33
Management of Patients with Spinal Injury
INTRODUCTION
Spinal cord injury (SCI) is a non-progressive neurological impairment which can result from a range of traumatic and non-traumatic causes. It predominates amongst adult males but could affect any one of us, or our children, at any time. Thanks to our modern understanding and management of this condition it has a low mortality and a reasonably good life expectancy. It is estimated that around 40,000 people are currently living in the UK with chronic spinal cord injuries. This chapter will consider the role of the nurse in the initial and continuing care of SCI patients within the hospital and community setting. NHS management of both traumatic and non-traumatic SCI includes referral to a specialist Spinal Cord Injury Centre (SCI centre) within 24 hours of diagnosis, although this is not necessary for every spinal cord injury patient. This chapter will emphasise the supporting role provided by the SCI centres and the Spinal Injuries Association (SIA – the national charity supporting spinal cord injured people) to nurses managing spinal cord injured people outside of specialist clinical environments and in the community.
EPIDEMIOLOGY
Spinal cord injury affects fewer than 1,000 UK citizens each year. Significant spinal column injuries can be present in up to 10% of all trauma admissions (Hu et al., 1996) and up to 20% of severely injured patients (NCEPOD, 2007) whose injuries have the potential to result in damage to the underlying spinal cord. 71% of spinal cord injuries are due to trauma (SIA, 2009). However, traumatic spinal cord injury is still a relatively rare event, representing approximately 2% of current trauma admissions (Banit et al., 2000). There is a 7:3 male:female ratio amongst current new SCI centre admissions. SCI is not confined to any particular age group; acute admissions to SCI centres ranged between 3 and 103 years in 2007–2008, with 20% of new injuries occurring between 21 and 30 years of age (SIA, 2009).
The area of the spinal column most commonly involved in the incidence of traumatic SCI is the cervical spine (50% of all SCI centre admissions) (see Table 33.1). The most common levels of cervical trauma resulting in SCI are C5–C8 (26% of all SCI centre admissions) with 37% of SCI admissions involving the thoracic spinal cord (T1–T12) and 11% involve the lumbar spinal cord (L1–L5) (SIA, 2009).
Table 33.1 Admissions to the UK Spinal Cord Injuries Centre by level of neurological Injury.
| Level of injury | Percentage of admissions |
| C1–C4 | 21% |
| C5–C8 | 26% |
| T1–T12 | 37% |
| L1–L5 | 11% |
| S1–S5 | 0.1% |
| Not recorded | 1.9% |
Source: Spinal Injuries Association, 2009.
Mortality amongst children and older people sustaining SCI is higher than other age groups as they are more susceptible to the effects of severe multi-trauma. Older people are much more vulnerable to cervical spinal cord compression resulting from minor trauma because of the presence of pre-existing age-related diseases such as ankylosing spondylitis and spinal stenosis (Roth et al., 1992).
AETIOLOGY
Traumatic spinal cord injuries
The most common cause of spinal cord trauma in the UK is an incident involving sudden, unexpected, impact, collision or deceleration. Speed at impact or height of fall are unreliable in predicting the potential for spinal injury at the scene. Therefore, current UK trauma management guidelines emphasise the need for rescuers to maintain a high index of suspicion of spinal injury within the initial management of most trauma scenarios along with the early implementation of appropriate spinal column protection strategies (BTS, 2003; Fisher et al., 2006; ACS 2008).
Moving vehicle collisions (MVC) are the most common cause of SCI. In the majority of these cases, vehicle occupants were unsecured within the vehicle, or were ejected. Such casualties usually present with complex multi-system trauma in addition to SCI. Falls are the second most common cause of SCI centre admissions (SIA, 2009). However, the potential for a domestic fall to cause SCI is still poorly appreciated within most trauma care pathways (Helling et al., 1999). Aquatic spinal cord injuries can often present as near-drowning episodes with accompanying respiratory compromise including cerebral anoxia. Equestrian injuries can also present with significant multi-trauma including head injury. Gunshot or penetration injuries often involve multiple organ trauma. A small percentage of SCIs are inflicted during failed attempts at suicide.
Non-traumatic spinal cord injuries
Not all spinal cord injuries are caused by trauma. Approximately 28% of all cases of spinal cord paralysis are due to non-traumatic causes resulting in a similar non-progressive neurological impairment (SIA, 2009). These include:
- Ischaemic vascular incidents such as a thrombosis or haemorrhage affecting the spinal cord blood supply
- Viral and bacterial infections and abscesses such as those associated with tuberculosis and meningitis
- Inflammatory conditions such as transverse myelitis
- Non-malignant growths resulting in spinal cord compression
- Congenital defects such as spina bifida
MECHANISMS OF INJURY
Forced flexion and flexion with rotation are the most common mechanisms of cervical injury. These occur in incidents such as when vehicle drivers are struck by unsecured rear-seat passengers or when a rugby scrum collapses. Hyperextension cervical injuries are common amongst vehicle occupants in rear-end collisions, especially where headrests are not in situ. They also occur frequently in domestic falls where the falling person most commonly falls forwards, striking their chin or head against furniture or a wall. Increased axial loading from being struck on the head by a falling object or diving into shallow water results in a burst compression fracture. Individuals falling from a height and landing on their feet or falling onto the base of their spine can present with multiple compression fractures in lumbar, thoracic or cervical zones. Crush fractures are common in industrial accidents where the person is trapped by moving machinery or a heavy vehicle. Penetration injuries to the spine can be due to gunshot or stabbing. High speed impact trauma such as ejection from a moving vehicle can result in gross disruption and distraction of the spinal column because all of the above mechanisms can occur.
PATHOPHYSIOLOGY
The traumatic displacement of one or more vertebral bodies results in compression of the underlying spinal cord (Iencean, 2003). Alternatively, the spinal cord may be stretched or ‘concussed’ without any visible disruption of the spinal column. The resulting post-traumatic inflammatory oedema and vascular disruption start a complex series of physiological and biochemical reactions within the spinal cord (Sapru, 2002). There is little room for swelling within the structural confines of the vertebral canal and the oedematous spinal cord is quickly compressed against the surrounding bone. Circulation of blood and oxygen within the spinal cord is disrupted and ischaemic tissue necrosis quickly follows. There is an almost immediate cessation of conductivity within the spinal cord neuones. This is termed ‘spinal shock’ (Nacimiento and Noth, 1999).
Spinal shock
Spinal shock is best described as the complete suppression of all autonomic, somatic, and reflex activity below the level of lesion. The term neurogenic shock refers specifically to the loss of sympathetic activity and the effects this has on the cardiovascular system. This is presented in Chapter 14. The effects of spinal shock are most profound in complete lesions above the level of T6.
The impact of spinal shock on specific systems of the body is presented in Table 33.2. On average, spinal shock usually resolves within 2–14 days of onset but can persist for up to 6 weeks (Nacimiento and Noth 1999; Ditunno et al., 2004).
Table 33.2 The impact of spinal shock on specific systems of the body.
| Body system | Impact of spinal shock |
| Respiratory | Respirations compromised by flaccid skeletal muscles
Inability to cough and expectorate Danger of vagal overstimulation during suctioning Nasal passages blocked due to vasodilation |
| Cardiovascular (see Chapter 14: Neurogenic shock) | Hypotension due to systemic vasodilation
Bradycardia due to vagal domination Poikilothermia – adopting environmental temperature due to loss of vasodilation, vasoconstriction, sweating, shivering and piloerection (goose flesh) |
| Genitourinary | Poor renal perfusion due to hypotension
Loss of ureteric peristalsis Atonic (flaccid) bladder and urethral sphincters Pseudopriapism in males due to passive vasodilation Amenorrhoea in females secondary to metabolic deficits |
| Gastrointestinal | Increased volume and concentration of gastric acid due to vagal domination
Paralytic ileus due to loss of peristalsis (usually only for approximately 48 hours following injury) Atonic (flaccid) ano-rectum and sphincters |
| Skin | Increased pressure marking due to vasodilatation
Reduced tissue density over bony prominences and weight-bearing areas due to redistribution of paralysed muscle bulk Dry skin as unable to sweat or produce sebum |
Source: Ash and Harrison, 2007.
Although most patients with spinal shock will present at the scene of the accident with the loss of all voluntary movement and sensation in addition to loss of autonomic and reflex activity below the level of the injury, it is not uncommon for the onset of clinical symptoms to be delayed by up to 72 hours after the original incident. Even when symptoms are present immediately post trauma it is often difficult to be certain of the extent or permanence of the functional loss. The presence of paralysis or paraesthesia does not imply any finality to the process. In some instances, when spinal cord oedema and spinal shock resolve there can be a subsequent improvement in neurological function.
Further neurological deterioration, resulting from lesion extension after the initial SCI, can occur naturally in about 5% of trauma cases (Marshall et al., 1987). A number of complications associated with the multisystem effects of SCI can lead to respiratory, cardiovascular or other system compromise which can further compromise the body’s attempts to limit nerve tissue death and can further reduce any inherent potential for any degree of spinal cord recovery. Nursing and medical management is directed at limiting these potential problems.
Complete and incomplete lesions
Spinal cord injuries will result in either complete or incomplete loss of function below the level of the injury. A complete spinal cord lesion is when the cord is completely transected or when the ischaemia affects the entire width of the spinal cord. There is complete loss of all voluntary movement and sensation below the level of the injury. There is also a complete loss of autonomic nerve function throughout the same area. The majority of patients will have incomplete spinal cord lesions with varying degrees of impairment of sensory and/or motor function consistent with the extent of their lesion (see Box 33.1 for the clinical presentation of each of the incomplete syndromes and Figures 33.1a–d).
Box 33.1 Incomplete spinal cord lesions
| Anterior cord syndrome | This is the most common form of incomplete lesion after a high-velocity impact trauma. Flexion-rotation results in pressure against the anterior grey horn (contains cell bodies of motor neurones) of the spinal cord, the anterior spinal artery, and the anterior columns (contain the spinothalamic and corticospinal tracts). The spinal cord lesion develops through a combination of physical trauma, bony compression and ischaemia. Anterior cord lesions result in loss of motor function and the sensations of pain and temperature below the level of the lesion. The senses of crude touch, position (proprioception) and vibration are preserved below the level of the lesion |
| Posterior cord syndrome | Posterior impact injuries or hyperextension forces compress or traumatise the posterior column and posterior grey horns (sensory) of the spinal cord. Posterior lesions present with the loss of deep touch, position and vibration below the level of the lesion, with preservation of motor function and sensations of pain and temperature. Unfortunately, the sense of proprioception (the unconscious awareness of a limb’s position in space) is lost, which can limit the patient’s potential for developing a functional gait |
| Brown-Séquard syndrome | This lesion presents as a hemisection of the spinal cord. It is most commonly associated with stabbing/penetration injuries, but it may also occur from gross lateral flexion injuries. Motor power is absent or reduced on the same side as the lesion, but pain and temperature sensation are preserved. This presentation is reversed on the uninjured side which has good power but absent or reduced pain and temperature sensation. This results from the fact that the spinothalamic tracts cross over within the spinal cord, enabling sensory signals to travel up the side opposite to that where the origin of the sensation is perceived to be |
| Central cord syndrome | Usually occurs in elderly or spondylotic patients after minor hyperextension trauma to the neck. Vertebrae, discs and ligaments, which have become stiffened or thickened with age, focus compression forces and ischaemia towards the central portion of the cord, where the cervical nerve tracts originate. Patients present with significant loss of function in their upper limbs and hands, and partial preservation of function in their lower limbs, usually with retained sacral sensation and partially preserved bladder and bowel function |
Adapted from: Gall and Harrison, 2007.
Figure 33.1 (a) Anterior cord syndrome; (b) posterior cord syndrome; (c) Brown-Séquard syndrome; (d) central cord syndrome.
Reproduced from Ash D, Harrison P (2007) Understanding spinal shock. In: Harrison P (ed) Managing Spinal Cord Injury: The First 48 Hours. Milton Keynes: Spinal Injuries Association (SIA), with permission.

Tetraplegia (not quadriplegia) is the preferred medical term for documenting any spinal cord lesion affecting all four limbs. Paraplegia refers to a lesion affecting only the lower body and without any effect upon the upper limbs (see Figure 33.2).
Figure 33.2 Level of injury and extent of paralysis.
Reproduced from Ash D, Harrison P (2007) Understanding spinal shock. In: Harrison P (ed). Managing Spinal Cord Injury: The First 48 Hours. Milton Keynes: Spinal Injuries Association (SIA), with permission.

Because of the dynamic nature of pathological processes that occur following the initial incident, the medium and long-term outcomes of any spinal cord lesion cannot be accurately predicted initially. However, detailed clinical assessments supported by diagnostic imaging during the first few days and weeks can often help an experienced clinician to predict what the most likely neurological and functional outcomes will be. MRI of the spinal cord can inform the clinical prognosis, as the extent of cord compression, swelling and bleeding are related to neurological outcome (Fehlings et al., 2007).
PRIORITISING MEDICAL INTERVENTIONS
Medical management of traumatic SCI after admission to hospital follows established ATLS principles (BTS, 2003; ACS, 2008). During resuscitation, team members will endeavour to maintain spinal alignment as best as circumstances allow but life-threatening injuries take priority. Until the patient’s condition has stabilised sufficiently to allow for medical imaging and detailed neurological assessment, the trauma team will assume the presence of a possible spinal injury. Early secondary complications that can occur include: lesion extension, respiratory insufficiency, cardiovascular insufficiency, hypothermia, pressure ulcers, urinary and faecal incontinence, and renal failure. Box 33.2 shows a summary of the immediate medical management and Box 33.3 gives guidance for patient transfer to a SCI centre.
Box 33.2 Medical management of acute spinal cord injury
Early recognition of actual or potential spinal cord injury utilising:
- Accident history and mechanism of Injury
- Patient history and presenting symptoms
- Clinical survey including neurophysiological examination (including sacral nerve pathways)
- Diagnostic Imaging
Implement initial management of patient in accordance with local trauma protocols:
- Prioritise interventions according to ATLS guidelines. Ensure the judicious administration of fluid and pharmaceutical resuscitation protocols in accordance with the diagnosis of actual or potential spinal cord injury as there is a significant potential for substantial overinfusion and subsequent pulmonary oedema if neurogenic hypotension is confused with hypovolaemia (see Chapter 14 for the management of neurogenic shock)
- Management of spinal shock may include the adaptation of local hospital early warning score indicators to incorporate the physiological impact of spinal shock to avoid inappropriate ‘triggering’ of unnecessary medical alerts or treatment interventions
Ensure accurate documentation of findings and actions undertaken:
- American Spinal Injury Association Standard Neurological Classification of Spinal Cord Injury Worksheet (available in publications section of www.asia-spinalinjury.org)
Telephone referral of actual or suspected spinal cord injured patient to spinal cord injury consultant at locally nominated spinal cord injury centre within 24 hours of diagnosis being made. Additional information sent by fax and/or electronic data transfer as soon as possible after referral.
Maintain close collaboration with spinal cord injury consultant regarding the options for surgical management of spinal trauma and the initial medical and multi-professional management of spinal shock until transfer or further case discussion. This may include seeking advice regarding the way in which the paralysis may mask the normal symptoms of underlying soft tissue trauma or visceral complications.
Inform patient’s next-of-kin accurately of the diagnosis and tentative prognosis (if appropriate) as advised by expert clinical peer. Where patient has been accepted for transfer, include within this discussion the need to eventually transfer their relative to a tertiary specialist centre which may be some distance away.
Where available, ensure referral has been made to local hospital SCI link-workers for additional advice and assistance in managing this patient until transfer.
Source: British Trauma Society, 2003; Ravichandran and El Masri 2005; British Orthopaedic Association, 2006; Harrison 2007; American College of Surgeons, 2008.
Box 33.3 Guidelines for transferring acute spinal cord injured patients
- The expected total journey time should usually not exceed 2.5 hours. If the journey is likely to exceed 2.5 hours, consider aeromedical transfer or consult with the receiving SCI centre regarding the possibility of delaying the transfer
- Inform the receiving SCI centre of the time that the patient left and their expected time of arrival at the SCI centre
- All relevant medical, nursing and therapy notes, diagnostic imaging files and laboratory reports must accompany the patient as either copies or originals
- An experienced and informed member of medical and/or nursing staff, who is familiar with the care that the patient has received before transfer to the SCI centre, is an essential requirement as an escort. An anaesthetist must escort the SCI patient at risk of respiratory difficulties
- It is possible for the SCI patient to vomit while being transferred, so their stomach should be emptied before transfer. Other potential complications that may be encountered en route are hypothermia, pressure ulcers, respiratory compromise, further neurological deterioration and autonomic dysreflexia. Additional patient distress may occur if the level of pain control is inadequately maintained during transportation
- Transferring a SCI patient on a spinal board is not always required and the planned use of any spinal protective devices during transfer should always be discussed in advance with SCI centre staff
Source: Ravichandran and El Masri, 2005; British Orthopaedic Association, 2006; Sarhan and Harrison, 2007.
Reduction and stabilisation of the spinal injury is the first priority in the stabilised SCI patient. In most instances, conservative methods will be exhausted first before considering surgical options.
Diagnostic imaging
Diagnostic imaging for potential spinal injury is directed by the accident history and clinical examination. It usually begins with a series of plain x-rays to provide anteroposterior and lateral views of the spinal column. Cervical views must include the odontoid peg and the C7–T1 junction. However, because of the difficulty in adequately imaging the spine in this way, many emergency departments now prefer to routinely CT the whole spine instead.
CT of the spine is essential for any patient who presents with a neurological deficit following trauma. MRI of the spine should follow at the earliest opportunity, especially if surgical stabilisation is being planned.
When head injured or multi-trauma patients are sent for CT scanning, every opportunity should be sought to extend the scan to include the whole spine to avoid the need to return later after admission (NICE, 2007).
Neurological examination
Neurological examination for both sensory and motor impairment should progress from head to toe. Ideally all clothing should be removed before or during the examination. Because of the danger of hypothermia, only the actual area of the body being examined should be exposed.
Both sides of the body should be examined separately as variation can occur. Sensation should be compared with an area of the body known to be unaffected by paralysis such as the face of a tetraplegic. Motor power should be assessed against age and any established pre-injury impairment. Refer to Chapter 11 for how to assess sensory and motor level and also see: Neurological systems.
The level of spinal cord injury is the level at which sensation is noted to be absent or altered or at which absence or weakness of movement is noted.
Steroid use
Evidence to support the administration of short-term, high-dose steroids such as methylprednisolone as a definitive strategy for reducing the impact of a traumatic SCI is extremely limited. The accumulated studies have been critically appraised within the framework of evidence-based practice (Short, 2001) and the routine administration of high-dose methylprednisolone can no longer be justified within current medical practice as a standard treatment for acute spinal cord injury (Ravichandran and El-Masri, 2005; BOA, 2006; Consortium for Spinal Cord Medicine, 2008; ACS, 2008 ). High dose methylprednisolone can prove hazardous for the patient and is associated with a significant increase in the incidence of unexpected sepsis and pneumonia leading to ventilation and admission to intensive care (McCutcheon et al. 2004). Nursing and medical staff facing requests to undertake or assist in the administration of high-dose methyprednisolone to traumatic SCI patients should consider their professional obligation to protect patients from the effects of inappropriate prescribing or administration of medicines.
Spinal surgery in the presence of SCI
The role of surgery in the management of acute traumatic SCI is controversial. The systemic impact of spinal shock means that patients in the acute phase of SCI management are at grave risk of neurological deterioration if oxygenation and blood pressure are not properly maintained during anaesthesia, surgery and post-operative recovery. In addition, post-operative oedema and swelling increases the potential for additional insult to the already traumatised spinal cord. Therefore, wherever possible, surgery (where intended) should be delayed to allow for better systemic stabilisation of the patient prior to surgery. Given the implications of wound infection, delayed healing and failed instrumentation for SCI patients, and their vulnerability to acquiring ‘preventable’ complications post-operatively such as pressure ulcers and chest infections, it is preferable for spinal surgery to be undertaken within, or in close association with, a specialist SCI centre (Ravichandran and El-Masri, 2005; BOA, 2006; Consortium for Spinal Cord Medicine, 2008; NHSSCGHSS, 2009; Spinal Task Force, 2010).
Cervical traction is the preferred method for the initial reduction and/or stabilisation of cervical dislocations and fractures. However, proper insertion of traction devices and regular neurological assessment by experienced practitioners are vital as the injured cord is particularly vulnerable to distraction (BOA, 2006). Also it is not unknown for traction tongs to be displaced and re-dislocation to occur during turning or repositioning of patients and transferring patients between surfaces.
Conservative reduction of thoraco-lumbar injuries is less likely to achieve satisfactory alignment and therefore surgical reduction and stabilisation is performed when the patient is stable.
There is no clear evidence to suggest that routine early surgical interventions such as surgical decompression are beneficial to patient outcomes and, if undertaken inappropriately, they have the potential to reduce a patient’s rehabilitation potential (Boerger et al., 2000). Early surgical intervention is usually only undertaken for the following: extradural lesions such as epidural haematomas, cauda equina syndrome, acute neurological deterioration, and specific injuries which cannot be resolved by conservative methods such as bilateral locked cervical facet joints (Kishan et al., 2005; BOA, 2006; Consortium for Spinal Cord Medicine, 2008).
Spinal cord protection programmes
The perception that a patient with an actual or suspected spinal or spinal cord injury can be completely ‘immobilised’ is a fallacy. An individualised spinal protection programme utilising a range of devices designed to reduce the impact of spinal column movement and weightloading through the length of the spinal column is implemented for all patients suspected of having a SCI. Failure to implement or maintain appropriate spinal protection or to deliver appropriately informed care in the presence of apparent or suspected neurological impairment can compound what is already ‘one of the worst disasters that can ever befall an individual’ (Ravichandran, 1989). There are three main scenarios in which hospital-based health care professionals may encounter the need for a spinal cord protection programme: acute, potential and uncleared spinal cord injury.
Actual spinal cord injury
Where symptomatic spinal cord trauma is present, it must be considered when undertaking all aspects of patient care and therapeutic interventions to prevent any deterioration in the patient’s neurological or physiological status. This patient scenario will form the basis for most of the following discussion on nursing management.
Potential spinal cord injury
The management of the conscious, orientated and cooperative patient who presents with an actual spinal column injury but without any symptoms of accompanying spinal cord trauma is well established in most trauma departments. However, each patient is still at risk of delayed onset of SCI symptoms or secondary neurological deterioration due to inappropriate management.
Uncleared spinal cord injury
Where doubt exists over the presence of spinal column or spinal cord trauma, experience has proved time and again that it is better to err on the side of caution. In the presence of multi-system trauma or traumatic brain injury, SCI should continue to be suspected until consciousness returns or a later tertiary review is concluded (Harrison, 2000; BTS, 2003; NICE, 2007). In the unconscious or sedated patient, a cervical collar often acts as a ‘flag’ to alert staff to the possibility of an uncleared cervical spine.
Concomitant injury in the presence of traumatic SCI usually necessitates a significant delay in transfer to the specialist SCI centre until the patient’s condition has been stabilised and the receiving centre can provide the appropriate level of care required. Such delays in transfer are associated with an increase in the potential for complications such as bowel impaction, pressure ulcers and joint contractures. Pre-transfer complications such as these result in an extended period of hospitalisation, protracted rehabilitation programmes, delayed discharge and, potentially, a reduced quality of life post discharge including early readmission to hospital (Carvell and Grundy 1994; Tator et al., 1995; Aung and El Masry, 1997). The additional impact of concomitant trauma upon morbidity and mortality after SCI has yet to be comprehensively audited and evaluated. It is therefore essential that local trauma teams refer such patients quickly in order to avail themselves of the additional clinical support available within a SCI centre.
Practical issues for nurses in the use and maintenance of spinal protective devices and cervical traction
The routine but uninformed use of spinal protective devices such as head restraints, cervical collars, cervical traction and halo bracing systems can prove disastrous for individuals with pre-existing spinal disorders or diseases. Age-related spinal diseases such as ankylosing spondylitis cause increased rigidity and deformity of the spinal column and it is well documented that trying to force an ageing spinal column into an inappropriate position during the fitting of a rigid cervical collar, or supine positioning on a spinal board, scanner couch or theatre table can result in spinal cord injury (Moreau et al., 2003). Where evidence or history of any such condition is available alternative methods of positioning the patient and protecting the spine, must be utilised.
Cervical traction and halo devices
Cervical traction is a non-invasive method for the reduction and stabilisation of spinal fractures and dislocations. It is the method of choice for patients unsuitable for surgery or for whom surgery is not a priority. Guidelines for the appropriate application and maintenance of cervical traction and halo brace systems are well established in most trauma and critical care environments.
Nursing management is orientated towards monitoring the patient after application for any discernible change in neurology and maintaining the condition and security of the traction cord, knots, weights and runners. The position of the traction tongs or halo ring should be checked for any signs of slipping from the original position. Pin-sites should be inspected daily for signs of excessive encrustation, pain, bleeding, swelling or obvious infection. Cleaning, dressing and swabbing of pin sites is undertaken in accordance with local policy, but normal saline usually suffices for routine cleaning.
Gardner–Wells traction tongs have an indicator pin situated within a pin head. Secure pressure is indicated whenever the pin stands proud of its base and set on insertion. The traction should be tightened whenever the pin is noted to be receding from this position. A compatible torque screwdriver is needed for checking and tightening halo pins as required. A suitably sized spanner, key or wrench, should always be available at the patient’s bedside in the event that the traction needs to be removed in an emergency.
During reduction of a cervical dislocation, traction weights are applied under x-ray guidance. These are increased in stages and weight in excess of 60 lb can be required before a successful reduction is achieved. During this time, the consultant may manually adjust the angle of the patient’s neck between extension and flexion. He may also request changes in the foot down angle of the patient’s bed to alter the amount of counter traction provided by the patient’s body. With so many staff circulating around the patient’s bed, care must be taken not to dislodge a traction weight onto a foot.
After reduction of the dislocation, the traction weight and bed angle will be reduced to support a minimum maintenance weight of only a few pounds. This weight must never be removed during turning unless it is first replaced by manual traction and/or a suitably prescribed cervical collar. In addition, the consultant will indicate the degree of neck flexion/extension required to maintain cervical alignment. This must be maintained during logrolling. Similar precautions should be applied during transfers between flat surfaces. When moving a patient in bed between departments, ensure the traction weight does not pendulum too much.
If a halo jacket is being used, check to ensure that the jacket has been fitted with the hinged access panel facing uppermost, for use in the event of cardiac arrest to provide chest compressions. Check fastenings for security and wear and tear daily. Also ensure that an appropriate wrench or hexagonal key to unfasten the frame struts is available (usually kept taped to the jacket) in the event of an emergency. The cutaway panels in modern jackets usually allow sufficient access for checking underlying skin condition. Skin under the jacket can be washed with a damp cloth and dried. To maintain the underlying skin and jacket lining in best condition, moisturising creams should be used sparingly and talcum powder should not be used. If necessary, with the patient supine, the lining of most jackets can be replaced or laundered as appropriate. Never use any part of the halo, jacket or framework as a handhold during turning or transferring between surfaces.
Cervical collars
The management of cervical collars for patients with SCI during an extensive period of supine bedrest continues to present nurses with many challenges (Hogan et al., 1997; Harrison, 2000). The decision to discontinue spinal precautions is a medical decision and the clinical and radiological investigations necessary to inform such a decision may take some time to complete. Therefore, the decision to discontinue spinal precautions should not be unduly influenced by the pressures and constraints that such a device places upon the delivery of nursing care procedures. Nursing staff, as much as doctors, need to understand that radiological clearance alone is insufficient to discontinue the use of spinal protective devices. The doctor authorising removal of a cervical collar must document this decision in the patient’s medical notes, citing the screening process which was followed before it is removed.
The two most commonly reported nursing concerns are the development of pressure ulcers beneath the collar and the potential for increasing intracranial pressure (ICP) in patients with accompanying or suspected traumatic brain injury (TBI) (Ho et al., 2002).
Reducing pressure ulcer incidence in cervical collar use
It is important for nurses to know how to measure and size a cervical collar and to be aware of the different types available. SCI patients are usually admitted wearing a rigid extraction collar that was measured and fitted at the accident scene or in A&E. These collars are often applied whilst a patient is sitting and need to be adjusted or replaced with a collar of a different size or type to accommodate supine-lying pressures, for greater security and patient comfort. Aspen and Philadelphia collars have been evaluated as having the lowest potential for causing pressure ulcers in longer-term use (Hogan et al., 1997) and should replace the hard extraction collars within 48 hours of admission. Most collars contain pre-cut access panels for observation of the position of the trachea, accessing the blood vessels of the neck and positioning tracheostomy tubes. Most pressure ulcers related to the wearing of cervical collars during supine bed rest can be prevented through a programme of regular turning and observation of the underlying skin, good skin hygiene and, where permitted, periods spent without the collar in situ (Harrison, 2000). Strict attention must also be paid to collar hygiene. The inner surface of the collar should be washed and dried, or its inner lining changed, every day.
Managing cervical collars in the presence of actual or suspected raised ICP
Collars should not be routinely discarded just because of the presence of actual brain injury. Any new or problematic increase in ICP or symptomatic neurological deterioration must first be identified as being related to the cervical collar in use. In such instances, close and regular liaison with local specialists enables staff to adapt their care provision over time, and to provide appropriate levels of spinal protection throughout the patient’s admission.
First establish that the collar has been sized and fitted appropriately. If loosening or adjusting the collar does not improve ICP within 15 minutes then the collar is not a contributing factor and should be re-secured appropriately (Ho et al., 2002). If ICP does improve following loosening of the collar then re-sizing the collar or changing it to a different type of collar may provide cervical support without compromising ICP.
Moving and handling patients with SCI
Wherever there is a reasonable suspicion of acute SCI, the aim is to maintain full spinal alignment during any moving and handling activity. Careful handling, positioning and turning, on every occasion, can reduce the potential for secondary spinal cord trauma during patient transfers and movements. Maintaining full spinal protection during logrolling involves at least four members of staff to maintain spinal alignment throughout the procedure. Additional staff will be required to undertake examination or care associated with the logroll such as washing and checking of skin, placement of pillows, insertion or removal of transfer devices, etc. Nurses required to undertake the turning or transferring of actual or potential SCI patients during the acute period must have supreme confidence in their ability to work as a team, especially when other health service staff are involved. It is essential that all moving and handling is coordinated by a nominated team leader.
Routine two-hourly turning and repositioning of SCI patients during the initial period of spinal shock can reduce the incidence of multi-system complications of acute bedrest by reducing the extent and duration of pressure on weight-bearing areas and also the duration of fluid stasis in the body (Hawkins et al., 1999). As spinal shock resolves and the patient’s systemic condition improves, a three to four hourly turning regime can be implemented until transfer to a SCI centre or specialist rehabilitation unit.
Logrolling
Four nurses are needed to log-roll an acute tetraplegic patient and this does not vary in the acute SCI patient even following surgery. Three nurses suffice for paraplegic patients as the head does not need to be held. All commands will come from the team leader who is always the nurse holding or nearest to, the patient’s head.
Logrolling patients with cervical SCI
After explaining how the turn is to be accomplished, the team leader positions their hands to support the patient’s head and cervical spine. Hands should be positioned to support the whole of the patient’s neck. The left hand of the second nurse is positioned at the patient’s shoulder and the right hand on the upper pelvis. The left hand of the third nurse is positioned on the patient’s upper hip and the right hand is under the patient’s upper (left) thigh. The fourth nurse supports the lower portion of the patient’s upper leg (see Figure 33.3a). When members of the turning team are of different heights, the bed height should benefit all and should be negotiated on each occasion.
Figure 33.3 (a) Position of hands for a log-roll; (b) turn to 90° side lying position; (c) posture of the nurse holding the head at the end of the log roll; (d) 30° side lying position.
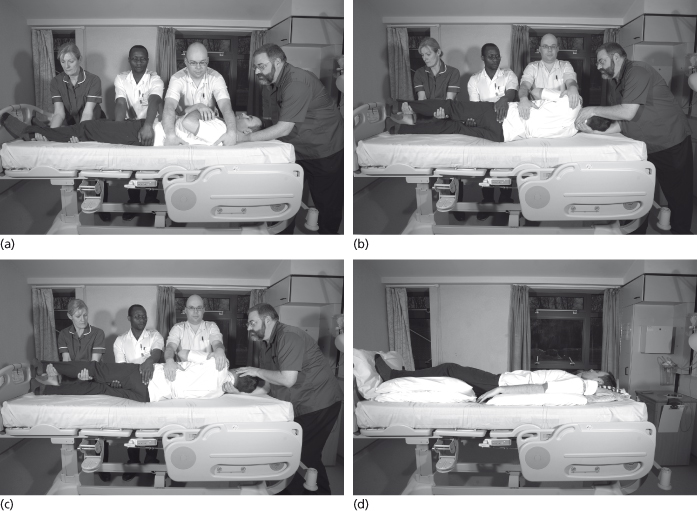
The logroll is undertaken using the commands: ‘Ready, Steady, Roll’. The patient is logrolled in unison by the turning team to a 90° side-lying position (see Figure 33.3b) Spinal alignment during logrolling includes maintaining lateral alignment, therefore the upper leg should always be held so that the outer malleolus of the heel is aligned with the trochanter of the upper hip. The nurse holding the head experiences a degree of lateral flexion during the maneouvre but adjusts to a more comfortable posture at the end of the logroll (Figure 33.3c). When the next turn is due it is imperative that staff members occupy a different position in the turning team to avoid repetitive strain injury. This is particularly so for the nurse holding the head.
After full examination of the underlying skin for signs of pressure damage, pillows or foam wedges can now be placed in situ, sufficient to maintain the patient in a 30° lateral position (see Figure 33.3d). Lateral positioning can vary between 30° and 90° dependent upon skin condition, comfort and clinical need, although a position between 20° and 30° is preferred in order to prevent excessive pressure being exerted upon the lower (right) trochanter of the hip.
Where staffing numbers make manual turning difficult, or where a patient presents with injuries to both the cervical and thoracolumbar spine, or with multiple injuries, which make lateral positioning difficult or painful, an electric turning bed such as Huntleigh’s Atlas (see Figure 33.4) can be used.
Figure 33.4 Electric turning bed.

NURSING MANAGEMENT OF THE ACUTE SCI PATIENT
Respiratory system
The impact of SCI upon the respiratory system creates the greatest potential for morbid complications. Complete traumatic paralysis above the level of C3 usually results in death for many before paramedic intervention or hospitalisation can occur. Survival at this level requires lifetime ventilator support following an extended period of rehabilitation. This requires the patient to adapt not only to depending on personal carers to maintain their activities of daily living, but also to their chronic dependency on a medical device to provide for their basic respiratory need – to breathe (North, 1999). However, the non-progressive nature of SCI coupled with advances in both respiratory medicine and medical device technology means that most ultra-high level SCI patients can realise a longevity and quality of life after discharge.
For those with lesions at C4 and below, the respiratory impact of SCI is relative to the extent of respiratory muscle paralysis. Most of the initial respiratory problems are due to accompanying trauma or pulmonary contamination at the scene. Careful monitoring of respiratory effort is required as patients can fatigue quickly, In addition to regular comprehensive respiratory assessments, forced vital capacity (FVC) should be measured (see Chapter 15 for respiratory assessment). Inspiratory effort in tetraplegics is usually better than expiration, therefore monitoring for carbon dioxide retention is essential to avoid hypercapnia.
During bedrest, the diaphragmatic excursion is enhanced if the patient is nursed supine; therefore, any need to nurse the patient in a semi-recumbent or sitting position should first be discussed with an SCI specialist as this can increase the risk of lower lobe consolidation. Regular side-to-side turning also improves the movement of pulmonary secretions, preventing pooling and the stasis of fluid which can increase the risk of bacterial growth.
Tetraplegics and high paraplegics are unable to cough effectively due to their non-functional abdominal muscles. Clearing secretions often requires the use of manual ‘assisted coughing’ techniques, intermittent positive pressure breathing (IPPB) and the use of suction equipment (see Chapter 15). During tracheal and pharyngeal suctioning the patient’s pulse should be closely monitored as tetraplegics are prone to vasovagal stimulation resulting in excessive bradycardia and cardiac syncope (refer to Neurogenic shock in Chapter 14).
Cardiovascular system
Much has already been said of the impact of neurogenic shock (see Chapter 14) on the cardiovascular system. Nurses must ensure that the new parameters established for ‘acceptable’ blood pressure, pulse and urinary output as well as ‘triggers’ prompting intervention are well documented and communicated at every handover and between disciplines. This should also be emphasised whenever the patient is transferred to another ward or department.
Hypotension and persistent bradycardia are prevalent in higher levels of SCI but after resuscitation and stabilisation of the patient, they remain relatively stable during the initial period of bedrest. However, blood pressure can still drop due to orthostatic hypotension during periods of sitting and/or standing necessitating a programme of gradual mobilisation which may include the use of recliner wheelchairs, abdominal binders and prophylactic vasoconstrictor drugs. Orthostatic hypotension usually resolves within the first month of mobilisation but severe orthostatic hypotension can both delay and extend the initial mobilisation and rehabilitation of SCI patients, and if ignored or overlooked can even lead to neurological deterioration in previously stable patients (El-Masry, 1993).
Due to loss of sympathetic activity temperature control will be significantly compromised. The patient’s temperature will be dependent on that of the environment (poikilothermia – see Chapter 14). It is therefore imperative for nurses to protect the patient from adverse environmental temperature, i.e. too hot or too cold. During bedbathing and neurophysiological examination, the minimum amount of flesh should be exposed in turn. During diagnostic imaging and surgery, extra layers of protection may be needed to protect against the effects of air conditioning. Heating coils and warm-air devices should be used cautiously and only if properly distanced or insulated from contact with any paralysed skin. In contrast, during hot spells the patient will need to be cooled.
Even in the presence of appropriate prophylaxis, enforced bedrest and systemic paralysis increase the risk of venous thromboembolism (VTE). Unexplained fever is often the first indicator of deep vein thrombosis (DVT) as the patient will not experience pain in the affected limb. A general feeling of malaise, sweating, confusion or bizarre speech or behaviour may be early indicators of pulmonary or fat embolism.
Prophylactic anticoagulation (unless contraindicated) and the application of properly sized thigh length thromboembolic device (TED) stockings, or the application of pneumatic compression devices, and passive movements are well established preventative strategies for SCI patients. Often overlooked however are the benefits of regular turning and repositioning of limbs by nursing staff throughout the day. Frequent turning during the day has a beneficial effect on circulation throughout the body. In addition, frequent handling of lower limbs enables earliest detection of ‘suspect’ areas of heat, swelling or discolouration. TEDs should be removed at least twice every day to check for pressure marking and should be resized regularly. The repositioning of lower limbs on pillows during turning and when the patient is supine also encourages venous drainage (see Figure 33.5). Remember that the patient also has a role to play in the prevention of VTE by complying wherever possible with active respiratory and limb exercises as outlined by the physiotherapist.
Figure 33.5 Tetraplegic in flat supine with legs supported.

VTE is not just a risk for the patient in bed. Thromboprophylaxis continues into the early mobilisation programme as the paralysed body adapts to new pressures during sitting. Proximal rather than distal vessels become the most prevalent site of DVT. As with turning in bed, the graduated mobilisation and/or standing programme incorporating regular position changes and pressure relief are also beneficial to the circulatory system.
Neurological system
The frequency at which neurological observations are performed is based upon the patient’s neuro-physiological presentation at admission, but generally they are recorded at fifteen minute intervals for the first two hours following admission and at less frequent intervals as the patient’s condition stabilises. Sensation is assessed at and around the point at which the patient says sensation has changed (see Chapter 11). This point should be clearly indicated on a dermatome chart to enable detection of deterioration in function between nurse observers. Motor power is assessed by asking the patient to demonstrate strength and range of movement in muscle groups at and above the level of paralysis. Patient effort is graded and recorded on a myotome chart. Inter-rater reliability between nurse observers is enhanced by asking the patient to demonstrate best motor effort during handover. Any noticeable degree of deterioration or improvement in neurophysiology should be reported immediately to a senior colleague or doctor for further evaluation.
Spasticity can occur during the acute period of SCI (see Chapter 11). It is most prevalent in patients with sensory incomplete lesions. Reflex spasms can be triggered by cuticular stimulation during daily patient care such as turning and bedbathing. The spasms can prove an additional challenge to maintaining spinal alignment and staff safety during turns and transfers. Spasms can also occur in response to a patient’s physical and emotional discomfort and thus can serve as a useful indicator of complications causing visceral discomfort below the level of lesion, such as bladder and bowel distension. The onset of spasticity should be anticipated and the patient and family educated about the cause and informed that it is not a sign of restoration of normal motor function. Spasm can prove useful to many SCI patients who can use it to assist in standing and transferring between sitting surfaces.
The initial management is usually focused upon correct positioning in bed, stretching and exercising of limbs by physiotherapists and small doses of antispasmodic medicines such as baclofen until such time that the potential benefits have been fully explored by the rehabilitation team.
Neuropathic pain
Pain is common following spinal cord injury; it is discussed in more detail in Chapter 17. Pain originating above the level of spinal cord injury can be managed in accordance with standard local pain management guidelines with the proviso that opiate use is initially avoided in non-ventilated tetraplegic patients for fear of suppressing voluntary respiratory effort. In addition, early opiate use may mask the initial detection of cervical lesion extension, which can also present as respiratory distress.
Pain perceived by the patient at or below the level of spinal cord lesion may arise from a local tissue injury or may have been referred from another region of the body or a visceral organ. Any complaint of pain below the level of lesion should first be investigated in order to exclude any pathology before a diagnosis of chronic neuropathic pain is made.
The guiding principle for pharmacological treatment of neuropathic pain after SCI is to first use those treatments that are simply administered and less likely to cause untoward side-effects. However, a variety of alternative treatment methods are available and a multi-faceted approach often proves more effective. All treatments tried, whether pharmacologically based or not, should be given an adequate trial and withdrawn appropriately if ineffective. Multidisciplinary teamwork is the cornerstone of effective pain management and the contributions of individual team members often overlap. Careful handling and correct positioning of the patient by nurses may make lying in bed more comfortable for the patient thereby potentially increasing the effect of current analgesia and possibly even reducing prescribed dose or frequency. Patient engagement in physical therapy may be enabled by timing exercise programmes to match the achievement of therapeutic levels of analgesia in the patient’s body. The formulation of a comprehensive treatment plan which is appropriately discussed with SCI centre staff and agreed to by the patient is essential if neuropathic pain is not to become a barrier to rehabilitation.
Gentitourinary system
The SCI patient initially will present with a flaccid bladder, which will require an indwelling catheter to protect the bladder and upper urinary tracts from the effects of overdistension, in addition to enabling close monitoring of urine output. A wider than usual bore of catheter (FG14–16) is required as the flaccid bladder is prone to accumulating sedimentation debris that will cause catheter obstruction. A programme of planned catheter changes is based on the patient’s catheter history as a prophylactic measure against obstruction. When spinal shock resolves and the patient’s condition is stable bladder training will begin (see: Bladder management following SCI).
Gastrointestinal system
The immediate impact of spinal shock is to temporarily suspend gut peristalsis for approximately the first 48 hours after onset. In the presence of this paralytic ileus, the SCI patient is kept nil enterally for this period. When bowel sounds return, fluids are gradually introduced leading to eventual restoration of normal diet and fluids. Failure to follow this guideline can result in abdominal distension, diaphragmatic splinting and aspiration pneumonia. As with suction catheters, nasogastric tubes should be passed cautiously in tetraplegic patients as they can cause vaso-vagal stimulation.
Prophylactic gastric protection medication should commence within 24 hours of diagnosis and continue until transfer to a SCI centre or specialist rehabilitation centre. Gastric ulceration is a long-term complication of SCI and prophylactic medicines should not be discontinued on commencement of diet or enteral feeding, even in critical care environments.
A daily per rectum (PR) check is required during the spinal shock period to check for the presence of faeces and to monitor the return of any reflex activity. Any faeces present must be removed to prevent overdistension of the bowel. (See: Bowel management following SCI).
Musculoskeletal system
During the initial period of bedrest it is necessary to protect the patient’s joints during handling as the joints of flaccid limbs are at risk of overextension. The use of footdrop splints is discouraged due to the high risk of causing pressure ulcers. Rather feet should be ‘blocked’ with pillows against a footboard during bedrest. Wrist splints should only be applied on the advice of a SCI centre occupational therapist to ensure the use of an appropriate type or design. Wherever splints are used for SCI patients, nurses must ensure that they apply and remove them as indicated in the patient’s therapy notes. Nurses should work collaboratively with physiotherapists to ensure correct positioning of the patient.
Integumentary system
At least a third of new SCI patients develop a pressure ulcer prior to being admitted to a SCI centre (Ash, 2002). These individuals are therefore required to enter rehabilitation with skin that has already been compromised. Pressure ulcers on admission cause significant delay and disruption to mobilisation and rehabilitation programmes, extend length of initial hospitalisation, increase the potential for readmission and increase hospital care costs (NICE, 2005). The prevention of pressure ulcers requires the careful management and rigorous monitoring of both intrinsic and extrinsic factors in tandem. During acute care and initial rehabilitation, the SCI patient is dependent upon nursing staff to provide regular pressure relief.
Manual turning and repositioning in bed, initially two hourly and progressing to three to four hourly intervals when spinal shock resolves (see: Moving and handling patients with SCI), is the principal method of relieving skin pressure in acute SCI patients. Mechanical turning beds are an alternative but still require the patient to be repositioned on the mattress between turns. Pneumatic alternating pressure mattresses should NEVER be used for acute SCI patients. Thermal contouring foam mattresses are appropriate but require careful monitoring of the patient’s heels to ensure that they are generating enough body heat to transform the underlying foam to its gel state.
Discolouration of the skin is often the first indicator of skin suffering under physical pressures (NICE, 2005). In hospital environments, whenever a pressure ulcer is detected in the early stages of its development, it usually resolves quickly if the patient is able to be positioned in bed in such a way as to avoid placing any further pressure on the affected area. Pressure ulcers occurring after patient mobilisation most often occur in those parts of the body which are under constant pressure during sitting. Therefore effective treatment requires the patient to stay out of their wheelchair until the damage is resolved and skin integrity is restored. This requires both the patient and the care team to accept and accommodate a temporary loss of mobility and the suspension of key rehabilitation activities.
Psychological and emotional support during acute care
The initial impact of spinal cord injury (SCI) on an individual is sudden and unexpected. Reactions will vary enormously, depending on the individual’s personality, coping style, personal circumstances and their beliefs about the future. Age, gender and previous background/experiences will have an important impact (Royle and Glass, 2007). In addition to struggling to cope with the impact of their own paralysis they may also have concerns regarding the loss of, or injuries to partners and family members involved in the same accident. Guilt and self-blame regarding the circumstances surrounding their own injury can also be present at this time.
It is not unusual for the patient and family to imagine a complete cure or recovery is possible. Media coverage of developments in research into possible cures is often exaggerated giving patients false hope of recovery. Sensitive and informed handling of this topic is essential.
Nursing staff can also feel overwhelmed and may feel inadequately prepared or supported to provide sufficient information about such a complex diagnosis (North, 1999). Experienced nursing staff may be able to support their patient with greater confidence based upon learned experience in their field of practice, but the low incidence of SCI means that outside of specialist centres such experience is often lacking. Psychological management and support of the SCI patient at this time should therefore aim to orientate both the patient and their family to the immediate hospital environment and to inform them regarding the immediate diagnosis, but sufficient only to gain their cooperation (Royle and Glass, 2007). Many patients report post-traumatic amnesia, especially in multi-trauma scenarios, so the initial information provided regarding their injury and initial impairment needs to be frequently reiterated by nursing and medical staff.
There is no established pattern or predictability of response to SCI (Trieschmann, 1988; Webster and Kennedy, 2007). The young age of many of these individuals can mean a lack of developed coping mechanisms. Therefore an initial withdrawal from or reluctance to engage in any discussion of their condition is a common defense mechanism, as is a declaration of wishing they had not survived the accident. Crying, shouting and swearing are normal responses to the frustration of immobility and the prospect of prolonged hospitalisation. Patients who perceive that their condition engenders fear and anxiety in nursing staff can also direct the same behaviours at staff because they feel threatened.
Unlike in specialist centres, where staff have chosen to work with SCI patients, nurses and medical staff unfamiliar with the post-discharge potential for quality of life after SCI may, quite reasonably, struggle to maintain a consistently informed and positive attitude in front of their patient. However difficult it may seem, nurses need to display a competence and confidence in their own abilities so that the patient feels safe in their hands. This is particularly important because the physical contact and movement experienced by the patient during turning and routine nursing care is an effective means for reducing sensory deprivation caused by the loss of touch and positional awareness.
It is important to ensure that information given to the patient and family is consistent. All SCI centres provide outreach clinical support and education. The Spinal Injuries Association website (www.spinal.co.uk ) is an appropriate means of support for patients and families. Nurses should anticipate relatives ‘Googling’ ’spinal cord injury’ and provide details of the association at admission.
Effective support requires a higher degree of understanding of both the human and social impact of the disability than is usually found in general hospital environments. Enabling patients with SCI to gain early access to specialist staff and environments is the only strategy available to reduce the potential for long-term psychological problems after injury (Trieschmann, 1988).
Autonomic dysreflexia
Autonomic dysreflexia (see Chapter 14) can occur at any time following the onset of spinal cord paralysis although its occurrence is usually observed after the period of spinal shock has resolved (Consortium for Spinal Cord Medicine, 2001). It occurs in patients with both complete and incomplete lesions. Up to 90% of people with tetraplegia or high paraplegia will experience it at some time in their lives (Braddom and Rocco, 1991). Following the first occurrence, the ‘expert’ patient becomes intimately familiar with the symptoms of AD. Autonomic dysreflexia alert cards are available from the SIA for patients at risk.
NURSING ROLE AND RESPONSIBILITIES WITHIN SCI REHABILITATION PROGRAMMES
Inpatient rehabilitation and education following SCI involves a lot more than simply re-learning new skills. People with newly acquired SCI will experience a series of challenges during their initial coping and adjustment phase. Aside from addressing the acute psychological dimension, life skills such as empowerment, self-efficacy and problem solving abilities are crucial if the patient is to be autonomous, confident and capable of dealing with day to day minor issues, or prepared to tackle major life issues such as further education, returning to work, managing finances and exploring personal and social relationships (Webster and Kennedy, 2007). The development of appropriate coping skills is known to contribute to positive mood, social integration and life satisfaction (Elliott and Warren, 2007). Individuals who are able to adjust to their new disability status, will have less dependency behaviour and this correlates positively with quality of life, while individuals with poor emotional adjustment tend to demonstrate negative coping styles (Elfstrom et al., 2005).
Elliott et al. (2006) suggest that individuals with poor problem solving abilities are more at risk of developing additional health problems and complications. Since the incidence of health problems increases with the ageing process, the more information and advice available to the individual, the better prepared they will be to cope with them.
During the initial rehabilitation phase, information needs to be delivered in a format that is relevant and appropriate to the individual. The nurse should always bear in mind the volume of information that the patient needs to digest at this time, and so information regarding changes to their status or disability as a consequence of ageing may not be appropriate or a priority. Expanding the individual’s knowledge base in such a direction should be saved for a more appropriate time (Elliott and Warren, 2007). Whilst health care professionals would like to explore with the patient the potential for future complications and problems and to some degree these are touched upon, the initial focus is much more on immediate concerns and initial post-discharge care issues and not what will happen as they age.
As an organisation predominantly run by people with SCI, the Spinal Injuries Association shares a commitment with other SCI service providers to deliver continuous and long term support, and their peer support scheme bears testament to this. Peer support officers are positive role models, whose unique insight enables newly paralysed individuals to acquire a more informed opinion of their future potential in the world after discharge. Unlike health care professionals, peer support officers are in a unique position to engage in facilitating problem-solving approaches with other spinal cord injured individuals and, because their role and relationship with them extends beyond the initial hospital experience, they can continue to provide their peer support ‘lifeline’ to the individual after hospital discharge.
Community Peer Support can include introducing the newly discharged individual to a more extensive peer network that can enable wider access to a range of vocational and non-vocational opportunities. Peer networks are an excellent source of post-discharge support and community integration, all of which are known to contribute to positive well-being (Beedie and Kennedy, 2002).
Bladder management following SCI
The change to bladder function after SCI is caused by the disruption of nerve supply and is therefore termed neurogenic bladder dysfunction. Some patients with incomplete lesions (e.g. Brown-Séquard), may have normal or near-normal bladder function. If the sacral segments of the spinal cord have been destroyed the bladder is totally disconnected from nervous control. People with complete sacral SCI effectively have an areflexic bladder, although the bladder muscle may recover some tone and therefore fail to relax during filling which results in high bladder pressures, kidney damage and infection. The urethral sphincter is weak, causing stress incontinence (leakage on coughing, straining, etc).
A typical patient with an areflexic bladder will have a lesion below T12/L1 level (Fulford et al., 2004). If the sacral segments survive, the bladder detrusor muscle and urethral sphincter maintain a nerve supply from the sacral micturition centre, but their actions are not coordinated by the pontine micturition centre because of the disruption in communication caused by the intervening SCI. If the bladder emptying process is not perfectly coordinated, detrusor–sphincter dyssynergia (DSD) can occur. This is where the detrusor may contract against a tight sphincter resulting in abnormally high bladder pressures which damage the upper tract. A further consequence of DSD is incomplete emptying of the bladder which can lead to retention of ‘stale’ urine and urinary tract infection (UTI) (Reynard et al., 2003). A typical patient with a reflex bladder potential will have a lesion above T12/L1 (Fulford et al., 2004).
There are a number of other patterns of bladder dysfunction. Many people with incomplete SCI may have some degree of sensory sparing or even voluntary control of the urethral sphincter. Urge incontinence (see Chapter 18) is common in central cord syndrome (Fulford et al., 2004). Others may have a degree of voluntary control over voiding but be unable to use a toilet or urinal independently because of a lack of hand function or reduced mobility. Yet others may experience unpleasant or painful sensations as the bladder fills. Those with partial damage to the sacral spinal cord may have a reflex bladder but a weak urethral sphincter causing both urge and stress incontinence (Fulford et al., 2004).
The primary aims of bladder management after SCI are to:
- Achieve a system of management that is safe and acceptable, arising from a process of negotiation with the patient
- Protect the upper tract by preserving a low-pressure system and ensuring adequate emptying
- Minimise the risk of renal and ureteric stone formation and chronic infection
- Preserve the capacity of the bladder.
Box 33.4 shows a summary of the methods available for managing neurogenic bladder.
Catheters (intermittent or indwelling) may cause urethral stricture. Erosion of the penile urethra is possible if indwelling catheters or external urinary collection devices are poorly managed. Bladder stones are common in patients with indwelling catheters (Fulford et al., 2004).
Box 33.4 Methods for managing the neurogenic bladder
| Intermittent self-catheterisation |
|
| Indwelling urethral catheters |
|
| Suprapubic catheterisation (SPC) |
|
| External urinary collection devices (e.g. penile sheath, bioderm) |
|
| Voiding with voluntary control |
|
For further detail see Chapter 18.
Management of urinary incontinence and bladder dysfunction is discussed further in Chapter 18.
Bowel management following SCI
As voluntary control is mainly conferred via the sacral nerves (S2–S4), any SCI is likely to affect neurological communication between the brain and the anorectal structures (Fulford et al., 2004). Lesions above T12/LI principally affect upper motor neurones, leaving the reflex pathways that utilise the lower motor neurones intact. In this type of dysfunction, the reflex functions of the anorectum are preserved, but sensation and voluntary control are abolished (Fulford et al., 2004). Areflexic dysfunction typically affects patients with neurologically complete lesions at L1 and below. As with reflex dysfunction, voluntary control and sensation are disrupted, but in addition, there is an absence of reflex function. This restricts the options for management (Fulford et al 2004). Some individuals with incomplete lesions may have a degree of preserved sensation or residual voluntary control. However this may not be sufficient to enable reliable bowel control (Consortium for Spinal Cord Medicine, 1998).
The primary aims of bowel management after SCI are to:
- Achieve a system or programme of management that is both safe and acceptable to the individual
- Prevent incontinence in order to enable social and recreational activities including employment and educational opportunities
- Enable sexual activity and other intimate human behaviours
- Protect skin integrity
- Enable the patient to complete a routine bowel management episode within a maximum of one hour from commencement
Involvement of community nurses is best avoided wherever possible because of the restrictions this places on the lifestyle of the individual. The capacity of local community nursing services to assist with bowel care in accordance with an established programme should always be confirmed beforehand and the alternative option of employing personal carers explored where available.
The components of a bowel management programme are as follows:
- Maintain continence, avoid constipation, and facilitate regularity of evacuation, by maintaining an appropriate consistency of stool through careful attention to diet, fluid intake and physical activity as well as the use of oral laxatives and rectal stimulants (Coggrave et al., 2006)
- Gentle abdominal massage can be used as a precursor to the insertion of rectal stimulants, and before and after digital stimulation, in order to aid evacuation (Coggrave, 2005)
- Most individuals with reflex bowel dysfunction can be established on an alternate day routine, while those with areflexic bowel dysfunction usually need, or prefer, to evacuate at least once a day
Box 33.5 shows a summary of the management of neurogenic bowel.
Box 33.5 Evacuation techniques for managing the neurogenic bowel
| Areflexic bowel management
Because the bowel will not respond to rectal stimulants, as there is no reflex activity, the principal evacuation technique in areflexic bowel dysfunction is digital removal of faeces (DRF) This is an established form of bowel management and there is no evidence that it is harmful if performed properly. For most patients there is no satisfactory alternative |
|
| Reflex bowel management
The nursing care strategy for long-term reflex bowel management is to achieve complete and timely evacuation by using the mildest form of a proprietary chemical stimulant initially (usually suppositories or microenemas), reserving stronger stimulants for future occasions when problems may arise |
|
Consortium for Spinal Cord Medicine, 1998; Multidisciplinary Association of Spinal Cord Injury Professionals, 2009.
There are comprehensive guidelines for neurogenic bowel management on the Multidisciplinary Association of Spinal Cord Injury Professionals (MASCIP) and SIA websites (MASCIP, 2009). Professional guidelines are also available from the Royal College of Nursing (RCN, 2005; RCN, 2008). The National Patient Safety Agency recommends that every hospital and community NHS trust has a policy in place which meets the need to provide digital rectal evacuation procedures for patients with established spinal cord injuries (NPSA, 2004). Failure by a nurse to provide for this fundamental patient care requirement may precipitate autonomic dysreflexia (see: Autonomic dysreflexia) and would constitute both professional and clinical negligence.
SCI people requiring continuing assistance with bowel care should have a discharge care plan which outlines:
- The specific interventions to be used
- The regularity and timing of interventions
- Who is going to perform interventions
- Where bowel evacuation will take place
- Equipment and any adaptations required
Any changes that are made to the bowel management programme need to be assessed for effectiveness, so it is best to adopt a systematic approach: change only one element of the programme at a time, and allow at least a week between each change. Problems are generally grouped under three headings:
- Incontinence (unplanned evacuations)
- Constipation
- Prolonged management episodes
Management of constipation and fecal incontinence is discussed further in Chapter 18.
Changes in bowel habit or stool formation (loose stools, constipation or the unexpected presence of blood) that have persisted for six weeks or have resisted three separate changes to the established bowel management programme may be indicative of bowel cancer and should be investigated without further delay (www.cancerscreening.nhs.uk). Persisting in the belief that symptoms are solely related to neurogenic bowel dysfunction can have serious implications for the SCI individual (Poduri and Schnitzer, 2001).
Other options for managing the neurogenic bowel
For a minority of individuals with SCI a conservative bowel management programme may fail to meet the aims outlined above. In this case, the suitability of transanal irrigation should be considered (Christensen et al., 2006). The Peristeen device for transanal irrigation (Coloplast UK) can improve bowel management and independence with bowel care in many SCI patients. However, its suitability for an individual should be carefully assessed by an experienced professional (MASCIP, 2009). Otherwise, the formation of a stoma may be required to maintain the individual’s personal comfort and holistic health. Stoma formation may be considered in the following cases:
- Intractable faecal leakage
- Pressure sores caused by prolonged toilet routine
- To deliver a continent solution that can be independently managed by an individual with limited hand function
Whenever a stoma is considered, it is essential that the SCI centre or rehabilitation team are consulted to ensure that the full implications of SCI, such as the impact of ageing on the neurogenic bowel and active wheelchair living with a stoma, have been appropriately considered to enable the SCI person to give his fully informed consent to surgery (Consortium for Spinal Cord Medicine, 1998).
Sexual issues and sexual dysfunction after SCI
Sexual function is highly complex, involving a continuous combination of psychological, hormonal, vascular and neurological factors (Frohman, 2002). The neurological changes following injury undoubtedly have the most profound and permanent effect on sexual function for individuals with a SCI. Possible concerns that they may have are listed in Box 33.6.
Box 33.6 Possible sexual and sexuality concerns of SCI people
- Projecting a confident and sexually attractive body image
- Maintaining relationships with existing sexual partners
- Finding new sexual partners and forming new relationships
- Loss of libido (sex drive)
- Perceived or actual loss of spontaneity
- Difficulties as a result of changing roles and expectations within relationships as a consequence of SCI
- Ability to achieve erections (erectile dysfunction) in men, or vaginal lubrication in women, as a response to sexual arousal
- The pleasurable sensations arising from diverse sexual activity
- The experience of orgasm
- The ability to ejaculate as a result of sexual activity (ejaculatory dysfunction) in men
- Reproductive ability and contraception
- Managing menstruation
- The ability to self-stimulate or masturbate
- The ability to adopt different sexual positions
- Maintaining continence during sexual activity
- Maintaining intact skin
- Pain and spasm during sexual activity
- Autonomic dysreflexia triggered by sexual stimulation
Source: Lobley, 2002.
Addressing the totality of the impact of SCI on the sexual function and sexuality of individuals is challenging, as many of the effects emerge relatively slowly as individuals readjust and reintegrate after initial hospitalisation. It is difficult to prepare a newly paralysed individual for what to expect, especially as the focus for professionals and SCI people alike is frequently on the more basic functional aspects of SCI rehabilitation (bowel and bladder care, self-care skills, etc.). In addition, many professionals feel that they do not have sufficient interpersonal skills or specialist knowledge to deal with such intimate and complex issues (Herson et al., 1999).
Initiating discussions about sexual issues, should, as far as is possible, be directed by the SCI person who should be enabled to choose someone they trust, at a time they feel appropriate and in a setting they feel suitable. For their part, professionals should be able and willing to respond to direct or indirect cues that the patient is ready to talk, and should find ways to let them know that it is acceptable to start talking about these intimate concerns (Herson et al., 1999). They should also be aware of sources of additional support and specialist knowledge and expertise either locally or nationally, such as that provided by the SIA.
Most SCI males have some degree of erectile dysfunction and in addition, any SCI is likely to disrupt ejaculatory function. However, there have been many advances in treatment over the past 20 years (Hatzichristou and Pescatori 2001) and it is now possible to treat most erectile and ejaculatory dysfunction in SCI men. Specialist clinics now exist in many hospitals to supplement the services provided by SCI centres. Skilled patient education and follow-up is essential for all of these treatments and can have a marked impact upon effectiveness.
After an initial period of amenorrhoea, which is a normal reaction to the severe metabolic disturbance that occurs immediately post injury, women with SCI usually regain their full reproductive abilities within their first post-injury year (Charlifue et al., 1992) although there is a slight chance that in some vulnerable mature women, the trauma of SCI may induce an early menopause. The same neurological pathways (both reflexogenic and psychogenic) that govern erectile function in men produce clitoral and vulval engorgement and vaginal lubrication in women in response to arousal (psychogenic) or cutaneous genital stimulation (reflex). The degree of dysfunction will depend on the level and degree of completeness of the SCI.
This emphasis on dysfunctions and their treatment may give SCI people and their partners the impression that sexual activity is no longer about having fun or sharing sensual pleasure, and can no longer be spontaneous. On the other hand, once the initial psychological and emotional impact of SCI subsides, arousal and desire are often fully restored. Though genital sensation is often absent, greatly diminished or altered, most spinal cord injured people report a magnification of the pleasurable sensations in erogenous zones above the level of injury. Individuals should be encouraged to explore the possibilities of such changes in sensation. Most spinal cord injured people also discover that a huge part of the pleasure of sexual activity comes from giving pleasure to their partner, and that this does not necessarily require penetrative sex or genital sensation.
The prevention and management of pressure ulcers
The risk of pressure ulcer development increases with age and 80% of people with a chronic SCI will experience at least one pressure ulcer of significance within their lifetime (Byrne and Salzberg, 1996). Pressure ulcers form at least 30% of SCI centre readmissions (Rodriguez and Garber, 1994; Krause, 1998) and up to 8% of spinal cord injured individuals will die as a direct consequence of developing a pressure ulcer (Byrne and Salzberg, 1996). For this reason, the education of patients and their carers in the care and protection of those areas of skin presenting with lost or reduced sensation is a fundamental and heavily promoted component of SCI rehabilitation programmes. Pre-discharge education programmes within SCI centres benefit from their ability to utilise peer support to enable patients to develop a more realistic appreciation of the true impact of developing a pressure ulcer. The true potential for pressure ulcer development after discharge is based upon the individual’s self-perception of risk (Arnold, 1994). Those who are able to establish and maintain a responsible attitude towards active living cannot avoid pressure ulcers but, by acting appropriately when one does occur, will experience fewer days adversely affected by them. In the initial period following discharge, compliance with precautionary skin care procedures is usually high as the perceptible risk increases with the return of the patient to a new environment. However, following discharge the routine monitoring of skin using a hand mirror may soon become perceived as an avoidable chore. To sustain this task over time requires a level of belief, motivation and discipline that may prove difficult to maintain in the face of the distractions which present after discharge.
In the community, the development of a pressure ulcer means that a previously independent paraplegic may find himself reliant upon family, friends, community professionals and home care services for a wide range of personal care tasks. It may be no different for tetraplegic individuals being nursed in bed at home with subsequent disruption of established care packages and family routines. Many SCI people express a distinct lack of confidence in community services and local general hospitals (Gibson, 2002). This is because they need to be convinced that nurses outside of specialist centres can demonstrate an appropriate knowledge of SCI in general and of the management of pressure ulcers in people with SCI in particular. As a consequence, most spinal cord injured people expect their pressure sore to be managed by SCI centre community liaison teams and to be readmitted to a SCI centre if hospitalisation is necessary. This additional reliance upon the SCI centre is in fact proving detrimental, resulting in an increased workload which should be the remit of community services (Gibson, 2002).
FUTURE RESEARCH
At this time, spinal cord injury results in a permanent loss of neurological function because, although the embryonic spinal cord can repair itself, the adult spinal cord does not regenerate spontaneously after injury. Research has identified a number of factors which currently influence our ability to repair or regenerate the spinal cord after injury. These include:
- The presence of inhibitory substances in the tissue that surrounds the damaged nerve fibres
- Changes inside adult nerve fibres that make them unable to respond well to growth-inducing signals that are effective in embryonic fibres
- The formation of cysts (cavities) within the injury site, that growing fibres cannot cross
- The lack of molecules (called nerve growth factors) in the surrounding area that stimulate nerve fibres to grow
- The formation of scar tissue at the injury site that forms a physical barrier to regrowth as well as containing additional inhibitory substances
Neuroprotective research is exploring the use of pharmaceutical agents or tissue factors administered very soon after the initial injury to limit the extent of cell damage, or to reduce the amount of scarring. The benefits of particular surgical techniques to stabilise or reduce pressure on the cord due to the column fracture are also being examined. Research into the repair or regrowth of the spinal cord is exploring the introduction of embryonic tissue, and attempting to transform nerve fibres in the adult spinal cord to a state that more resembles embryonic nerve fibres. Attempts are also being made to increase the growth of new nerve fibres into and around spinal cord lesion sites by overcoming the inhibitory factors described above. The current scientific consensus believes that combining individual treatments into a form that is appropriate clinically is the next step in developing an effective therapy for spinal cord repair (Adams and Cavanagh, 2004).
The core aim of most of this research is to protect or restore spinal cord functions for the benefit of a future population. Research intended to benefit chronically injured individuals is still in the early stages of development. A realistic expectation for this population is that small advances in the primary research projects outlined above will enable a similar level of discrete clinical opportunities such as regaining upper limb dexterity in tetraplegia, improving bladder, bowel or sexual function, or the relief of chronic pain (Adams et al., 2007). Individuals seeking further insight into this research are best directed to the websites of the International Spinal Research Trust (www.spinalresearch.org) and The International Campaign for Cures in Spinal Cord Injury Paralysis (ICCP) (www.campaignforcure.org) as the two most credible authorities on this subject.
In addition to these attempts to repair or regrow the spinal cord we should not overlook the efforts of other researchers whose intentions are to bypass the effects of a spinal cord lesion through the use of implantable computerised nerve stimulators.
Spinal cord injury: patient perspective
Spinal cord injury (SCI) is a complex lifetime condition, it is rarely fully understood by clinicians working outside its field, although in my experience many find it hard or refuse to admit this.
As a SCI patient of over 40 years I have both experienced and seen, through my peers, what happens when our condition is managed badly. Not through intent or neglect, rather through lack of knowledge, understanding and expertise, and often through a lack of willingness on the part of the treating clinicians, at all levels, to liaise with and consult with expert peer clinicians (within a SCI centre), who fully understand the condition and the serious (life-threatening) consequences that can and do arise from inappropriate treatment. Perhaps you could call that neglect, or perhaps professional pride being put before the welfare of the patient. Whatever label you give, the consequences for someone with SCI can be devastating. I am fortunate in having a GP who despite being extremely capable recognises my expertise in my condition. He listens to me. He listens to my SCI centre.
That is not to say I would want all my acute treatments to be carried out in my SCI centre, only those that directly relate to my SCI. For instance, if I were to suffer a heart attack I would want my treatment to be in a coronary unit. But I would want my care whilst I was in that unit to be managed under the guidance of my SCI centre, particularly my nursing care. Similarly, if a member of my family were to suffer a suspected SCI, I would want/expect the A&E unit they were taken to, to contact the nearest SCI centre immediately, and be guided by them, setting up a programme of care to manage their condition, a condition that may include trauma incidences other than SCI, until such times as they could be safely transferred to a specialist SCI centre.
In my 40 years of being SCI I have, like many of my peers, retrained, gone back to full employment, married, integrated fully into my community, travelled the world, lived an ordinary life under extraordinary circumstances. Being comfortable in the knowledge that I have the constant support of a SCI centre, that knew me and my condition was, and still is, a major factor in allowing me to live a relatively normal life.
Written by a C5 tetraplegic
SUMMARY
A spinal cord injury is a life changing event because it impacts on every aspect of the individual’s life. The challenges the individual faces are numerous and are often complex, requiring skilled and experienced professionals to manage them. The support of a specialist multidisciplinary team is essential to give the individual the best possible chance to reach their full potential and to equip them with the necessary skills to enable them to be autonomous and confident in dealing with day to day challenges that they will face.
USEFUL WEBSITES
| www.iscos.org.uk | The International Spinal Cord Society (ISCoS) is an international association whose purpose is to study all problems relating to traumatic and non-traumatic lesions of the spinal cord and to support health care professionals involved in SCI patient care throughout the world. |
| Spinal Cord Injury UK (website pending in 2010) | A new consortium of the core professional associations and charities outlined below intended to develop and promote a national consensus regarding core issues for government, professionals and the public. |
| www.mascip.co.uk | The Multidisciplinary Association of Spinal Cord Injury Professionals provides peer support and consensus guidelines for multi-professional teams caring for spinal cord injured individuals both within and outside of UK and Irish SCI centres. |
| www.bascis.pwp.blueyonder.co.uk | The British Association of Spinal Cord Injury Specialists incorporates UK and Irish SCI centre consultants and clinicians associated with SCI medicine and rehabilitation. BASCIS provides peer support and consensus guidelines for the medical and surgical management of spinal cord injured individuals. |
| www.spinal-research.org | Spinal Research is the UK’s leading charity funding medical research around the world to develop reliable treatments for paralysis following spinal injury. |
| www.campaignforacure.org | The International Campaign for Cures of SCI Paralysis (ICCP) is a body of affiliate nonprofit organisations, working to fund research into cures for paralysis caused by spinal cord injury. The ICCP coalition mission is ‘to expedite the discovery of cures for SCI paralysis.’ |
| www.escif.org | The European SCI Federation represents a federation of national self-help SCI organisations working to safeguard and promote the interests of spinal cord injured people. |
| www.spinal.co.uk | The website of the Spinal Injuries Association, the UK charity for people living with spinal cord injury. An excellent resource for patients and families. |
| www.sisonline.org | Spinal Injuries Scotland is a charity supporting people living with spinal cord injury in Scotland. |
| www.aspire.org.uk | Aspire is a charity which offers practical support to the people living with SCI in the UK so that they can lead fulfilled and independent lives in their homes, with their families, in work-places and leisure time. |
| www.backuptrust.org.uk | The Back-Up Trust is a charity which provides a range of activity-based services for people with SCI as well as their friends, family and volunteers to encourage independence, self-confidence and motivation following a life changing injury. |
REFERENCES
Adams M, Cavanagh JFR (2004) International campaign for cures of spinal cord injury paralysis (ICCP): another step forward for spinal cord injury research. Spinal Cord 42:273–280.
Adams M, Carlstedt T, Cavanagh J et al. (2007) International Spinal Research Trust research strategy III: A discussion document. Spinal Cord 45:2–14.
American College of Surgeons’ Committee on Trauma (ACS) (2008) Advanced Trauma Life Support Manual for Physicians. (8th edition). Chicago: ACS.
Aung S, El-Masry WS (1997) Audit of a British centre for spinal injury. Spinal Cord 35:147–150.
Arnold N (1994) Clinical study: the relationship between patient perceived risk and actual risk of for the development of pressure sores. Ostomy Wound Management 40(3):36–45.
Ash D (2002) An exploration of the occurrence of pressure ulcers in a British spinal injuries unit. Journal of Clinical Nursing 11:470–478.
Ash D, Harrison P (2007) Understanding spinal shock. In: Harrison P (ed). Managing Spinal Cord Injury: The first 48 hours. Milton Keynes: Spinal Injuries Association.
Banit DM, Grau G, Fisher JR (2000) Evaluation of the acute cervical spine: A management algorithm. Journal of Trauma: Injury, Infection and Critical Care 49:450–456.
Beedie A, Kennedy P (2002) Quality of social support predicts hopelessness and depression post spinal cord injury. Journal of Clinical Psychology in Medical Settings 9:227–234.
Braddom RL, Rocco JF (1991) Autonomic dysreflexia: A survey of current treatment. American Journal of Physical Medicine and Rehabilitation 70:234–241.
Boerger TO, Limb D, Dickson RA (2000) Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures? Journal of Bone and Joint Surgery (Br) 82-B:629–635.
British Orthopaedic Association (BOA) (2006) The Initial Care and Transfer of Patients with Spinal Cord Injuries. London: BOA.
British Trauma Society (2003) Guidelines for the initial management of spinal injury. Injury 34:405-425.
Byrne DW, Salzberg CA (1996) Major risk factors for pressure ulcers in the spinal cord disabled: a literature review. Spinal Cord 34:255–263.
Carvell J, Grundy, D (1994) Complications of spinal surgery in acute SCI patients. Paraplegia 32:1389–1395.
Charlifue SW, Gerhart KA, Menter RR et al. (1992) Sexual issues of women with spinal cord injuries. Paraplegia 30:192–199.
Christensen P, Bazocchi G, Coggrave M et al. (2006) A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 131(3):738–747.
Coggrave M (2005) Management of the neurogenic bowel. British Journal of Neuroscience Nursing. 1(1):6–13.
Coggrave M, Wiesel PH, Norton C (2006) Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database of Systematic Reviews Issue 1. Art. No.: CD002115. DOI: 10.1002/14651858.CD002115.pub3
Consortium for Spinal Cord Medicine (1998) Neurogenic Bowel Management in Adults with SCI: Clinical practice guidelines. Washington DC: Paralyzed Veterans of America.
Consortium for Spinal Cord Medicine (2001) Acute Management of Autonomic Dysreflexia: Individuals with spinal cord injury presenting to health care facilities. Washington DC: Paralyzed Veterans of America.
Consortium for Spinal Cord Medicine (2008) Early Acute Management in Adults with Spinal Cord Injury. Washington DC: Paralysed Veterans of America.
Ditunno JF, Little JW, Tessler A et al. (2004) Spinal shock revisited: a four-phase model. Spinal Cord 42:383–395.
El-Masry WS (1993) Physiological instability of the spinal cord following injury. Paraplegia 31:273–275.
Elliott T, Warren AM (2007) Why psychology is important in rehabilitation. In: Kennedy P (ed). Psychological Management of Physical Disabilities. London: Brunner-Rutledge Press.
Elliott T, Bush B, Chen Y (2006) Social problem solving abilities predict pressure sore occurrence in the first three years of spinal cord injury. Rehabilitation Psychology 51:69–77.
Elfstrom M, Ryden A, Kreuter M et al. (2005) Relations between coping strategies and health-related quality of life in patients with spinal cord lesion. Journal of Rehabilitation Medicine 37(1):1650–1677.
Fehlings MG, Miyanji F, Furlan JC et al. (2007) Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome – prospective study with 100 consecutive patients. Radiology 243:820–827.
Fisher JD, Brown SN, Cooke MW (eds) (2006) UK Ambulance Service: Clinical practice guidelines. Joint Royal Colleges Ambulance Liaison Committee and the Ambulance Services Association: London. Available from (http://www.jrcalc.org.uk). Accessed July 2010.
Frohman EM. (2002) Sexual dysfunction in neurologic disease. Clinical Neuropharmacology 25:126–132.
Fulford S, Coggrave M, Wright L (2004) Bladder and Bowel Management for People with Spinal Cord Injury. London: Spinal Injuries Association.
Gall A, Harrison P (2007) The process of spinal cord lesion formation. In: Harrison P (ed) Managing Spinal Cord Injury: The First 48 Hours. Milton Keynes: Spinal Injuries Association.
Gibson L (2002) Perceptions of pressure ulcers among young men with a spinal injury. British Journal of Community Nursing 7:451–460.
Harrison P (2000) Spinal Injury: Critical care. London: Spinal Injuries Association.
Harrison P (2007) (ed) Managing Spinal Cord Injury: The first 48 hours. Milton Keynes: Spinal Injuries Association.
Hatzichristou DG, Pescatori ES (2001) Current treatments and emerging therapeutic approaches in male erectile dysfunction. British Journal of Urology International 88 (Suppl 3):11–17.
Hawkins S, Stone K, Plummer L (1999) An holistic approach to turning patients. Nursing Standard 14:(3)52–56.
Helling TS, Watkins M, Evans LL et al. (1999) Low falls: an underappreciated mechanism of injury. Journal of Trauma 46:453–456.
Herson L, Hart KA, Gordon MJ (1999) Identifying and overcoming barriers to providing sexuality information in the clinical setting. Rehabilitation Nursing 24:148–151.
Ho M, Fung KY, Joynt GM et al. (2002) Rigid collar and intracranial pressure of patients with severe head injury. Journal of Trauma: Injury, Infection and Critical Care 53:1185–1188.
Hogan BJ, Blaylock B, Tobian TL (1997) Trauma multidisciplinary QI project: evaluation of cervical spine clearance, collar selection and skin care. Journal of Trauma Nursing 4:60–67.
Hu R, Mustard CA, Burns C (1996) Epidemiology of incident spinal fracture in a complete population. Spine 21:492–499.
Iencean SM (2003) Classification of spinal injuries based on the essential traumatic spinal mechanisms. Spinal Cord 41:385–396.
Kishan S, Vives MJ, Reiter MF (2005) Timing of surgery following spinal cord injury. Journal of Spinal Cord Medicine 28:11–19.
Krause JS (1998) Skin sores after spinal cord injury: relationship to life adjustment. Spinal Cord 36:51–56.
Lobley K (2002) Sex Matters. Milton Keynes: Spinal Injuries Association.
Marshall LF, Knowlton S, Garfin SR et al. (1987) Deterioration following spinal cord injury. A multicenter study. Journal of Neurosurgery 66:400–404.
Multidisciplinary Association of Spinal Cord Injury Professionals (2009) Guidelines for Management of Neurogenic Bowel Dysfunction after Spinal Cord Injury. Peterborough: Coloplast Ltd. (see also http://www.mascip.co.uk/)
McCutcheon EP, Selassie AW, Gu JK et al. (2004) Acute traumatic spinal cord injury, 1993–2000. A population-based assessment of methylprednisolone administration and hospitalization. Journal of Trauma: Injury, Infection and Critical Care 56:1076–1083.
Moreau APM, Willcox N, Brown MF (2003) Immobilisation of spinal fractures in patients with ankylosing spondylitis: two case reports. Injury 34:372–373.
Nacimiento W, Noth J (1999) What, if anything, is spinal shock? Archives of Neurology 56:1033–1035.
National Confidential Enquiry into Patient Outcome and Death (2007) Trauma: Who Cares? A report of the National Confidential Enquiry into Patient Outcome and Death. London: NCEPOD.
National Institute for Health and Clinical Excellence (2005) The Management of Pressure Ulcers in Primary and Secondary Care. London: NICE.
National Institute for Health and Clinical Excellence (2007) Head Injury: triage, assessment, investigation and early management of head injury in infants, children and adults. London: NICE.
National Patient Safety Agency (2004) Improving the Safety of Patients with Established Spinal Injuries in Hospital. Patient Safety Information Bulletin. London: NPSA. Available from: http://www.npsa.nhs.uk
NHS National Commissioning Group for Highly Specialised Services (NHSSCGHSS) (2009) Specialised Services: National definitions set (3rd edition). Definition No 6: Specialised spinal services (all ages). London: Department of Health. Available from: www.specialisedcommissioning.nhs.uk Accessed July 2010.
North NT (1999) The psychological effects of spinal cord injury: a review. Spinal Cord 37:671–679.
Poduri KR, Schnitzer EM (2001) Carcinoid tumour mistaken for persistent neurogenic bowel symptoms in a patient with paraplegia: a case report. Archives of Physical Medicine and Rehabilitation 82:996–999.
Ravichandran G, El-Masri WS (2005) Management of Individuals with Spinal Cord Injury in General Hospitals: Good practice guide. Oswestry: British Association of Spinal Cord Injury Specialists.
Ravichandran G (1989) Errors and omissions in the acute management of spinal cord injury. Journal of the Medical Defence Union 5:14–16.
Reynard JM, Vass J, Sullivan ME et al. (2003) Sphincterotomy and the treatment of detrusor-sphincter dyssynergia: current status, future prospects. Spinal Cord 41:1–11.
Rodriguez GP, Garber SL (1994) Prospective study of pressure ulcer risk in spinal cord injury patients. Paraplegia 32 (3):150–158.
Roth EJ, Lovell L, Heinemann AW et al. (1992) The older adult with a spinal cord injury. Paraplegia 30:520–526.
Royle J, Glass C (2007) Psychological and emotional support. In: Harrison P (Ed) Managing Spinal Cord Injury: The first 48 hours. Milton Keynes: Spinal Injuries Association.
Royal College of Nursing (2005) The Procedure for Digital Removal of Faeces. London: RCN.
Royal College of Nursing (2008) Bowel Care: Including digital rectal examination and manual removal of faeces. London: RCN.
Sapru H (2002) Spinal cord: anatomy, physiology and pathophysiology. In: Kirschblum S et al. (eds). Spinal Cord Medicine. Philadelphia: Lippincott, Williams and Wilkins.
Sarhan F, Harrison P (2007) Transferring patients to SCI centres. In: Harrison P (ed). Managing Spinal Cord Injury: The first 48 hours. Milton Keynes: Spinal Injuries Association.
Short D (2001) Is the role of steroids in acute spinal cord injury now resolved? Current Opinion in Neurology 14:759–763.
Spinal Injuries Association (2009) Preserving and Developing the National Spinal Cord Injury Service: Phase 2 – seeking the evidence. Milton Keynes: Spinal Injuries Association.
Spinal Task Force (2010) Organising Quality and Effective Spinal Services for Patients: A report for local health communities by the Spinal Taskforce. London: Department of Health. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_114528 Accessed July 2010.
Tator CH, Duncan EG, Edmonds VE et al. (1995) Neurological recovery, mortality and length of stay after acute spinal cord injury associated with changes in management. Paraplegia 33:254–262.
Trieschmann RB (1988) Spinal Cord Injuries: Psychological, social and vocational rehabilitation. New York: Demos.
Webster G, Kennedy P (2007) Spinal cord injuries. In: Kennedy P. (Ed) Psychological Management of Physical Disabilities. London: Brunner-Rutledge Press.