 3
3 
 3
3 
Oil and water may not mix, but with a little help, the two can join together in an emulsion. You can have an emulsion of fats in water, such as milk, cream, or most salad dressings, or you can have an emulsion of water in fat, as you do in butter and peanut butter.
It is common to say that oil and water don’t mix, but lots of things don’t mix. Air and water, air and oil, sand and water, sand and oil, sand and air—all of these will separate into layers if you put them in the same container.
What makes things separate is the attraction that molecules have to each other. Water molecules bind to one another using bonds that are stronger than the ones that air molecules bind with. So the water molecules join up and leave the air behind. Water molecules by themselves are lighter than air (a water molecule has a molecular weight of 18, while air is made of nitrogen molecules that weigh 28, and oxygen molecules that weigh 32). But when the water molecules bind together, the bond is strong enough to pull the molecules close, and the density goes up, and it rains.
The molecules in sand or steel bind even tighter than water, and these materials become dense solids. The molecules in oils and fats bind to one another only with weak bonds. As with air, the water molecules bind together, leaving the oil behind. Oil and fat molecules have long chains of atoms that are bulky and tangle together, making them less dense than water, more viscous, and in the case of fats with really long chains, solid.
When you make foams, you get air and water to mix and stay stable. You can use the same tricks to make oil and water mix and stay stable—just use a molecule that has both a part that likes water and a part that doesn’t.
Proteins work, and so do smaller molecules, such as soap and detergent. A soap is basically a fat attached to a water-loving element, such as sodium or potassium. While good at making emulsions in the dishwater, soaps have a flavor most people dislike and are not used in fancy French sauces.
Luckily for the cook, plants and animals find these double-ended molecules very handy as well, and they produce them in large quantities. Cell walls are made of things called lipid bilayer membranes. These are sheets of molecules called phospholipids. They have water-loving sides and water-avoiding sides. In water (in the cell) they join up back-to-back, keeping their water-loving sides facing the water—thus the “bilayer” part of the name.
 Chemistry Lesson
Chemistry LessonSome atoms, such as chlorine, fluorine, oxygen, and nitrogen, have strong affinities for electrons. When these atoms covalently bond to hydrogen, the electrons seem to spend more time near the heavier atom than near the hydrogen.
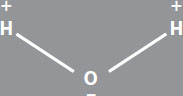
This makes one part of the resulting molecule a little bit positive, and the rest a little bit negative. When molecules like this get together, their negative sides are attracted to one another’s positive sides, and a weak bond is formed. This is called a hydrogen bond.
Water forms hydrogen bonds because the oxygen side is more negative than the side that has the hydrogens. The water molecules can also form hydrogen bonds with other molecules that have similar areas of different charges. Molecules that have a positive side and a negative side are called polar molecules.
If you grind up the cell walls, and mix in a little bit of oil, you can get those layers to open up to make a single membrane around the oil droplet, with the water-loving side out, facing the water. This water-loving side prevents other oil droplets from combining with this one.
A common phospholipid is lecithin. It is found in the yolk of eggs, but commercially it is extracted from soybeans, which are cheaper. But there are phospholipids in almost every living cell, whether plant or animal.
When you mention emulsion to a cook, he or she will think of mayonnaise. In mayonnaise, the emulsion is stabilized by the lecithin and proteins in the egg yolk, and the phospholipids in the ground mustard. Other emulsified sauces are stabilized by the phospholipids from garlic or some other plant material.
Another stabilizer for emulsions is vegetable gum. Gums are starchlike large molecules that form thick colloidal suspensions in water. These have the effect of keeping the oil droplets from recombining simply by getting in the way, forming semirigid walls between the droplets. The gums in mustard and in garlic help to stabilize emulsions that contain them.
Sometimes it seems like people want to make traditional emulsion sauces just because they are notoriously hard to make. Using a double boiler to prevent the eggs in a hollandaise sauce from scrambling is considered cheating by some, despite the results being identical to the traditional method. Adding a pinch of xanthan gum when making a beurre blanc sauce will prevent it from separating, but purists would never hear of doing so.
The advantage to the practical cook is that you can use whatever methods you like, and then name the resulting sauce after yourself, even if it tastes just like hollandaise or béarnaise sauce.
There are plenty of recipes and long discussions about how to make traditional sauces in the traditional ways. I’d rather talk about the cheats.
Getting the emulsion started is one common problem. You are advised to add the oil to the beaten eggs and mustard very slowly at first, and to add only a small amount of the acidic vinegar or lemon juice at first as well. Later, when the emulsion has started, you can pretty much dump oil and vinegar in wholesale. But I have never heard anyone suggest the obvious solution: just start with a dollop of yesterday’s mayonnaise beaten into the egg and mustard, then dump in the other ingredients. That works.
Adding 1/8 of a teaspoon of xanthan gum to any oil and water emulsion will remove most of the barriers to success. You can be much more relaxed about amounts and rates, and it will be much more resistant to separation.
Some emulsions, such as hollandaise sauce, call for cooking an egg-stabilized emulsion. The proteins in an egg are easy to denature with heat. That is what you see happening when an egg white turns from transparent to actually being white. The egg proteins start out carefully folded up so that they can do the job they do in the cell. You heat them to get them to open up, so that they tangle together and prevent the flow of water in the mixture, and so the oil-loving parts can find the oil. If you heat them too much, they will start bonding together into a strong rubbery network, giving you scrambled eggs instead of a thick sauce.
Eggs begin to coagulate at 160°F to 170°F (70°C to 77°C). Not coincidentally, this is also the temperature needed to coagulate the proteins in salmonella and other pathogens, thus killing them. In order to cook the eggs well enough to kill bacteria, but still prevent them from scrambling, you can use a well-known trick from both chemistry and your mother’s cookbooks: add an acid to the eggs.
Acids prevent some of the chemical bonds from forming between the proteins until the temperature gets much higher, closer to 195°F (90°C). So adding some lemon juice or vinegar to the sauce will prevent the eggs from curdling, at least if you keep the temperature well below boiling.
Let’s walk through the creation of a hollandaise sauce and see what steps there are and why they are needed.
Some recipes start with clarified butter, while others use whole butter. Clarified butter is butter that has been heated until the emulsion breaks and the water and milk solids fall to the bottom, leaving pure butterfat at the top.
If the recipe uses clarified butter, you lose the parts of the butter that help it emulsify, and you lose the flavor elements of the milk solids, as well as some important water. An emulsion needs water as much as it needs oil. So a recipe using clarified butter will need more water or lemon juice than one that does not.
To clarify butter, the heat should be very low. You just want to separate the emulsion; you don’t want to burn the milk solids or boil the water in the butter. There will be some froth on the top of the clarified butter. This is discarded, along with the milk solids and water, since you only want the butterfat in clarified butter.
While the butter is clarifying, you make a reduction of white wine vinegar, crushed white peppercorns, white wine, and minced scallions. Simmer these until you have reduced the liquid by half, to about a tablespoon or two.
Once the butter is clarified and the wine reduced, bring water in the bottom pot of a double boiler to a simmer. The water should not be high enough to touch the top pot of the double boiler. You only want steam touching the top pot. This will keep the temperature low enough to prevent the eggs from curdling.
When the water has come to a simmer, take the pot off the burner. Put two egg yolks into the top of the double boiler, along with a tablespoon of the reduction. Immediately start whipping the egg yolks. Whip the yolks until they lighten to the color of butter, and they start to thicken.
Now you start drizzling in the butterfat. Just a few drops at first, allowing the butter to be absorbed as you continue to whip the eggs. When the eggs have absorbed about 4 ounces (8 tablespoons) of butterfat, you have started the emulsion, and you can add the rest of the butterfat more quickly.
Once all of the butterfat has been whisked in, you can add other flavorings, such as lemon juice, cayenne pepper, Tabasco sauce, or Worcestershire sauce, to your taste.
The sauce is generally served right away, since it must be kept warm enough to prevent the butterfat from congealing and separating the emulsion. This obviously means that it can’t be refrigerated, so there is no safe way to keep it for another day.
Lecithin, the phospholipid found in eggs and cell walls, is one example of an emulsifying agent. We have also discussed proteins and detergents. Another class of emulsifier comes from taking apart a fat molecule (or not completing the building of a fat molecule).
Fats are triglycerides. This means that they have glycerin as a backbone, and attached to the glycerin are three (hence the “tri-”) fatty acids. If you remove one or two of those fatty acids, then part of the glycerin molecule is left available for attaching to something else, such as water. This would leave a molecule that has a water-loving end (where the glycerin is) and a fat-loving end (the remaining tails of the fatty acids). This would make a good emulsifying agent.
These partial fats are called monoglycerides or diglycerides, depending on whether there are one or two fatty acids attached to the glycerin. You may have read a food package label that mentions mono- and diglycerides. Now you know that they are there to stabilize emulsions or foams. They are just partial fats. Glyceryl stearate is an example you might see on a package label.
Various polysorbates are also commonly used as emulsifying agents. Again, a fatty acid is attached to a water-loving molecule. Polysorbates come in a wide variety, and the number after the name indicates the length of the fatty acid chain. Polysorbate 20 and polysorbate 80 are common examples; the latter is used in ice cream to modify how the proteins coat the fat droplets. Polysorbate 60 is used in hot cocoa mix.
Similar compounds are ceteareth alcohol, cetyl alcohol, or stearyl alcohol. These are compounds known as emulsifying waxes.
Emulsifying agents generally dissolve better in one part of the emulsion than the other. Molecules such as proteins, which dissolve better in water than in oil, will help to make oil-in-water emulsions, such as milk and cream. Molecules that dissolve better in fats and oils help to make water-in-oil emulsions, such as butter and margarine. Churning cream helps to denature some of the proteins in the cream until they fold in a way that makes them dissolve better in fat, and the emulsion becomes one of water in solid butterfat, and you get a solid.
To make a water-in-oil emulsion like margarine, you would use an emulsifying agent that dissolves better in fat. Something with a longer fatty acid chain would dissolve better in fat than one with a shorter fatty acid chain.