Niels Bohr
1885–1962
Niels Bohr (1885–1962) was one of the greatest physical chemists of the twentieth century. He proposed the ‘solar system’ model of atomic structure, in which electrons orbit the central nucleus – a model which still underpins our understanding of matter.

BOHR WAS AN INSPIRED THINKER with immense powers of concentration. He was dedicated to his work and was known for his staying power, but was also a generous and softly spoken man. His promise was evident early in his life; a fellow student wrote of him in 1904: ‘It is very interesting to know a genius and I do, I am even together with him every day. I am talking about Niels Bohr … and besides he is the best, most modest human being that can be imagined.’ His humanitarian spirit came to the fore in later life when he, like many other scientists of his day, campaigned against indiscriminate nuclear arms development.
Bohr won a Nobel Prize for physics for his groundbreaking work on the structure of the atom. The element bohrium is named after him, and the Institute for Theoretical Physics in Copenhagen, which he headed in his lifetime, was renamed in his honour.
Early life
Niels Bohr was born on 7 October 1885 in a stately mansion owned by his maternal grandmother, a woman from a wealthy and influential Jewish banking family. His father, Christian Bohr, was appointed professor of physiology at the University of Copenhagen. Niels, his brother and sister grew up in a stimulating atmosphere in which the pursuit of knowledge was respected and encouraged, surrounded by intellectual discussion and culture. Bohr later said that philosophical discussions amongst his father’s friends at the house inspired him to look for unifying principles in human knowledge – a quest which he surely fulfilled in applying quantum theory to chemistry.
Bohr was not academically exceptional at school, usually coming third or fourth in a class of twenty. He was a skillful footballer, but in this he was outshone by his brother Harald, who played for Denmark in the 1908 Olympics, winning a silver medal. Despite keen competition on the football field, Niels and Harald were best friends, and remained inseparable all their lives. Some of Bohr’s discoveries were first related to his brother, in the letters they exchanged frequently.
A promising start
Bohr studied at the University of Copenhagen, but because there was no physics laboratory in the university, he was only able to carry out experimental work by using his father’s physiology laboratory. Even so, in 1906 he won the Gold Medal from the Royal Danish Academy of the Sciences for his measurement of the surface tension of water.
Bohr completed his PhD in 1911 and later in the same year went to England, intending to work with J.J. Thomson at the University of Cambridge. However, the two did not get on well and Bohr looked for an escape route.
He was fortunate to find one quickly. Ernest Rutherford had just published his discovery that most of the mass of an atom is in its nucleus (the centre). He was in Cambridge to give a talk on his work in October 1911, and Bohr heard him and was greatly impressed. When Bohr went to Manchester a month later to visit a friend of his father, Rutherford was invited to dinner. The meeting was a success, and in March of the following year Bohr joined Rutherford’s team in Manchester working on the structure of the atom. He adopted Rutherford as something of a role model, both professionally and personally. The two became firm friends, though they had very different characters, and after Bohr left Manchester they wrote to each other frequently until Rutherford’s death in 1937.
A new atom
While at Manchester, Bohr worked with quantum theory developed by Einstein and Planck to explore his own theories about atomic structure and fix the faults he could see in Rutherford’s model. Although Rutherford’s model was a brilliant innovation and represented a huge leap forward, it didn’t quite work. In Rutherford’s atom, the electrons would slowly spiral into the middle, or could be knocked out of position by a nearby positive particle.
Bohr left Manchester after 6 months and returned to Copenhagen where he married his fiancée Margrette Nørlind in the summer of 1912. They were to have six sons, two of whom died young. The fourth, Aage, would eventually follow his father into physics and win his own Nobel Prize in 1975.
Back in Copenhagen, Bohr carried on working on his theory of the atom, publishing his theories in three papers in England in 1913. It was for this explanation of atomic structure that he was awarded the Nobel Prize for Physics in 1922. His work became the foundation of quantum mechanics, which developed during the 1920s, largely centred around his institute in Denmark.
In 1914, Bohr was to take up a new a professorship in theoretical physics in Copenhagen, but the start of the First World War delayed the creation of the post until 1916. In the meantime, Rutherford offered him a readership for 2 years at Manchester. He and his wife endured a dangerous journey by sea in the middle of the war to take up the position. The readership gave him the opportunity to continue with his research without having to devote time to teaching in the university.
On returning to Denmark and his new professorship, Bohr was elected to the Royal Academy of Sciences. In 1921 he became president of the newly established Institute of Theoretical Physics (sponsored by the Carlsberg brewery), which he had petitioned to open. He held this post until his death, and the institute was later renamed in his honour. His son Aage succeeded him as director in 1963.
The quantum atom
The key difference in Bohr’s model of atomic structure was that the electrons occupied distinct orbits, or shells, rather than whirling arbitrarily around the nucleus in a cloud.
An electron can jump between orbits but is never in between two orbits. Jumping to an orbit further from or closer to the nucleus is associated with absorbing or giving out energy.
The innermost orbit contains up to 2 electrons. The next may contain up to eight electrons. If an inner orbit is not full, an electron from an outer orbit can jump into it. Energy is released as light (a photon) when this happens. The energy released is a fixed amount, a quantum.
Hydrogen emission spectra – lines showing the light given out by hydrogen when bombarded with alpha-particles – provided evidence for Bohr’s model. The emission spectra show light is emitted in regular patterns as the hydrogen molecules’ electrons move between orbits.
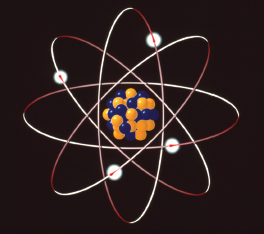
A diagram of Bohr’s model of the atom, with electron shells.
Unravelling the elements
Besides his work on quantum theory, Bohr pursued the implications of his model of atomic structure for the periodic table of elements. He showed that the characteristics of an element could be accounted for and even predicted by the configuration of electrons in its atoms, and so by its position in the periodic table.
Under Bohr’s guidance, the institute in Copenhagen attracted some of the leading physicists from abroad and became a world centre for work on atomic physics and quantum theory. Bohr himself travelled and lectured in Europe, the United States and Canada.
All change
In the mid-1920s, Bohr stressed that the new models of the atom on which he was working were theoretical – he foresaw a new shift and synthesis of ideas coming together. It arrived the same decade with the emerging field of quantum mechanics, which was grounded in Bohr’s quantum model of the atom. In 1927, Heisenberg published his uncertainty principle, which says that it is impossible to measure the position and energy of a particle, since the act of measuring will affect the particle and so alter its state.
In September 1927, Bohr took account of Heisenberg’s principle in explaining the concept of complementarity (see below).
Albert Einstein was doubtful about Bohr’s new interpretation of quantum theory, though in the end Bohr’s version prevailed. The two men debated the issue over many years, and although Einstein never agreed with him, Bohr acknowledged the huge value of their discussions in refining his ideas. In 1927 he wrote: ‘Anyone who is not shocked by quantum theory does not understand it.’
Atomic structure and the chemistry of the elements
Each of the elements has an atomic number, starting with hydrogen, with an atomic number of one. The atomic number corresponds to the number of protons in the element’s atoms.
Bohr had already shown that electrons inhabit fixed orbits around the nucleus of the atom. Atoms strive to have a full outer shell (allowed orbit), which enables them to have a stable structure.
They may share, give away or receive extra electrons in order to achieve stability. The way that atoms will form bonds with others, and the ease with which they will do it, is determined by the configuration of electrons.
As elements are ordered in the periodic table by atomic number, it can be seen that their position in the table can be used by scientists to predict how they will react in combination with others.
Complementarity
Bohr’s theory of complementarity states that electrons may be both a wave and a particle, but that we can only experience them as one or the other at any given time. He showed that contradictory characteristics of an electron can be proved in separate experiments and none of the results can be accepted singly – we need to hold all the possibilities in mind at once. This requires a slight adjustment to Bohr’s original model of atomic structure, in that it means we can no longer say that an electron occupies a particular orbit, but can only give the probability that it is there.
A difficult war
During the 1930s Bohr became interested in nuclear fission and the possibility of gaining energy from it. Nuclear fission involves the splitting of an atomic nucleus, causing a release of energy. Work on nuclear fission rapidly became part of the race to develop an atomic bomb as the Second World War unfolded. Lise Meitner, who had escaped from Nazi-occupied Austria, brought news that the Germans were researching nuclear fission. And on a visit to Bohr, Heisenberg revealed that Germany was working on an atomic bomb – indeed, Heisenberg was in charge of the project. He later claimed that he and Bohr came to an understanding that Heisenberg would undermine the project if it looked as if it would succeed, but Bohr denied such an agreement was ever made.
When Hitler began persecuting Jews in Germany, Bohr offered a safe haven at the Institute in Copenhagen for many Jewish scientists, and after the outbreak of war he even donated his gold Nobel medal to the Finnish war effort. When the Germans invaded Denmark in 1940, Bohr’s Jewish descent made life difficult for him, especially as he made no secret of his anti-Nazi feelings. In a daring escape, he and his family fled to Sweden in a fishing boat provided by the Resistance movement. From there they went to England, hiding in the empty bomb rack of a British Mosquito plane sent to pick them up.
Safely in England, Bohr joined the war effort to develop the atom bomb ahead of the Germans. He and his son Aage later moved to Los Alamos in the United States with the rest of the British research team to join the Manhattan Project. But Bohr was not happy about the development of nuclear weapons, and in 1944 tried to persuade both Roosevelt and Churchill that international cooperation would be a better path forwards in the development of nuclear fission. Churchill was annoyed that Bohr thought their knowledge should be shared with the Russians and that he favoured post-war arms control. In 1950, Bohr wrote to the United Nations to put his case against unilateral development of nuclear arms.
In 1955 Bohr organised the Atoms for Peace Conference in Geneva. He was also a leading figure in the foundation of CERN, the Centre for Nuclear and Particle Physics Research in Switzerland, founded in 1954. He died in Copenhagen after a stroke on 18 November 1962.