 6
6 
 6
6 
Solutions are just mixtures in which two or more substances are well mixed (homogeneous). We are used to thinking of liquid solutions for which a solid substance (the solute) is dissolved in a liquid substance (the solvent). But liquids can be dissolved in other liquids, and solids can be dissolved in solids, and gases can be dissolved in liquids.
Some questions this section will attempt to answer are:
To begin, look at table salt dissolving in water. Water is a polar molecule, with one side slightly positive and one side slightly negative.
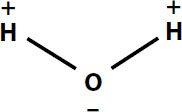
Ionic bonds were discussed earlier. An ion is formed when an atom loses an electron, or gains one, and becomes charged. Table salt is an ionic solid composed of positively charged sodium ions and negatively charged chlorine ions. Their opposite charges make them stick together closely, making the result a dense solid. The ions are created when sodium atoms lose electrons to chlorine atoms.
When solid table salt is dropped into water, the positive ends of the water molecules are attracted to the negative chloride ions, and the negative ends of the water molecules are attracted to the positive sodium ions. The attraction goes both ways—the electrons in the chloride ions move closer to the water molecules, and the electrons in the water molecules move closer to the sodium ions. This makes the attraction between the sodium ions and the chlorine ions weaker. Several water molecules exert a stronger attraction to the ions than the ions have for one another, and the ions move into the water. The salt has dissolved.
The reaction is reversible. Ions in the water attract one another when there are not enough water molecules to overcome the attraction, and salt crystals grow. As the area next to a dissolving crystal of salt becomes saturated with ions, the rate of dissolving matches the rate of crystal growth. Things would come to a standstill if it weren’t for the motion of the water molecules. That motion carries the ions farther away and allows more water molecules to get close to the salt, so the dissolving process continues.
When the solution eventually becomes so full of ions everywhere that the rate of crystal growth matches the rate of dissolving, the solution is said to be saturated. The saturation point is affected by the heat of the solution. Heat is just the motion of molecules, so when the solution is hotter, the molecules jostle around more. This makes it more likely that an ion will leave the crystal than that one will stick to the crystal, so more salt will dissolve in hot water than in cold water.
To make the crystals dissolve faster, stir the water. This mixes the pure water with the saturated solution close to the crystal, so there are more water molecules next to the crystal than there would have been without stirring.
A similar effect happens when sugar is dissolved in water. Sugar is not an ionic solid like salt. Instead, it is a polar molecule, like water. There are places on the molecule that are slightly more positive and places that are slightly more negative. These areas attract one another, and because there are many of them—the molecule itself is somewhat large—sugar is a solid.
In water, the positive and negative parts of a sugar molecule attract the negative and positive parts of the water. Again, the attraction of a lot of water molecules is stronger than the attraction of the sugar molecules to one another. The sugar dissolves. It takes energy to break the bonds that make salt or sugar solid. The energy can come from many sources. One is the heat of the water and the salt itself. Another is the attraction of the water molecules to the molecules of the salt or sugar. Just as the gravitational attraction between the earth and the water behind a dam can provide energy for generating electricity, the attraction between the polar water molecules and the polar molecules in the solid can weaken the strength of the bonds in the solid.
Something like sand does not dissolve in water. The molecules in sand are bound so tightly together that their attraction to one another is stronger than their attraction to water, so nothing happens.
When a nonpolar molecule like Styrofoam dissolves in a nonpolar solvent such as gasoline, similar processes take place. The bonds involved are generally weaker, and diffusion becomes the most apparent force. Diffusion is the tendency of things that are separate to become mixed up when randomly shaken by the motion of molecules (heat). If you drop a bit of food coloring into a glass of water you can watch it slowly diffuse until the entire liquid is a uniform color.
In the same way, the Styrofoam molecules gradually diffuse into the gasoline until the two are completely mixed. The same effect happens when salt or sugar dissolves in water, of course, but without water’s polar nature, the relatively strong bonds between the salt molecules or the sugar molecules would prevent diffusion from happening. This is why salt and sugar do not dissolve in oil.
Higher temperatures generally increase solubility, but the effect differs for different substances. At just above freezing, about 356 grams of salt will dissolve in a liter of water. At just below boiling, 390 grams will dissolve, which is not a big increase. For sugar, however, the amounts go from 1,790 grams at near freezing to 4,870 at near boiling.
Adding other substances can affect solubility. After you have dissolved as much sugar as possible into water, adding a little salt will allow more to dissolve. In effect, some of the sugar is dissolving in some of the salt, and vice versa. The various salts in mineral water can thus make a difference in candy making.
It is possible to supersaturate a solution. You can dissolve as much sugar as possible into hot water and then carefully cool it without stirring, and the sugar will remain dissolved. Drop a few crystals of sugar into the solution at that point, and they will grow into large sugar crystals (rock candy) as the sugar crystallizes out of the solution. The sugar molecules are more strongly attracted to one another than to the smaller number of water molecules available in a supersaturated solution.
Gases also dissolve in liquids. Carbon dioxide dissolves in water to make carbonated water. Here, temperature has the opposite effect. Since carbon dioxide is a gas, there are no bonds holding it together that have to first be broken before it can dissolve. But the bonds between the gas and the liquid break more easily with increased temperature. Thus more carbon dioxide will dissolve in cold water than in warm or hot water.
The nitrous oxide in whipped cream cans, or “whippits,” exhibits the same effect. More of it will go into solution in very cold cream than in warm or room temperature cream.
When making ice, you sometimes want to prevent bubbles of air from forming in the ice so it stays clear. As water freezes, crystals of solid ice form. Crystals are, in general, very pure—as layers of molecules accumulate in the crystal, foreign molecules are left in the remaining liquid. Eventually, they exceed the saturation point and come out of solution. If the foreign material is dissolved air, bubbles in the ice are the result.
To prevent the bubbles, boil the water before freezing it. The high temperature makes most of the air come out of solution, so there is little left in the water when it is subsequently chilled. Since air can dissolve back into the water as it cools, cover the water with a sheet of plastic wrap to prevent contact with the air.
There are many solutions in the kitchen. Beef broth is a solution of salt, proteins, sugars, and other small molecules. Sodas are solutions of sugar, flavorings, and carbon dioxide. Vinegar is a solution of acetic acid and small flavor molecules in water.
In the previous discussions about colloids, sauces were thickened using small amounts of proteins, starches, or other large molecules. But because you can dissolve almost four and a half pounds of sugar in a quart of water, even a small molecule like sugar will thicken water if there is enough of it. This is no surprise—at those ratios, there are only about 10 molecules of water per molecule of sugar. In boiling water, where you can dissolve even more sugar, there are only about four molecules of water per molecule of sugar.
The density of maple syrup, honey, and corn syrup is about 1.3 times that of water. Thus a liter of syrup has about 300 grams of sugar in it, about 10 ounces per quart.
The sugar in corn syrup is glucose. It is made by using enzymes to break down cornstarch, which is made up of chains of glucose molecules. Glucose is not as sweet as sucrose, so corn syrup does not taste as sweet as sugar syrup does. Adding to the viscosity are long chains of glucose molecules that were not completely broken apart by the enzymes used to change starch into glucose.
High-fructose corn syrup is made by using other enzymes to convert some of the glucose into the sweeter simple sugar fructose. To get a syrup that has the same sweetness as sucrose syrup, a ratio of 55 percent fructose to 45 percent glucose is used. This is called HFCS 55. This is the same ratio of fructose to glucose that honey has. Because US corn is subsidized and sugar has tariffs and quotas, high-fructose corn syrup is less expensive than sugar. But for the most part, it is flavorless honey.
Honey itself is a supersaturated solution of fructose, glucose, maltose, and sucrose. The water and sugars make up 99.5 percent of the weight. The other 0.5 percent is a mix of protein, amino acids, vitamins, and minerals, although the amounts are minute in comparison to the recommended daily allowances of those nutrients. Honey is between 25 percent and 44 percent fructose and between 24 percent and 36 percent glucose, with the other sugars making up less than 9 percent by weight.
Sucrose syrup can be heated with a small amount of acid to separate the fructose and glucose. The result is invert syrup, which is slightly sweeter than the original sucrose syrup. It is also less likely to crystallize. Both of these properties come into play when making jams and jellies, where the fruit adds the acid needed.
Solutions are not always liquid. Many hard candies are solid solutions of sugar, water, and flavorings.
Rock candy, where sugar crystals are carefully grown to large size, is not a solution. But lollipops, suckers, and other clear, hard sugar candies are a form of glass made from sugar.
In making hard candies, several tricks are used to ensure that the sugar does not crystallize into an opaque, gritty texture. Corn syrup is used along with the sugar because the unbroken chains of glucose left over from converting starch help to prevent crystallization. Acids such as tartaric acid or citric acid are used to convert the sucrose into fructose and glucose. Having two dissimilar simple sugars in the solution also helps to prevent crystallization, which occurs best with pure substances.
The water, sugar, corn syrup, and acids are cooked together until the amount of water falls to a very low level. When the solution comes to a boil, no further stirring or agitation is done, to prevent the fructose and glucose from joining back together into sucrose. Any crystals of sucrose are washed from the walls of the pan to prevent them from acting as “seed” crystals, and making the whole batch of candy suddenly crystallize into a gritty or sandy texture.
Flavorings boil away if added too soon, so generally once the mixture has been cooked to the hard-crack stage (310°F, 154°C), it is cooled to 275°F (135°C) before the volatile flavorings such as vanilla or mint oils are stirred in.
The mixture is then cooled quickly to prevent crystallization, often on a marble slab or a baking pan turned upside down to speed the cooling. Fast cooling freezes the molecules in place, giving them no time to rearrange themselves into precise crystalline order.
Liquids can also dissolve in other liquids. Some liquids behave like any of the solids previously discussed when dissolving. They will only dissolve a certain amount and then the solution saturates. No more will dissolve, leaving the liquids in two distinct phases.
Other liquids, such as ethanol and water, will dissolve in one another in any proportions. They are said to be completely miscible in one another. This feature of ethanol and water allows alcoholic beverage makers the entire range of potencies—from less than 1 percent alcohol content to 100 percent alcohol.
Many flavor elements are nonpolar oils and fats. Because they are nonpolar, they dissolve in alcohol better than they do in water. But if there is not enough alcohol (too much water), they come out of solution. Absinthe becomes cloudy, or oils begin to float on the surface of the liquid. Sometimes this is beneficial, bringing the aromatic oils closer to the nose as ice melts in a glass.
The opposite effect happens with sugar, which dissolves better in water than in alcohol. Having both solvents in the same glass allows a sweet drink to contain aromatic oils and other flavors that do not dissolve in water.