et al., 2007).
Particularly dangerous neurological sequelae of stroke are:
- Raised intracranial pressure (see Chapter 7)
- Cerebral oedema
- Hydrocephalus (see Chapter 25)
Serial neurological assessment is therefore vital in the first hours and days following stroke (see Chapters 7 and 8).
Blood pressure
In acute stroke, autoregulation is impaired so that blood flow to the ischaemic brain becomes dependent on systemic blood pressure. A rapid fall may result in decreased blood flow to an already underperfused brain (Hyde and Dowell, 2002). High blood pressure could, therefore, play a major role in maintaining blood flow in the acute stroke patient so it should not be pharmacologically treated. Only in hypertensive emergencies involving cerebral oedema or cerebral haemorrhage, or if the patient is a candidate for thrombolysis, should a reduction of blood pressure to 185/110 mmHg or lower be effected (RCP, 2008).
Respiratory
Hypoxia will exacerbate ischaemic brain damage so it is crucial to monitor respiratory function. Current recommendations suggest that oxygen saturation levels should be maintained above 95%, either by sitting the patient up as soon as their condition permits (RCP, 2008), or by oxygen therapy. Oxygen therapy should only be administered if blood saturation levels drop below 95% (RCP, 2008).
Respiratory assessment and management is presented in Chapter 15.
Blood glucose
Between 10 and 20% of acute stroke patients are hyperglycaemic on admission and high blood glucose levels can increase neuronal damage within the ischaemic penumbra; potentially impacting on stroke outcome. Blood glucose should be maintained within normal limits (RCP, 2008) and close monitoring in the acute phase is required, to detect changes in plasma glucose concentrations (Jones et al., 2007).
Body temperature
Pyrexia following stroke is common, due either to infection or the effect of the stroke on central temperature regulation. As a strong link has been established between pyrexia and poorer stroke outcomes, temperature monitoring and treatment with antipyretic medication are recommended (Jones et al., 2007; RCP, 2008).
In addition to physiological monitoring, nurses also undertake specific risk assessments relating to: moving and handling, nutrition, pressure sores, falls and deep vein thrombosis (DVT) (Cross, 2008). However, the acutely ill stroke patient requires assessment by all members of the stroke team who can also intervene to prevent complications and promote recovery.
STROKE REHABILITATION
Preventing complications and promoting recovery
Early mobilisation
Although the exact association between positioning and neurological recovery has yet to be established, correct positioning of a patient following stroke (see Figure 22.4) is important in preventing potential complications arising from impaired movement. These include skin breakdown, muscle spasticity, urinary tract infections, chest infections, mobility-related falls, shoulder pain and deep vein thrombosis (RCP, 2004; Arias and Smith, 2007) (see also Chapters 8 and 11).
Figure 22.4 The typical chart used to guide the positioning of stroke patients with hemiplegia (shown in black) whilst in bed.
Reproduced from Charles P. Warlow et al., Stroke: Practical Management 3e, Wiley-Blackwell, with permission.

Nurses’ ability to position patients correctly following a stroke is essential and improvements in nursing practice have been achieved through a multiprofessional, collaborative stroke training programme reported by Dowswell et al. (1999) and Forster et al. (1999a,b). However, this was tempered by the fact that there were no significant improvements in patient outcomes and in a larger, multi-site study, Jones et al. (2005) were unable to demonstrate any impact on patient outcomes following a teaching intervention to improve nurses’ positioning of patients following a stroke.
There is no standard definition of what constitutes early mobilisation, but it would appear to include sitting the patient up, being out of bed within 24 hours, being assessed by a physiotherapist between eight and 24 hours after admission, and training in transfer, sitting and walking between 24 and 72 hours (Arias and Smith, 2007). Early mobilisation can be facilitated by appropriately trained nurses or a physiotherapist. Early mobilisation is particularly important in preventing venous thromboembolism (VTE), a common complication for patients with weak or paralysed legs which can result in deep venous thrombosis (DVT), or the most serious consequence of DVT: pulmonary embolism (Harvey, 2003). Although antithrombotic medication should be avoided in patients with haemorrhagic stroke, aspirin and the use of graduated elastic compression stockings (GECS) are recommended in the NICE guidelines (NICE, 2008). More recently published findings from the Clots in Legs Or sTockings after Stroke (CLOTS) trial 1, provide insufficient evidence for the routine use of GECS in patients with acute ischaemic stroke. Findings showed that GECS can even present a greater risk to patients of skin breaks, blisters, ulcers and skin necrosis (CLOTS Trial Collaboration, 2009).
Another potential complication of stroke which can be prevented by careful positioning and early mobilisation is spasticity. It has been estimated to occur in 19% of patients and, although the clinical presentation will vary between patients, spasticity is associated with pain and interferes with rehabilitation interventions (McCrea et al., 2008). Accurate assessment and careful management are essential (Jarrett, 2006) (see Chapter 11). Pharmacological interventions with anti-spasmodics are widely used but the evidence base has yet to be established (McCrea et al., 2008). Local botulinum toxin injections do appear to be effective in reducing spasticity, in combination with a range of motion exercises and splinting (RCP, 2004; Ozcakir and Sivrioglu, 2007).
Dysphagia, nutritional management and hydration
Dysphagia occurs in 64–90% of people affected by a stroke and, because all patients with swallowing difficulties are at risk of developing aspiration pneumonia, their swallowing should be screened on admission by a dysphagia trained professional (RCP, 2008). Malnutrition is also a significant risk and is associated with slower recovery and a poorer outcome for the patient (RCP, 2008). All patients should have their nutritional status assessed within 48 hours of admission, using a validated screening method (RCP, 2004) (see Chapter 16). The assessment and management of dysphagia is discussed in Chapter 12.
The nutritional management of a person with dysphagia involves close liaison with both the speech and language therapist (SLT) and dietitian, but nurses are responsible for the patient’s ongoing assessment, weight monitoring, providing assistance for patients who cannot eat and drink unaided and for managing alternative methods of feeding. The maintenance of a patient’s oral hygiene is also important (RCP, 2004), as this is not only essential for the patient’s comfort, but there is also an established link between poor oral hygiene and an increased risk of pneumonia (Brady et al., 2006). Evidence is lacking regarding oral care interventions in hospital (Brady et al., 2006), but adherence to local practice standards should be expected (see also Chapters 8 and 12).
In the event of a patient being unable to swallow safely, particularly in the early days, all nurses will need to be able to pass a nasogastric (NG) tube following local clinical guidelines (Best, 2007), although ensuring that the NG tube remains in place is challenging (Horsburgh et al., 2008) (see Chapter 16). In addition, nurses also need to ensure that medication is administered in the most appropriate form. Crushing tablets and opening capsules to facilitate medication administration via enteral feeding tubes is generally unsafe and liquid preparations should be used whenever possible (Morris, 2005; Wright et al., 2006).
Bowel and bladder management
Between 40 and 60% of patients with moderate to severe stroke experience urinary and faecal incontinence in the initial stages. Detrusor overactivity can result in frequency, urgency and urge urinary incontinence (Thomas et al., 2008). The persistence of these problems can present a significant challenge to carers on discharge from hospital. The quality of the continence care that a person receives following stroke can impact on their overall quality of life, therefore, the management of bowel and bladder problems are essential activities in stroke acute care and rehabilitation. The National Clinical Guideline for Stroke (RCP, 2008) recommend that established assessment and management protocols for urinary and faecal incontinence and constipation should be in place, that these conditions should be actively managed from admission and that all nurses should be able to assess incontinent patients. A structured approach and input from specialist continence nurses are recommended (Thomas et al., 2008).
Enabling a person to regain continence following stroke will need to be individualised but general principles include the avoidance of catheterisation which should only be used after alternative management methods have been considered (RCP, 2004). Early mobilisation and taking a person to the toilet as soon as they are stable can assist a person to regain a sense of control (Nazarko, 2007a) and independence in toileting has been identified as important in avoiding both the need for assistance and the feelings of decreased self-esteem (Clark and Rugg, 2005). Management of bladder and bowel problems is discussed in detail in Chapter 18.
Communication difficulties
Around one-third of people who are affected by a stroke will experience some degree of dysphasia or aphasia (Parr et al., 1997). Dysarthria, caused by damage to the muscles involved in speaking; and dyspraxia, difficulty in co-ordinating the movements required to produce speech, also challenge the communication abilities of people affected. Unfortunately, even in health care settings, people with communication difficulties are often excluded from decision-making, or from social conversation, because health care professionals lack the knowledge and skills to engage them effectively (Knight, 2005). Assessment by a SLT is essential so that appropriate communication strategies can be identified and taught to both staff and relatives (RCP, 2004) (see Chapter 12).
For people who have difficulty understanding and following verbal conversation or written language, a visual format is more easily processed and enables them to control what they wish to communicate (Murphy and Cameron, 2005). A particularly helpful and comprehensive visual aid to communicating essential clinical information has been developed by Cottrell and Davies (2006). Connect, the communication disability network (www.ukconnect.org), offers a range of interactive programmes which are extremely helpful in promoting confidence in the use of ‘total communication’ strategies; particularly as practical sessions are led and evaluated by people with aphasia (Fursland, 2005).
Cognitive, perceptual and sensory deficits
Cognitive and perceptual impairments include hemi-inattention/neglect, memory, attention, praxis, and executive function deficits, which can significantly affect both a person’s ability to engage with rehabilitation following stroke and their longer term recovery. Despite such impairments, patients can still derive significant benefit from participating in rehabilitation (Rabadi et al., 2008). Sensory impairments, particularly visual changes, can also impact on motor and functional recovery (Patel and Taylor, 1999; Nazarko, 2007b). Although no specific strategies have been identified as being more successful than others in the management of these often ‘invisible’ effects of stroke (Bowen and Lincoln, 2007; Nair and Lincoln, 2007), nurses need to be aware of their potential impact on rehabilitation and to incorporate appropriate techniques, wherever possible, into their interventions with patients and their families.
Neglect/inattention
A stroke affecting the right hemisphere (more specifically the parietal lobe) can affect a person’s awareness of the space around them and can cause difficulty with attending to one side of space, usually the left (see also Chapter 11) (Bowen and Lincoln, 2007). This can result in ‘neglect’ of a person’s left arm and a failure to attend to things positioned on the left (RCP, 2004), causing difficulty with tasks such as washing, dressing and eating, which in turn restricts independence (Bowen and Lincoln, 2007). Visual scanning and ‘retraining’ patients to attend to the neglected field by using visual, verbal and tactile cues are described as the mainstay of current therapy (Bowen and Lincoln, 2007). For example a patient may be taught to turn their plate one quarter turn after each mouthful until the complete meal is eaten.
Memory
Memory can be affected in a number of ways following a stroke, such as when learning new information or skills, or remembering and retrieving information. Prospective memory, i.e. remembering to do something in the future, can also be impaired (RCP, 2004). Using techniques which capitalise on preserved ability, or compensatory strategies such as notebooks, diaries and electronic organisers, memory training and participation in rehabilitation, are all recommended in the absence of evidence for specific techniques (RCP, 2008; Nair and Lincoln, 2007).
Attention
Focusing and sustaining attention on a task are essential for many cognitive and motor functions, and impairments can negatively impact on rehabilitation. Careful planning of nursing and therapy sessions to minimise the demands placed on patients with attention deficits, and avoidance of background distractions are recommended (RCP, 2008) (see Chapter 9). Lincoln et al. (2000) suggest that training may improve alertness and sustained attention.
Praxis
Apraxia, or dyspraxia affects a person’s ability to organise the actions required to perform a skilled activity such as dressing (Walker et al., 2003). The effects of dyspraxia can be seen in assessment, when a patient may put on clothes in a disorganised manner: upside-down or back-to-front (Walker and Walker, 2001). How dyspraxia influences a person’s ability to relearn to dress remains unclear, but in aspects of patient care such as washing and dressing there is a clear indication that nurses require awareness and understanding of facilitation techniques to support patients with dyspraxia (Booth et al., 2001; Booth et al., 2005).
Executive function
Frontal lobe damage can result in impairments to executive functioning, specifically affecting a person’s ability to initiate behaviour, such as planning and problem-solving; and the ability to self-monitor social behaviour. Executive function deficits are common in stroke patients and incorporating executive function rehabilitation into patient interventions can provide opportunities for training in problem-solving and information processing (Struder, 2007) (see Chapter 9).
Sensory deficits – visual changes
Around 60% of patients affected by a stroke will experience visual problems and they are more common in those who have had right hemisphere strokes (Nazarko, 2007b). Blurred or double vision can often be remedied with corrective lenses, prisms, or eye patches and nystagmus can be treated pharmacologically (Nazarko, 2007b). However, hemianopia, a sensory impairment resulting in loss of half of the visual field, presents a significant barrier to motor and functional recovery following stroke. As it often co-exists with visuo-spatial neglect, the two conditions can be difficult to distinguish (Patel and Taylor, 1999) (see Chapter 11). Compensatory strategies such as scanning the environment or placing things on the person’s affected side are helpful and spectacles with built-in prisms can also help (Nazarko, 2007b). As many visual problems following stroke can be easily corrected or can improve with interventions, patients would benefit from formal screening for visual problems.
Pain and fatigue
Physical barriers to engagement in rehabilitation following a stroke include pain and fatigue, both of which may be overlooked as symptoms of stroke, but they are common, potentially treatable and need to be addressed (Appelros, 2006). Pain can be a particular problem following a stroke, affecting recovery and rehabilitation, but it is often not recognised and is poorly managed. Two particular causes of pain deserve specific consideration because of the difficulty they present in their effective management; these are hemiplegic shoulder pain and neuropathic (central post-stroke) pain.
Hemiplegic shoulder pain
Hemiplegic shoulder pain, or post-stroke shoulder pain, occurs in at least 30% of patients (RCP, 2004); although another estimate placed it at 70% (Bender and McKenna, 2001). Post-stroke shoulder pain can adversely affect both length of in-patient rehabilitation and overall functional outcome (Pomeroy et al., 2001). Yet, contrary to the established belief that it results from subluxation, the relationship remains unclear and incorrect handling is more likely to be a contributing factor in both its development and exacerbation (RCP, 2004). Because the causes have not been clearly identified, post-stroke shoulder pain is difficult to define (Bender and McKenna, 2001) but four categories of pain have been identified, including joint pain caused by an incorrectly aligned joint, muscle pain, altered sensitivity resulting from CNS damage, and shoulder–hand syndrome (Bender and McKenna, 2001).
Effective interventions and strategies for the prevention and management of post-stroke shoulder pain have not been conclusively established; but strapping the shoulder might reduce the incidence of pain (Figure 22.5). The use of foam supports, correct positioning and education of staff and carers regarding correct handling of the hemiplegic arm are essential (Bender and McKenna, 2001).
Figure 22.5 By supporting the weight of the arm, glenohumeral subluxation can be reduced. This may be achieved when the patient is sitting using an arm support (a) which attaches to the chair or wheelchair or, alternatively, a perspex tray (b) which, because it is transparent, allows the patient to check on the position of their feet. Both are better than pillows which invariably end up on the floor. Several designs of sling (c) are available to reduce subluxation when patients are upright.
Reproduced from Charles P. Warlow et al., Stroke: Practical Management 3e, Wiley-Blackwell, with permission.
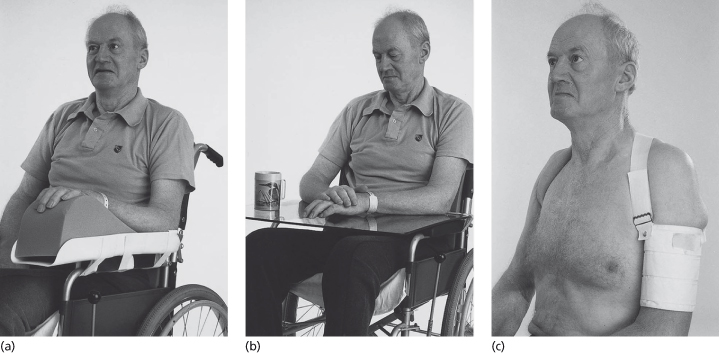
Neuropathic pain
Post-stroke, neuropathic pain is caused by damage to the central or peripheral nervous system, or both. It differs from physiological pain in both its complexity and the approaches to its management (Pasero, 2004). The frequency of neuropathic pain following stroke has been estimated between 5 and 20% (RCP, 2008) and approaches to management involve the use of anticonvulsant or antidepressant medication (see also Chapter 17).
Fatigue
In addition to pain, fatigue can be a common and persistent problem following a stroke and can prevent a person’s full participation in rehabilitation, delay their functional improvement and accentuate their physical and cognitive symptoms (Michael, 2002). Therefore, nurses need to assess the presence and severity of fatigue and to identify the potential causes, such as underlying nutritional or metabolic deficiencies and physiological co-morbidities such as anaemia or cancer. Several fatigue assessment scales are available (Mead et al., 2007) and in both the short and longer terms, developing structured daily routines and strategies to conserve energy can help (Michael, 2002). Strategies for the management of fatigue following stroke are similar to those used with people with multiple sclerosis (see Chapter 28).
Supporting the process of rehabilitation: emotional well-being, motivation and education
Once a person’s condition has stabilised, their needs and those of their carers become essentially educational. Successful adjustment and adaptation depend upon how well they acquire the new knowledge and skills that they need in order to get on with their lives. The challenge for the rehabilitation team is how to help them to learn. However, the suddenness of a stroke can be emotionally overwhelming and a person’s ability to engage in the rehabilitation process, particularly in the early stages, can be inhibited or delayed because emotional and psychological needs may be a greater priority. A person may need time to grieve for the losses incurred by the stroke before they can get on with their physical rehabilitation, and nurses need to be aware of this.
In the context of rehabilitation, readiness to change needs to be assessed, in order to prepare the patient for the work ahead of them and, according to Dalton and Gottlieb (2003), nurses have a key role in assessing and supporting the patient during this transitional phase. This can be achieved by acknowledging the patient’s concerns, listening to their perspective and valuing their opinions about what needs to change. Dalton and Gottlieb (2003) identify a number of factors in a person which may trigger this readiness, including:
- Perceiving that their health concern is not going to resolve
- The change in their physical condition taking on a new significance
- Feeling able to manage their stress
- Having sufficient energy
- Perceiving that they have adequate support in undertaking change
Motivation also plays a vital role in determining patient outcomes (Maclean et al., 2000a). However, the concept of motivation is poorly understood, and labeling patients who fail to engage in a rehabilitation programme as lazy (Maclean et al., 2000b) or apathetic (Resnick, 1998) is not only unhelpful but also fails to recognise the role that professionals play in supporting patients through what is often a long and difficult process. Indeed, it has been suggested that motivation is more to do with the way in which a patient evaluates their chances of successful rehabilitation and that this is influenced by social or external factors; suggesting that there are factors which can positively or negatively affect a person’s motivation. Strategies that are likely to enhance motivation include setting clear and revisable goals, making the patient feel that their views are valid and welcome, and acceptance of the patient’s idiosyncrasies and avoiding clashing with the patient’s value system.
In addition, a warm, approachable and competent manner are valuable and reminding the patient that goals exist beyond the ward setting (Maclean and Pound, 2000). It is most important to avoid placing the responsibility for motivation and recovery solely on the individual patient. Nurses play a key role in identifying barriers to rehabilitation and enhancing the patient’s readiness to learn.
Education
The provision of information and patient and carer education are essential in improving knowledge of stroke, enhancing satisfaction with services and reducing emotional distress (Smith et al., 2008), and the content, timing and method of presentation are all important (Wachters-Kaufmann et al., 2005). Garrett and Cowdell (2005) identified that initially, the focus for information may be on understanding the diagnosis, the results of investigations and likelihood of recovery. Whilst medical staff are normally approached, information about the control of symptoms is sought from nurses and nurses can assist in identifying and accessing the most appropriate sources of information (Garrett and Cowdell, 2005). Over time, information needs become more diverse and wide ranging, and include reducing the chance of another stoke, and longer-term issues such as financial matters and social services.
The means by which information is provided also requires careful consideration and it should be made available in a range of formats and languages. Verbal information should be supported by written sources, although the readability and suitability of written information should not be assumed (Hoffmann et al., 2004; Tooth and Hoffmann, 2004). Pictorial formats, models, videos, audiotapes and web-based sources of information can be provided to accommodate individual learning needs and abilities, and education strategies should aim to actively involve patients and carers (Garrett and Coldwell, 2005).
End of life care
Although improvements in early treatment and rehabilitation have significantly reduced the numbers of people who die following a stroke, it is estimated that around 20% of people affected will die within one month of the event (Rogers and Addington-Hall, 2005). Many of these deaths will occur in hospital, including acute stroke wards and stroke rehabilitation units, where the needs of dying patients may not be fully acknowledged. In recognition of this, the National Clinical Guidelines for Stroke (RCP, 2008) recommend staff training in the principles and practice of palliative care and access to specialist palliative care expertise. The development of a model of service interaction, relating neurology, rehabilitation and palliative care (neuropalliative rehabilitation), should present opportunities to enable nurses to successfully embed palliative care principles into stroke care (Sutton, 2008).
Transfer of care and long-term management
Whilst the roles of stroke nurse specialists tend to focus on acute stroke services, the consultant and coordinator roles are more diffuse, often working across the stroke care continuum, from primary prevention to long-term care. Once the acute recovery phase has passed, continuing rehabilitation and longer term management are essential. The role of family caregivers should be considered from the outset and their needs for support must not be neglected (RCP, 2008).
The psychosocial effects of stroke
Personal accounts of stroke highlight the intense emotional distress experienced and the ensuing struggle to cope during the period of rehabilitation and beyond and it is not surprising that mood disorders following stroke are common.
Emotionalism
Although mood disturbances following stroke have been generally well-documented, emotionalism – characterised by difficulty controlling crying or laughing – has been relatively neglected (House et al., 2004). However, in the first six months following stroke, between 20 and 25% of people will experience emotionalism and report crying or laughing ‘in situations that would have not previously have provoked such behaviour’ (House et al., 2004). These emotional outbursts can be sudden and unpredictable; causing distress and embarrassment. They can negatively impact on contact with family and friends and lead to social avoidance. Emotionalism is also associated with an increase in depressive symptoms, although, fortunately, the majority of people affected do not experience diagnosable depression (House et al., 2004).
Therapeutic interventions for emotionalism have tended to focus on pharmacological approaches. Numerous clinical trials with antidepressant medication have been reported and the use of antidepressants is recommended (Stroke Association, 2006; RCP, 2008). However, no specific antidepressant has been identified as the most effective and House et al. (2004) concluded that further data are required before recommendations can be made regarding the pharmacological treatment of post-stroke emotionalism. Other approaches have sought to explore the interpersonal dimensions of emotionalism and the ways in which emotional displays are managed by those closest to the person affected, but again no specific strategies have been identified as more successful than others (Manzo et al., 1998). Acknowledging that the behaviour is due to the stroke is important and empathy and understanding are essential. Advice for relatives and friends focuses on distraction, or treating an emotional display as ‘a minor inconvenience’ and continuing with the conversation that may have provoked the emotionalism (Stroke Association, 2006). With time, the frequency and severity of emotionalism declines.
Anxiety
Anxiety can be provoked by specific activities or events, or might occur without provocation. Whatever the cause, anxiety can exert seriously adverse effects on daily functioning, personal relationships and the quality of life for a person affected by a stroke. Anxiety following stroke might also be associated with post-traumatic stress disorder (PTSD) and can be characterised by intrusive memories or flashbacks to the traumatic event of the stroke (McCoy, 2006). The person may feel that they are re-experiencing the stroke event and do not want to think about what happened because of the unpleasant feelings this generates (McCoy, 2006). For both generalised anxiety disorder and PTSD, psychological therapies might be of value; possibly in combination with benzodiazepines or antidepressant medication (RCP, 2004)
Depression
Whilst emotionalism and anxiety are undeniably distressing, depression has received most attention from researchers and practitioners. This is justifiable because it is the most debilitating mood disturbance following stroke, and is associated with increased morbidity, delayed rehabilitation, longer periods of hospitalisation and generally poorer outcomes for the person affected (Turner-Stokes and Hassan, 2002). Estimates of depression following stroke vary radically. O’Rourke et al. (1998) suggests that 23–60% of stroke survivors will experience depression and Lincoln et al. (2003) indicated an even wider range of 25–79%. However, Hackett et al. (2005) suggest a pooled estimate of 33% for all stroke survivors who experience depression.
Depression can occur in response to any life-changing event where a person has experienced a catastrophic loss of independence, self esteem and perceived status in society; and not everyone is equipped to cope with the grieving and adjustment processes (Rochette et al., 2007). There is also an increased risk of stroke-related mood disturbance in patients who are unable to process loss events due to a decrease in cognitive ability (Rickards, 2005) It has been proposed that areas of the brain responsible for the control of mood may be damaged by a stroke, causing a neurochemical imbalance resulting in mood disturbance (Robinson et al., 1990), but systematic reviews of the literature have identified no conclusive evidence for this theory (Aben et al., 2001; Carson et al., 2000).
Reported risk factors for the development of depression include previous history of depression or psychiatric illness, living alone, lack of social support and social isolation (Ouimet et al., 2001). In females, the estimated risk of developing depression following stroke is 7–10% greater than in males (Paolucci et al., 1999). A person experiencing depression following a stroke is more likely to die over the course of 5 years, than a non-depressed person (Jorge et al., 2003) and there is also a significantly increased risk of suicide, particularly in people under the age of 60 and in females (Teasdale and Enberg, 2001).
Screening for anxiety and depression should occur within the first month following a stroke, together with keeping the patient’s mood under review. However, although there is widespread agreement that the early recognition and management of depression following stroke are essential, consensus regarding the most appropriate assessment tools, diagnostic criteria and effective interventions for the prevention and treatment of depression following stroke has yet to be reached. The Hospital Anxiety and Depression Scale (HADS) remains a popular screening tool, possibly because it can be administered by nurses and other members of the multiprofessional team. For patients with communication impairments, the Stroke and Aphasia Depressions Questionnaire is more appropriate (Bennett and Lincoln, 2006).
Supporting carers
It is important to recognise that the emotional costs of providing long-term care to a person affected by a stroke can also be great, and that carers are more prone to the development of depression than the wider population (Wyller et al., 2003). Informal caregivers provide indispensable support following a person’s return home and their ability to manage this caring role is crucial (Ski and O’Connell, 2007). However, it has been identified that the greater the care giving burden, the greater the level of emotional distress for carers and interventions to support carers are essential in maintaining their emotional wellbeing (Brereton et al., 2007).
Ensuring that appropriate support is available to a person experiencing a stroke and their carers has been highlighted as a ‘key area of challenge’ in the National Stroke Strategy (DH, 2007). Emotional support features highly in nursing interventions within the context of community-based stroke services (Burton and Gibbon, 2005; Bennett and Greensmith 2007) as well as in hospital-based contexts (Dundas, 2006; Bennett et al., 2007). For patients with severe or persistent depression, psychological therapies or antidepressant medication might be considered (RCP, 2008)
SUMMARY
Care of patients following stroke is complex and requires skilled and knowledgeable nurses to coordinate care of the patient with other members of the multidisciplinary team. While several guidelines have been produced addressing stroke care, there is still a paucity of evidence supporting some aspects of care and further research is required. The profile of stroke and TIA care has been raised and developments in hyperacute care and thrombolysis mean that patients who suffer from stroke should now benefit from improved outcomes providing symptoms are recognised and acted upon as a medical emergency.
Stroke: patient perspective
I am 62 years old and I am a window fitter by trade. On the morning of my stroke I got up early to ring my bosses to let them know I was unable to come into work again that day. I made a cup of tea for my wife and when I took it downstairs, I was unable to carry the cup without spilling the tea on each step. I did feel peculiar, but was unaware that I had had a stroke. My wife noticed instantly that my face was drooping on the right side and thought something serious had happened. She arranged an emergency appointment with the doctor who then told us to go to the hospital immediately since my heart rate was 130 and I was in atrial fibrillation.
By this stage, I was confused and unsure of what was happening around me and I had lost power in my right arm. After some initial tests in A&E, I was admitted to the Stroke Unit. By the following day, the power had returned to my arm and I was feeling less confused but my speech was still affected and I had difficulties with numbers and telling the time.
Therapy
My occupational therapist helped me with my measurements and we worked on some drawings and site plans to obtain accurate measurements for the buildings. I found this much more difficult as it was something I took for granted prior to my stroke. I had an ability to know measurements by sight before and when I looked at an architect’s drawing, my mind had the ability to understand immediately what size windows would be needed and where they would go without hardly thinking about it. After my stroke, I stared at architect’s drawings for hours and they made little or no sense. I still find it difficult now to work out measurements when doing DIY for example. I have to keep checking and re-checking that I have the correct measurements.
I have found that when I am tired, my speech is not as fluent as it is when I am well rested. I did also suffer some side effects from my medication and I found that I was easily depressed about my condition. I become impatient and frustrated and wanted everything to go back to how it was before my stroke.
The Future
I am hopeful that I can return to work soon. I may not be able to do the same work as before as I have dizzy spells, which is obviously dangerous on a building site. I still feel impatient sometimes when things don’t go right or if I cannot say what I want to say. However, I am a lot better than I was so I am grateful for that.
For further examples of stroke survivor’s experiences please refer to the Different Strokes website available at: www.differentstrokes.co.uk
REFERENCES
Aben I, Verhey F, Honig A et al. (2001) Research into the specificity of depression after stroke: a review on an unresolved issue. Progress in Neuropsychopharmacology and Biological Psychiatry 25:671–689.
Adamson J, Beswick A, Ebrahim S (2004) Is stroke the most common cause of disability? Journal of Stroke and Cerebrovascular Diseases 13(4):171–177.
Allender S, Colquhoun D, Kelly P (2006) Competing discourses of workplace health. Health (London) 10(1):75–93.
Amarenco P, Bogousslavsky J, Callahan A et al. (2006) High-dose atorvastatin after stroke or transient ischaemic attack. New England Journal of Medicine 355:549–559.
Appelros P (2006) Prevalence and predictors of pain and fatigue after stroke: a population-based study. International Journal of Rehabilitation Research 29(4):329–333.
Arias M, Smith L (2007) Early mobilization of acute stroke patients. Journal of Clinical Nursing 16:282–288.
Baker M, Opatowsky M, Wilson J et al. (2001). Rheolytic catheter thrombolysis of dural venous sinus thrombosis: A case series. Neurosurgery 48(3):487–494.
Bamford J, Sandercock P, Dennis M et al. (1990) A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981–1986. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. Journal of Neurology, Neurosurgery and Psychiatry 53:16–22.
Bamford J, Sandercock P, Dennis M et al. (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 22 337(8756):1521–1526.
Bender L, McKenna K (2001) Hemiplegic shoulder pain: defining the problem and its management. Disability and Rehabilitation 23(16):698–705.
Bennett B, Barnston S, Smith R (2007) Emotional support after stroke, part 1: Two models from hospital practice. British Journal of Neuroscience Nursing 3(1):19–23.
Bennett B, Greensmith C (2007) Emotional support after stroke, part 2: A community practice model. British Journal of Neuroscience Nursing 3(2):61–64.
Bennett H, Lincoln N (2006) Potential screening measures for depression and anxiety after stroke. International Journal of Therapy and Rehabilitation 13(9):401–406.
Best C (2007) Nasogastric tube insertion in adults who require enteral feeding. Nursing Standard 21(40):39–43.
Birns J, Fitzpatrick M (2005) secondary prevention of stroke. British Journal of Neuroscience Nursing 1(1):32–37.
Booth J, Davidson I, Winstanley J et al. (2001) Observing washing and dressing of stroke patients: nursing intervention compared with occupational therapists. What is the difference? Journal of Advanced Nursing 33(1):98–105.
Booth J, Hillier V, Waters K et al. (2005) Effects of a stroke rehabilitation education programme for nurses. Journal of Advanced Nursing 49(5):465–473.
Bowen A, Lincoln N (2007) Cognitive rehabilitation for spatial neglect following stroke. Cochrane database of Systematic Reviews (2):CD003586.
Brady M, Furlanetto D, Lewis S et al. (2006) Staff-led interventions for improving oral hygiene in patients following stroke. Cochrane Database of Systematic Reviews (4):CD003864.
Brereton L, Carroll C, Barnston S (2007) Interventions for adult family carers of people who have had a stroke: a systematic review. Clinical Rehabilitation 21(10):867–884.
Brodal P (2004) The Central Nervous System: Structure and function. (3rd edition). Oxford: Oxford University Press.
Burton C, Gibbon B (2005) Expanding the role of the stroke nurse: a pragmatic clinical trial. Journal of Advanced Nursing 52(6):640–650.
Carson A, MacHale S, Allen K et al. (2000) Depression after stroke and lesion location: a systematic review. Lancet 356(9224):122–126.
Clark J, Rugg S (2005) The importance of independence in toileting: the views of stroke survivors and their occupational therapists. British Journal of Occupational Therapy 68(4):165–171.
CLOTS Trial Collaboration (2009) Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. The Lancet 373(9679):1958–1965.
Cottrell S, Davies A (2006) Stroke Talk: A communication resource for hospital care. London: Connect Press.
Cross S (2008) Stroke care: a nursing perspective. Nursing Standard 22(23):47–56.
Dalton C, Gottlieb L (2003) The concept of readiness to change. Journal of Advanced Nursing 42(2):108–117.
Department of Health (2001) National Service Framework for Older People. London: DH.
Department of Health (2007) National Stroke Strategy. London: DH.
Dirnagl U, Pulsinelli W (1990) Autoregulation of cerebral blood flow in experimental focal brain ischaemia. Journal of Cerebral Blood Flow and Metabolism 10:327–336.
Dowswell G, Forster A, Young J et al. (1999) The development of a collaborative stroke training programme for nurses. Journal of Clinical Nursing 8:743–752.
Dundas J (2006) An evaluation of use of the HADS scale to screen for post-stroke depression in practice. British Journal of Neuroscience Nursing 2(8):399–403.
Forster A, Dowswell G, Young J et al. (1999a) Effects of a physiotherapist-led stroke training programme for nurses. Age and Ageing 28:567–574.
Forster A, Dowswell G, Young J et al. (1999b) Effect of a physiotherapist-led training programme on attitudes of nurses caring for patients after stroke. Clinical Rehabilitation 13:113–122.
Fursland E (2005) Finding the words. Nursing Standard 20(1):24–25.
Garrett D, Cowdell F (2005) Information needs of patients and carers following stroke. Nursing Older People 17(6):14–16.
Hackett M, Yapa C, Parag V et al. (2005) Frequency of depression after stroke: a systematic review of observational studies. Stroke 36(6):1330–1340.
Hankam D, Spence D (2007) Combining multiple approaches for the secondary prevention of vascular events after stroke. Stroke 38:1881–1885.
Hankey GJ, Warlow CP (1999) Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations. Lancet 354(9188):1457–1463.
Harvey R (2003) Prevention of venous thromboembolism after stroke. Topics in Stroke Rehabilitation 10: 61–69.
Hoffmann T, McKenna K, Worrall L et al. (2004) Evaluating current practice in the provision of written information to stroke patients and their carers. International Journal of Therapy and Rehabilitation 11(7):303–310.
Horsburgh D, Rowat A, Mahoney C et al. (2008) A necessary evil? Interventions to prevent nasogastric tube-tugging after stroke. British Journal of Neuroscience Nursing 4(5):230–234.
House A, Hackett M, Anderson C et al. (2004) Pharmaceutical interventions for emotionalism after stroke. Cochrane Database of Systematic Reviews (2):CD0033690.
Hyde S, Dowell M (2002) Acute stroke management: the importance of vital signs. Nurse 2 Nurse 2(12):10–12.
Jarrett L (2006) The nurse’s role in assessing and measuring spasticity. Nursing Times 102(15):26–28.
Johnston SC, Rothwell PM, Nguyen-Huynh MN et al. (2007) Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 369(9558):283–292.
Jones A, Tilling K, Wilson-Barnett J et al. (2005) Effect of recommended positioning on stroke outcome at six months: a randomised controlled trial. Clinical Rehabilitation 19:138–145.
Jones S, Leathley M, McAdamm J et al. (2007) Physiological monitoring in acute stroke: a literature review. Journal of Advanced Nursing 60(6):577–594.
Jorge R, Robinson R, Arndt S et al. (2003) Mortality and poststroke depression: a placebo-controlled trial of antidepressants. American Journal of Psychiatry 160(10):1823–1829.
Knight G (2005) Better Conversations: A guide for relatives. London: Connect – the Communication Disability Network.
Lincoln N, Majid M, Weyman N (2000) Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews (4):CD002842.
Lincoln N, Nicholl C, Flannaghan T et al. (2003) The validity of questionnaire measures of assessing depression after stroke. Clinical Rehabilitation 17(8):840–846.
Lip GY, Barnett AH, Bradbury A et al. (2007) Ethnicity and cardiovascular disease prevention in the United Kingdom: a practical approach to management. Journal of Human Hypertension 21(3):183–211.
Maclean N, Pound P (2000) A critical review of the concept of patient motivation in the literature on physical rehabilitation. Social Science and Medicine 50:495–506.
Maclean N, Pound P, Wolfe C et al. (2000a) The concept of patient motivation. A qualitative analysis of stroke professionals’ attitudes. Stroke 33(2):444–448.
Maclean N, Pound P, Wolfe C et al. (2000b) Qualitative analysis of stroke patients’ motivation for rehabilitation. British Medical Journal 321(7268):1051–1054.
MacWalter RS, Shirley CP (2003) Managing Strokes and TIAs in Practice. London: Royal Society of Medicine Press Ltd.
Manzo J, Heath R, Blonder L (1998) The interpersonal management of crying among survivors of stroke. Sociological Spectrum 18:161–184.
McCoy K (2006) Even a minor stroke might lead to stress and anxiety. Neurology 66:15–16.
McCrea J, Langhorne P, Pandyan A et al. (2008) Systemically-acting pharmacological interventions for spasticity after stroke (Protocol). Cochrane Database of Systematic Reviews (3):CD006874.
McMahon A, Irwin P, Redehan E (2003) Identifying priorities for strengthening and developing the nursing contribution in the field of practice: A case study in stroke care. Nursing Times Research 8(3):202–212.
Mead G, Lynch J, Greig C et al. (2007) Evaluation of fatigue scales in stroke patients. Stroke 8(7):2090–2095.
Michael K (2002) Fatigue and stroke. Rehabilitation Nursing 27(3):89–94,103.
Morris H (2005) Administering drugs to patients with swallowing difficulties. Nursing Times 101(39):28–30.
Murphy J, Cameron L (2005) Talking Mats: A resource To enhance communication. Stirling: University of Stirling.
Nair R, Lincoln N (2007) Cognitive rehabilitation for memory deficits following a stroke. Cochrane Database of Systematic Reviews (3):CD002293.
National Audit Office (2005) Reducing Brain Damage: Faster access to better stroke care. London: The Stationery Office.
National Institute for Health and Clinical Excellence (2008) Stroke: national clinical guidelines for diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). London: Royal College of Physicians.
Nazarko L (2007a) Stroke and continence: The benefits of assessment. Nursing and Residential Care 9(5):203–206.
Nazarko L (2007b) Visual impairment in stroke sufferers. Nursing and Residential Care 9(3):109–111.
Office of National Statistics (2001) Stroke incidence and risk factors in a population-based cohort study. In: Office of National Statistics Health Statistics Quarterly (12) Winter.
O’Rourke S, MacHale S, Signorini D et al. (1998) Detecting psychiatric morbidity after stroke: comparison of the GHQ and the HAD Scale. Stroke 29(5):980–985.
Ouimet M, Primeau F, Cole M (2001) Psychosocial risk factors in poststroke depression: a systematic review. Canadian Journal of Psychiatry 46:819–828.
Ozcakir S, Sivrioglu K (2007) Botulinum toxin in poststroke spasticity. Clinical Medicine and Research 5(2):132–138.
Paolucci S, Antonucci G, Pratesi L et al. (1999) Poststroke depression and its role in rehabilitation. Archives of Physical Medicine and Rehabilitation 80:985–990.
Parr S, Byng S, Gilpin S (1997) Talking About Aphasia: Living with loss of language after stroke. Buckingham, Open University Press.
Pasero C (2004) Pathophysiology of neuropathic pain. Pain Management Journal 5(4):(Suppl 1):3–8.
Patel S, Taylor L (1999) Only half of the world: hemianopia or neglect. British Journal of Therapy and Rehabilitation 6(7):327–329.
Perry L, Brooks W, Hamilton S et al. (2004) Exploring nurses’ perspectives of stroke care. Nursing Standard 19(12):33–38.
Pomeroy V, Niven D, Barrow S et al. (2001) Unpacking the black box of nursing and therapy practice for post-stroke shoulder pain: a precursor to evaluation. Clinical Rehabilitation 15:67–83.
Poulter N, Thorn S, Kirby M (2000) Shared Care for Hypertension. London: Isis Medical Media.
Rabadi M, Rabadi F, Edelstein L et al. (2008) Cognitively impaired stroke patients do benefit from admission to an acute stroke unit. Archives of Physical Medicine and Rehabilitation 89(3):441–448.
Resnick B (1998) Motivating older adults to perform functional activities. Journal of Gerontological Nursing 24(11):23–30.
Rickards H (2005) Depression in neurological disorders: Parkinson’s disease, multiple sclerosis and stroke. Journal of Neurology, Neurosurgery and Psychiatry 76(Suppl 1):148–152.
Robinson R, Morris P, Fedoroff J (1990) Depression and cerebrovascular disease. Journal of Clinical Psychiatry 51(7)Suppl:26–31.
Rochette A, Bravo G, Desrosiers J et al. (2007) Adaptation process, participation and depression over six months in first-stroke individuals and spouses. Clinical Rehabilitation 21:554–562.
Rogers A, Addington-Hall J (2005) Care of the dying stroke patient in the acute setting. Journal of Research in Nursing 10(2):153–167.
Rothwell P (2007) Making the most of secondary prevention. Stroke 38(6):1726.
Rothwell P, Eliasziw M, Gutnikov S et al. (2004). Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. The Lancet 363(9413):915–924.
Rothwell PM, Gutnikov SA, Warlow CP for the European Carotid Surgery Trialists’ Collaboration (2003) Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 34:514–523.
Royal College of Physicians (2004) National Clinical Guidelines for Stroke. (2nd edition). London: RCP.
Royal College of Physicians (2008) National Clinical Guidelines for Stroke (3rd edition). London: RCP.
Ski C, O’Connell B (2007) Stroke: the increasing complexity of carer needs. Journal of Neuroscience Nursing 39(3):172–179.
Smith J, Forster A, House A et al. (2008) Information provision for stroke patients and their caregivers. Cochrane Database of Systematic Reviews (2):CD001919.
Stroke Association (2006) Psychological Effects of Stroke Fact sheet 10 Available from: www.stroke.org.uk
Struder M (2007) Rehabilitation of executive function: to err is human, to be aware – divine. Journal of Neurologic Physical Therapy 31(3):128–134.
Sudlow CL, Warlow CP (1997) Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke 28(3):491–499.
Sutton L (2008) Addressing palliative and end-of-life care needs in neurology. British Journal of Neuroscience Nursing 4(5):235–238.
Teasdale T, Enberg A (2001) Suicide after stroke: a population study. Journal of Epidemiology and Community Health 55(12):863–866.
Thomas L, Cross S, Barrett J et al. (2008) Treatment of urinary incontinence after stroke in adults. Cochrane Database of Systematic Reviews (1):CD004462.
Thorvaldsen P, Davidsen M, Brønnum-Hansen H et al. (1999) Stable stroke occurrence despite incidence reduction in an aging population: stroke trends in the danish monitoring trends and determinants in cardiovascular disease (MONICA) population. Stroke 30(12):2529–2534.
Tooth L, Hoffmann T (2004) Patient perceptions of the quality of information provided in a hospital stroke rehabilitation unit. British Journal of Occupational Therapy 67(3):111–117.
Touzani O, Roussel S, MacKenzie ET (2001) The ischaemic penumbra. Current Opinions in Neurology 14(1):83–88.
Turner-Stokes L, Hassan N (2002) Depression after stroke: a review of the evidence base to inform the development of an integrated care pathway. Part 1: Diagnosis, frequency and impact. Clinical Rehabilitation 16(3):231–247.
Wachters-Kaufmann C, Schuling J, Hauw T et al. (2005) Actual and desired information provision after a stroke. Patient Education and Counseling 54:211–217.
Walker C, Walker M (2001) Dressing ability after stroke: a review of the literature. British Journal of Occupational Therapy 64(9):449–454.
Walker C, Walker M, Sunderland A (2003) Dressing after a stroke: a survey of current occupational therapy practice. British Journal of Occupational Therapy 66(6):263–268.
Wardlaw JM, del Zoppo G, Yamaguchi T et al. (2003) Thrombolysis for acute ischaemic stroke (Cochrane review). Cochrane Database of Systematic Reviews Issue 3. Review updated in Issue 4 (2008).
Warlow C, Dennis M, van Gijn J et al. (2008) Stroke: A practical guide to management. (3rd edition). Oxford: Blackwell Publishing.
Williams B, Poulter N, Brown M et al. (2004) Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society. Journal of Human Hypertension 18:1991–1994.
Wolf P (2003) Cerebrovascular risk. In: Izzo J and Black H (eds) Hypertension Primer: The essentials of high blood pressure. (3rd edition). Philadelphia: Lippincott, Williams and Wilkins.
Wolf PA, D’Agostino RB, O’Neal MA et al. (1992) Secular trends in stroke incidence and mortality: the Framingham Study. Stroke 23:1551–1555.
Woodward S (2007) Antiphospholipid (Hughes) syndrome. British Journal of Neuroscience Nursing 3(1):16–18.
World Health Organisation (1988) World Health Organisation MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. Journal of Clinical Epidemiology 41:105–141.
Wright D, Chapman N, Foundling-Miah M et al. (2006) Consensus Guideline on the Medication Management of Adults with Swallowing Difficulties. London: Medendium Group Publishing.
Wyller T, Thommessen B, Sødring K et al. (2003) Emotional well-being of close relatives to stroke survivors. Clinical Rehabilitation 17(4):410–417.