 11
11 
 11
11 
We heat food for many reasons; cooking the food is only one of them.
Water for tea or coffee is heated because the volatile oils and flavors dissolve better in hot water, or are released into the water when fats melt or membranes break. Volatile components that are vaporized by the heat then reach our nose, creating aroma and flavor.
Heating starches changes crystallized starch molecules into gels. Bread becomes stale when the starches crystallize, and warming the bread returns them to their soft gel state, making the bread taste and feel fresh. Stale bread is not dry; it just feels that way because of the crystallized starches. In raw potatoes, the starch is compact, but heating makes the starch granules swell and absorb water, becoming soft and easier to digest.
Heating meat causes the tough collagen connective tissue to denature and soften into a gel. Heating it more causes the other proteins to harden, and we get crisp bacon.
There are two ways that foods become brown: through the action of enzymes, as when a sliced apple turns brown, and without enzymes. In the latter category, you can break the browning process down further into three groups: caramelization, where sugars react with one another; ascorbic acid oxidation, where vitamin C reacts with oxygen; and Maillard reactions, where sugars react with amino acids.
The Maillard reaction is complex. The reaction of the sugar glucose and the simple amino acid glycine gives more than 24 reaction products. In many foods, there are five or more sugars and as many as 20 or more amino acids, and they can all react when heated to create the brown color found on toast. That color is made up largely of melanoidins—large molecules that polymerize from the products of the Maillard reaction. Melanoidins have antioxidant properties, like many other food colors, and can bind to metal ions like iron and take them out of solution (a process called chelation, named for the chelae, the claw of a crab, since they grab onto the ions).
Five-carbon sugars such as ribose react more easily than six-carbon sugars like glucose, or disaccharides such as sucrose or lactose. The amino acid lysine produces the most color when reacting with sugars, and cysteine produces the least color. Foods that are rich in lysine, such as milk proteins, brown easily. This is why milk is used as invisible ink. It does not take much heat to make the milk turn brown, so the ink browns before the paper does.
Maillard reactions produce flavors as well as colors. Melanoidins have their own flavors, but their long chains full of stickyside groups allow them to hold onto smaller flavor molecules that are also produced by the Maillard reaction, such as isobutyraldehyde and furfural, and hydroxymethylfurfural. These are then slowly released into the air as the aroma of toast, coffee, beer, and other foods that owe much of their flavor to the roasting or steeping process.
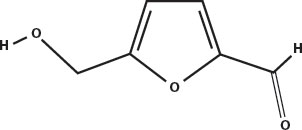
Hydroxymethylfurfural
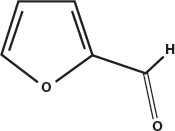
Furfural
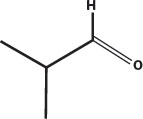
Isobutyraldehyde
Maillard reactions happen at room temperature (one of the reasons soils are brown), but they happen faster as temperatures rise. Above 248°F (120°C), the sugars in food combine with one another in the process known as caramelization. Fructose caramelizes at the lowest temperature, about 230°F (110°C). Maltose doesn’t caramelize until it reaches about 356°F (180°C).
Caramelization is another complex reaction, involving the breakdown of complex sugars into simple sugars, followed by polymerizations into larger molecules, oxidations, isomerizations (changing the shape of the molecule without changing the number and type of the atoms in it), and other reactions. The result is a brown color and the familiar odors and flavors of burned sugar.
As sugar is cooked, it first breaks down into glucose and fructose, and then these simple sugars combine by losing water molecules (an OH group on one molecule reacts with an H on the other, and they join up as the resulting H2O molecule boils away). The result is a type of molecule called a sucrose anhydride (meaning “without water”).
Heating sucrose at 392°F (200°C) for 35 minutes results in the loss of one water molecule for every molecule of sucrose, producing a molecule called isosacchrosan. After another 55 minutes, the total water loss is four molecules per molecule of sucrose, and the molecule caramelan is formed. Caramelan is a bitter-tasting molecule with the formula C24 H36 O18 Yet another 55 minutes of cooking causes every three sucrose molecules on average to lose eight molecules of water, and the molecule caramelen is formed, with the formula C36H50O25.
Heating the sugar further creates an even bigger molecule that is very dark and does not dissolve well, called caramelin, with the formula C125 H188 O80
Heating proteins destroys their carefully folded, three-dimensional structure and makes them no longer able to perform their normal functions. Egg white becomes a firm opaque solid, collagen loses its ability to connect bone and muscle and becomes gelatin, and enzymes lose their ability to efficiently catalyze chemical reactions. In the process, eggs become more palatable, meats become tender, and foods spoil less easily.
Heat is used to change the structure of proteins in many ways. When making cheese, curds are heated. This causes the proteins, which were already denatured by acid and the rennet enzyme, to bunch closer together and expel the whey to make the cheese firm and less likely to spoil.
In making custard, egg proteins are heated to make a firm gel, but we try not to overheat them. Overheating would cause the protein to contract and expel too much moisture, turning the custard into sweet scrambled eggs.
In making bread with milk, recipes sometimes tell you to scald the milk first. This denatures a protein in the whey that interferes with the volume of the loaf as it rises and bakes. Of course, overheating the milk scorches it, welding the milk proteins to the bottom of the pan, making it very difficult to scrub clean. Pasteurization and scalding perform the same functions, and many recipes that call for scalded milk were developed before most milk was pasteurized.
Sometimes foods are heated simply to drive off extra water to thicken the sauce, soup, or stew. In the process you get various reactions that change the flavor, color, and texture of the food, for the better or for the worse, but the main goal is reduction of volume and concentration of flavor.
Heating is a fast way of drying foods. Sliced apples dried in a 250°F (121°C) oven become crisp and light and are much less likely to spoil. Actually cooking the apples using a higher heat destroys the cell walls and some of the nutrients, while drying slowly without heat allows time for enzymes and microbes to spoil the food.
Heating food seldom makes it more sweet, sour, or salty. The other sensors on the tongue—those for bitter and savory tastes—are more often involved, especially if the food is burned. Heating sucrose can cause it to break down into glucose and fructose, and that combination is sweeter than the original sucrose. However, reactions like that are not the general rule.
For the sensors in the nose, however, heating is more often a benefit, as volatile molecules are freed and aromas are created and released. Maillard reactions and caramelization produce both bitter tastes and many volatile aroma molecules.
Heating also causes chemical reactions that don’t happen at lower temperatures, or happen much more slowly. Many of those chemical reactions create flavor molecules. We have already looked into the most famous reactions—the Maillard reaction and caramelization—but other effects also create flavor when foods are heated.
Fats release flavor when they melt. Fats with higher melting temperatures retain volatile flavor and aroma molecules until the temperature exceeds their melting point. Vegetables release flavors and aromas when the pectins in the plant cell wall dissolve. Onions benefit from more than just the browning reactions. Heating deactivates the tear-producing molecules in them and makes them more pleasant to eat.
But not all heating produces beneficial results. Volatile flavor and aroma molecules can escape during heating and not be available when the food is served. Fats can oxidize, causing bad tastes or smells. Overcooking cabbage produces hydrogen sulfide, the foul-smelling gas that makes rotten eggs unpleasant.
Muscles in cattle, pigs, birds, and fish contain a compound called phosphocreatine. At high temperatures—well above boiling, near 400°F (204°C) and above—it reacts with the amino acids in the proteins of the meat to create compounds called heterocyclic amines.
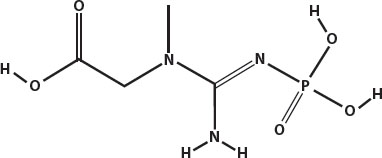
Phosphocreatine
Studies at the National Cancer Institute have shown that there is a link between people with stomach cancer and the consumption of cooked meats. These studies showed that the risk of stomach cancer was three times higher in people who cooked their meat well done than in people who cooked it medium rare. Other studies linked well-done meats to colorectal, pancreatic, and breast cancers.
The principal heterocyclic amines in overcooked muscle meats are imidazoquinolines (called IQ for short) and imidazopyridines (called IP for short). Five of the most prevalent are shown below. The word heterocyclic in this case means that they have a five-carbon ring attached to a six-carbon ring. The amine part is the nitrogen at the left that is attached to two hydrogens.
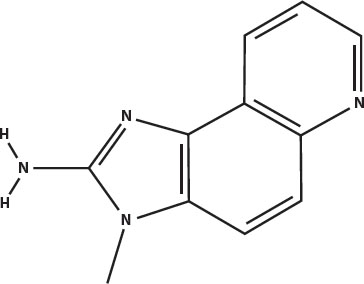
IQ: 3-methylimidazo(4,5-f)quinoline-2-amine
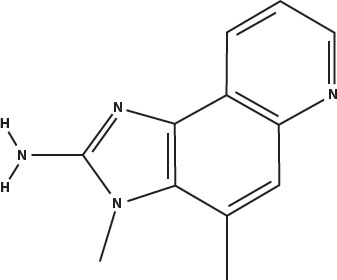
Methyl IQ: 2-amino-3,4-dimethylimidazo(4,5-f)quinoline
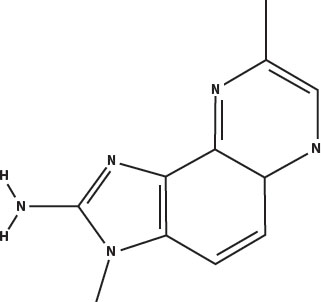
Methyl IQx: 2-amino-3,8-dimethylimidazo(4,5-f)quinoxaline
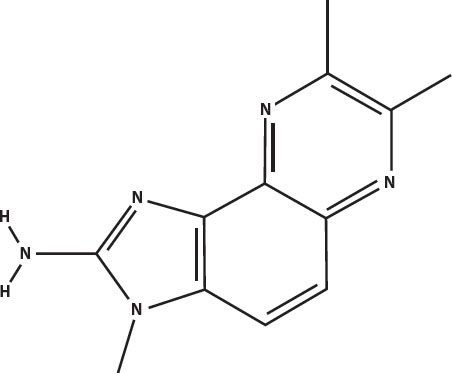
Dimethyl IQx: 2-amino-3,7,8-trimethylimidazo(4,5-f)quinoxaline
Phenyl IP: 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine
Eating well-done meat increases your risk of cancer by 0.011 percent. For comparison, smoking cigarettes increases your risk of cancer by 7.9 percent.
Stewing the meat, where the temperature never rises above the boiling point of water, does not create these molecules. Microwaving the meat before grilling it reduces the precursors of these molecules, so much less is created during the high heat treatment on the grill. This reduces the cancer risk by 95 percent (to 0.00055 percent).
The same Maillard reactions that produce the flavors and aromas of heat-browned foods can also produce another type of carcinogen. Found especially in deep-fried potatoes such as french fries and potato chips, acrylamide has been shown to cause cancer in laboratory animals.
High-carbohydrate foods heated to above 250°F (121°C) are what create acrylamide, by reacting the amino acid asparagine with sugars.
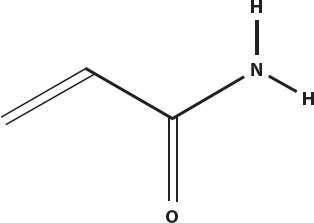
Acrylamide
However, studies in Swedish men found no connection to prostate and colorectal cancer when normal dietary amounts of acrylamide were eaten, and other studies showed no link to lung cancer in men, or endometrial cancer in women, and showed that it may in fact be protective against adenocarcinomas in women. One study of men and women in the Netherlands found some indications of a positive association between acrylamide and renal cancer risk.
So, while acrylamide is considered to be a probable human carcinogen, epidemiological studies have not shown any association between dietary acrylamide and cancer risk. Humans and rodents may have different absorption rates for this molecule, which might explain why laboratory animals get cancer from it while humans don’t seem to.
One of the more obvious effects of heating foods is that they often change color. The browning of the Maillard reaction is one type of color change, but many others are also apparent.
Meat contains myoglobin, a red pigment that gives meat its color even when the red blood has been removed. When myoglobin is heated, it reacts with oxygen and turns brown, giving cooked meat the color we see when we slice into a well-done steak or pot roast.
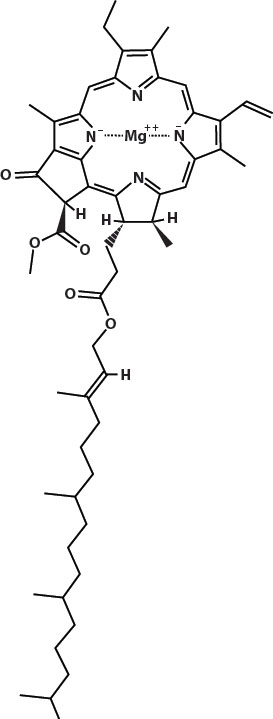
Chlorophyll, the pigment that gives green vegetables their color, contains a central atom of magnesium. In cooking, that magnesium atom may be lost and replaced with a hydrogen atom. This changes the molecule enough that it turns a more grayish pale or yellow green. The color change also allows the red and orange carotenoid pigments to show through.
Plant cells have pockets of air in them that hide some of the green color of the chlorophyll. When heated, these gases expand and escape, and the result is the bright green color of lightly steamed broccoli. Cooking too long, or in an acid, will result in the loss of the magnesium and the dull color of overcooked vegetables.
Cooking makes many nutrients in foods more available. Starches swell and soften, tough connective tissue turns into gelatin, and the pectins in plant cell walls soften into jelly.
Heating food can also protect some nutrients like ascorbic acid from being destroyed by natural plant enzymes. Heating can also destroy some antinutritional factors such as tannins in food and thus make the food more nutritious.
Heating can also destroy nutrients. Thiamin decomposes when heated in alkaline solutions, and eggs are slightly alkaline and become more alkaline with age. Boiling an egg results in an average loss of 15 percent of the thiamin in the raw egg.
And the Maillard reactions make the amino acids in proteins unusable as protein building blocks, although in general only a tiny portion of the protein in something like a steak participates in the reactions at the surface.
Gases expand when heated. This is an important effect in the baking of any foam, such as cakes and breads. Cooks call the extra rising that occurs in the first few minutes of baking “oven spring.”
Some baked goods such as popovers get all of their leavening when the water in the batter turns to steam, and the steam continues to expand with extra heat.
Another effect of heat comes into play in “double acting” baking powders. These powders are made from baking soda and two different powdered acids. One acid reacts immediately when water is added, and the acid then combines with the baking soda to create carbon dioxide gas. But the second acidic powder, sodium aluminum sulfate, reacts slowly at room temperature, but reacts much more during baking. This allows more time for a batter to be mixed or blended in the kitchen before baking. It ensures that the gases lost as the batter is prepared are replaced as it cooks in the oven.